Land-Use Redistribution Compensated for Ecosystem Service Losses Derived from Agriculture Expansion, with Mixed Effects on Biodiversity in a NW Argentina Watershed
Abstract
:1. Introduction
2. Materials and Methods
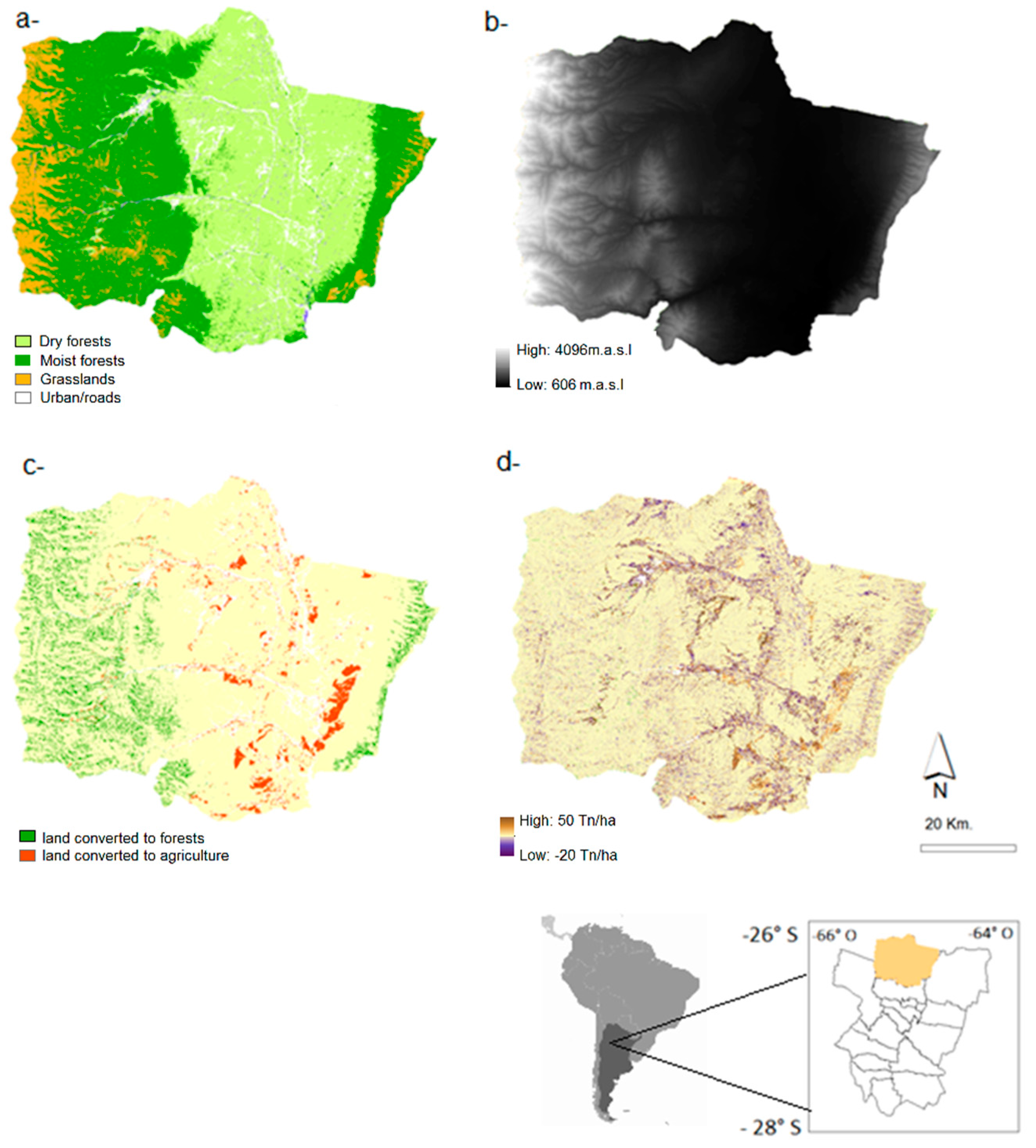
2.1. Study Area
2.2. Land-Cover Change, Ecosystem Services, and Diversity Estimations
2.2.1. Land-Cover Maps
2.2.2. Ecosystem Services
- R = rainfall erosivity factor (J/ha), the erosion potential of rainstorms;
- K = soil erodability factor; mean soil loss (Tn/J) by unit of rainfall erosivity;
- LS = slope length and slope steepness (adimensional);
- C = vegetation cover factor (adimensional); and
- P = conservationist practices factor (adimensional).
2.2.3. Biodiversity: Birds and Medium-Large Mammals
- We compared the diversity and composition of both groups among dry forests, moist forests, and montane Alnus acuminata forests, respectively, using ANOVA and ANOSIM, to assess changes in species composition along the topographic gradient.
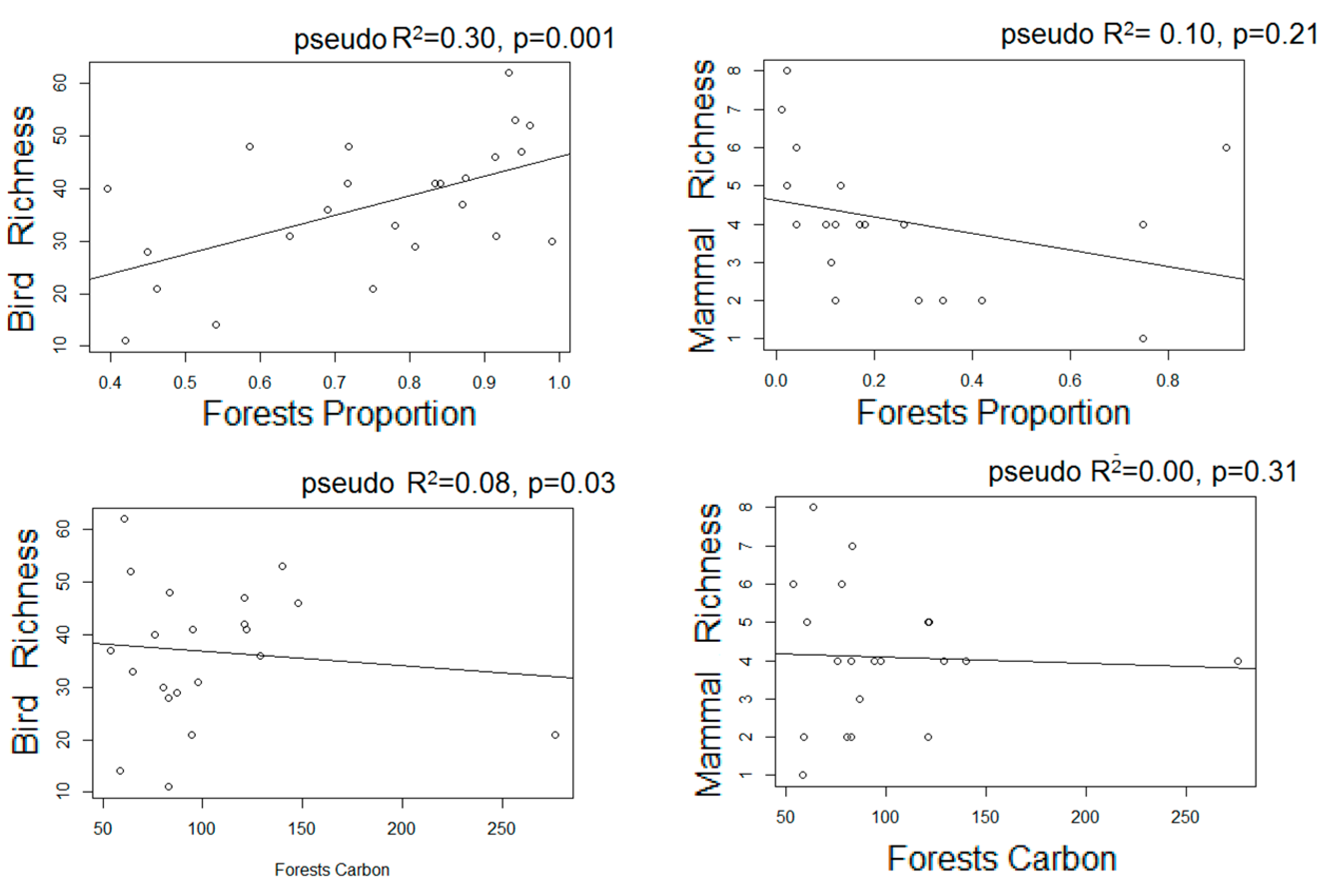
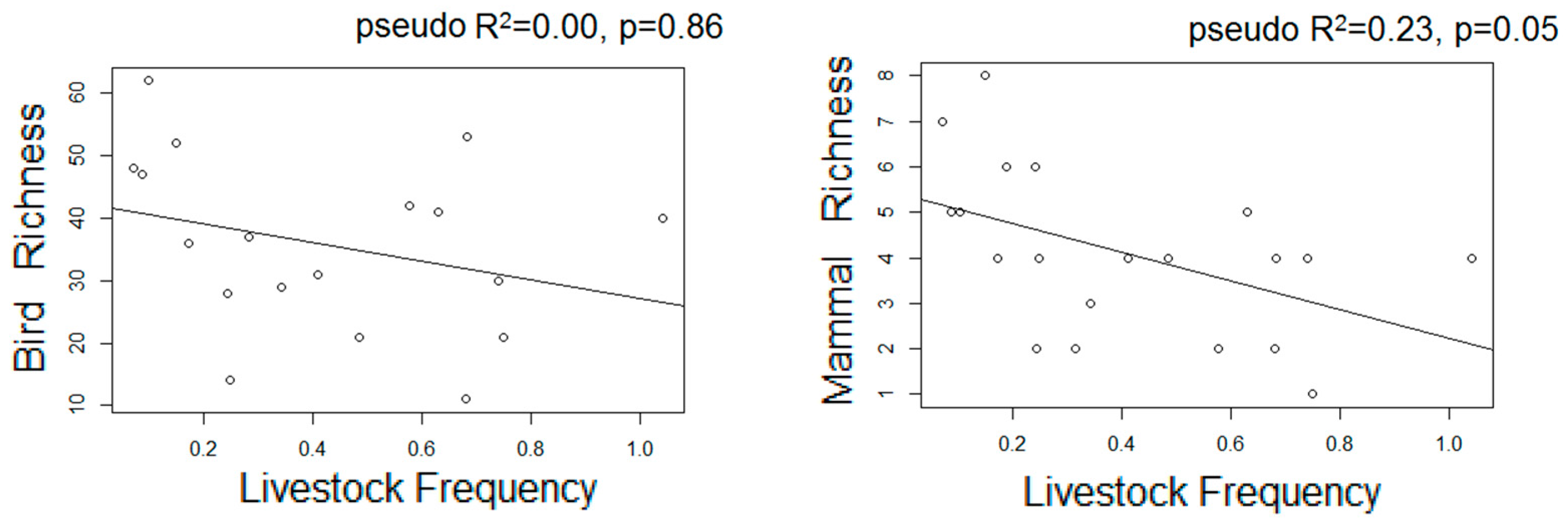
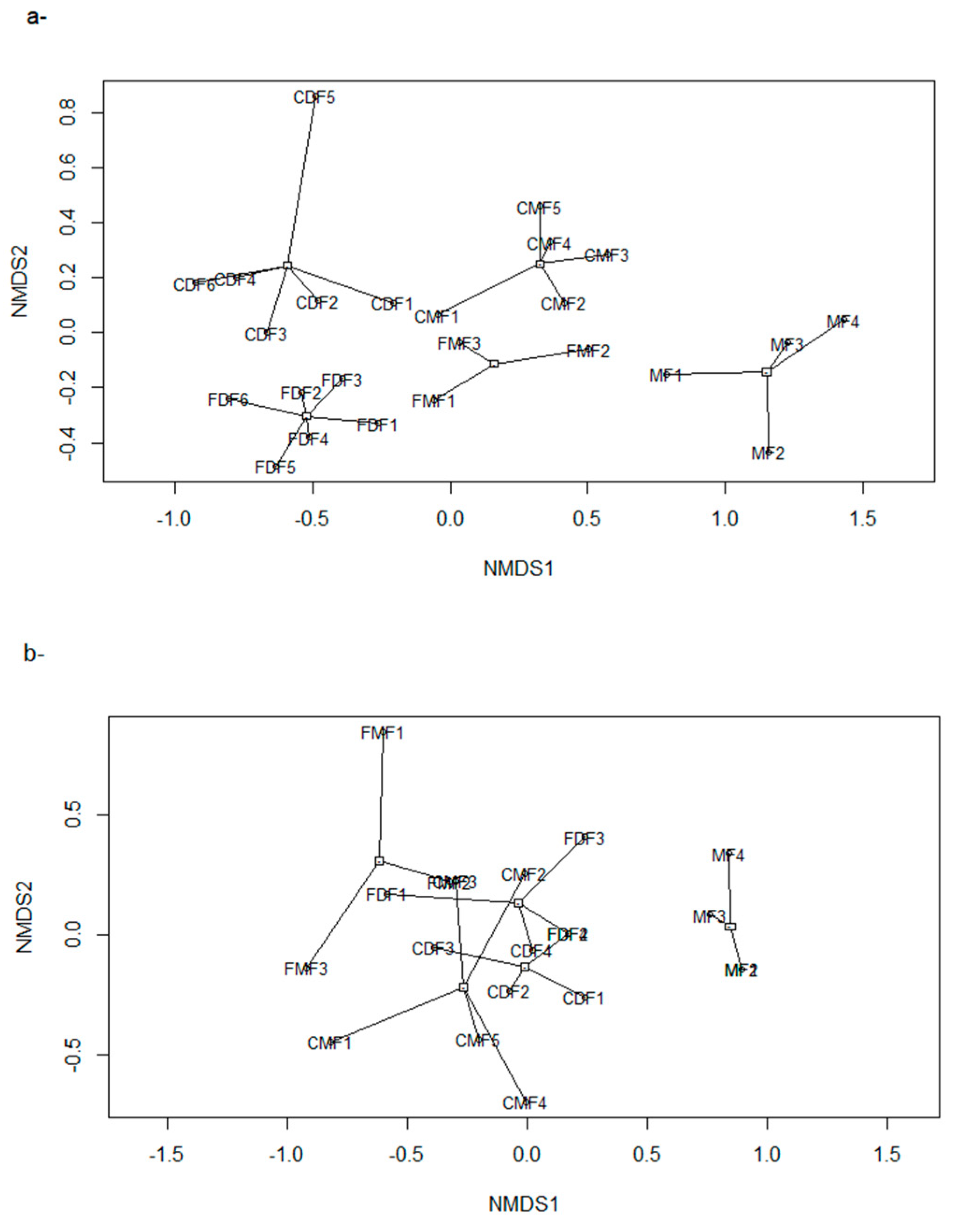
- We used Poisson generalized linear models to evaluate the relationship between species richness and proxies of intensive and extensive land-use practices: proportion of forested area, forests carbon, and frequency of extensive cattle ranching. Additionally, we performed non-metric multi-dimensional scaling ordinations based on a matrix of Bray–Curtis distances [50] between pairs of sites two describe their similarity in terms of composition. We chose the number of dimensions of the final MDS configuration based in stress values (the index of concordance between the distances in the graphic configuration and the distances in the data matrices) lower than 0.2 and the lower number of axes possible. We then assessed, a posteriori, the influence of the land-use proxies and altitude over the ordination of the sites in the multidimensional space by means of correlations between the values of the axes of the ordination and the values of variables, assessing their significance with random permutations [51]. Field sampling design and the proceedings for obtaining the variables are described below: based on the land cover maps for 2006 and Google Earth®, we located 24 sampling units corresponding to forested areas with varying degrees of continuity and fragmentation, and evenly distributed along the elevational gradient of the area (n = 10, 10, and 4 in dry, moist, and montane forests, respectively). At each sampling unit, birds were sampled in observational transects from the edge to the interior of the forests at hours of higher bird activity (8:00–10.30 am; [52]), in which the first author recorded the richness and abundance of all the individuals heard or seen. Bird sampling extended for two consecutive years (2012–2014) and was repeated in the dry and wet seasons, obtaining four sub-samples per site. Medium to large mammal sampling took place in 2014–2015 in 18 of the 24 sampling units selected for bird sampling, and two additional sampling units to totalize 20 sampling units. In each one, we established a Moultrie M-880 camera trap deployed > 100 m from the nearest unpaved road, placed 50 cm above-ground and attached to a tree trunk, set to be active 24 h a day. Total sampling effort was 1000 days, and camera traps remained active for 50 ± 13 days in each sampling unit.
3. Results
3.1. 1986–2006 Land Cover Change
3.2. Changes in Ecosystem Services
3.3. Estimated Changes in the Diversity of Birds and Medium-Large Mammals
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Verburg, P.H.; Crossman, N.; Ellis, E.C.; Heinimann, A.; Hostert, P.; Mertz, O.; Nagendra, H.; Sikor, T.; Erb, K.; Golubiewski, N.; et al. Land system science and sustainable development of the earth system: A global land project perspective. Anthropocene 2015, 12, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Mather, A.S.; Needle, C.L. The forest transition: A theoretical basis. Area 1998, 30, 117–124. [Google Scholar] [CrossRef]
- Aide, T.M.; Clark, M.L.; Grau, H.R.; López-Carr, D.; Levy, M.A.; Redo, D.; Bonilla-Moheno, M.; Riner, G.; Andrade-Núñez, M.J.; Muñiz, M. Deforestation and reforestation of Latin America and the Caribbean (2001–2010). Biotropica 2013, 45, 262–271. [Google Scholar] [CrossRef]
- Redo, D.J.; Grau, H.R.; Aide, T.M.; Clark, M.L. Asymmetric forest transition driven by the interaction of socioeconomic development and environmental heterogeneity in Central America. Proc. Natl. Acad. Sci. USA 2012, 109, 8839–8844. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.S.; Grau, H.R. Agricultural adjustment, population dynamics and forests redistribution in a subtropical watershed of NW Argentina. Reg. Environ. Chang. 2014, 14, 1641–1649. [Google Scholar] [CrossRef]
- Phalan, B.; Onial, M.; Balmford, A.; Green, R.E. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science 2011, 333, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Kuemmerle, T.; Macchi, L. Beyond ‘land sparing versus land sharing’: Environmental heterogeneity, globalization and the balance between agricultural production and nature conservation. Curr. Opin. Environ. Sustain. 2013, 5, 477–483. [Google Scholar] [CrossRef]
- Geist, H.J.; Lambin, E.F. What drives tropical deforestation. LUCC Rep. Ser. 2001, 4, 116. [Google Scholar]
- Niamir-Fuller, M.; Kerven, C.; Reid, R.; Milner-Gulland, E. Co-existence of wildlife and pastoralism on extensive rangelands: Competition or compatibility? Pastor. Res. Policy Pract. 2012, 2, 1. [Google Scholar] [CrossRef]
- Berry, P.M.; Rounsevell, M.D.A.; Harrison, P.A.; Audsley, E. Assessing the vulnerability of agricultural land use and species to climate change and the role of policy in facilitating adaptation. Environ. Sci. Policy 2006, 9, 189–204. [Google Scholar] [CrossRef]
- Cumming, G.S. Spatial resilience: Integrating landscape ecology, resilience, and sustainability. Landsc. Ecol. 2011, 26, 899–909. [Google Scholar] [CrossRef]
- Pianka, E.R. Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 1966, 100, 33–46. [Google Scholar] [CrossRef]
- Bruun, T.B.; De Neergaard, A.; Lawrence, D.; Ziegler, A.D. Environmental consequences of the demise in swidden cultivation in Southeast Asia: Carbon storage and soil quality. Hum. Ecol. 2009, 37, 375–388. [Google Scholar] [CrossRef]
- Powers, J.S.; Corre, M.D.; Twine, T.E.; Veldkamp, E. Geographic bias of field observations of soil carbon stocks with tropical land-use changes precludes spatial extrapolation. Proc. Natl. Acad. Sci. USA 2011, 108, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.S.; Gasparri, N.I.; Grau, H.R. Redistribution of forest biomass in a heterogeneous environment of subtropical Andes undergoing agriculture adjustment. Appl. Geogr. 2015, 62, 107–114. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environ. Res. Lett. 2007, 2, 045023. [Google Scholar] [CrossRef]
- Farley, K.A.; Jobbágy, E.G.; Jackson, R.B. Effects of afforestation on water yield: A global synthesis with implications for policy. Glob. Chang. Biol. 2005, 11, 1565–1576. [Google Scholar] [CrossRef]
- Turner, M.G.; Donato, D.C.; Romme, W.H. Consequences of spatial heterogeneity for ecosystem services in changing forest landscapes: Priorities for future research. Landsc. Ecol. 2013, 28, 1081–1097. [Google Scholar] [CrossRef]
- Garrido, H.B. Población y tierra en la cuenca de Trancas provincia de Tucumán (República Argentina). Cuad. Desarro. Rural 2005, 54, 31–60. [Google Scholar]
- Minetti, J.L.; Lamelas, C.M. Respuesta regional de la soja en Tucumán a la variabilidad climática. Rev. Ind. Agríc. Tucuman 1997, 72, 63–68. [Google Scholar]
- Aráoz, E.; Grau, H.R. Fire-mediated forest encroachment in response to climatic and land-use change in subtropical Andean treelines. Ecosystems 2010, 13, 992–1005. [Google Scholar] [CrossRef]
- INDEC Argentina. Censo Nacional Agropecuario 1988; Instituto Nacional de Estadísticas y Censos de la República Argentina: Buenos Aires, Argentina, 1988. [Google Scholar]
- INDEC Argentina. Censo Nacional Agropecuario 2002; Instituto Nacional de Estadísticas y Censos de la República Argentina: Buenos Aires, Argentina, 2002. [Google Scholar]
- Observatorio Ganadero. Caracterización regional: Noroeste argentino. In Observatorio de la Cadena de la Carne Bovina de Argentina. Argent. Informe N°3; Observatorio Ganadero: Buenos Aires, Argentina, 2013; p. 14. [Google Scholar]
- INDEC Argentina. Censo Nacional de Población, Hogares y Vivienda; Instituto Nacional de Estadísticas y Censos de la República Argentina: Buenos Aires, Argentina, 2010. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Pebesma, E.J.; Bivand, R.S. Classes and methods for spatial data in R. R News 2005, 5, 9–13. [Google Scholar]
- Cabrera, A.L. Enciclopedia Argentina de agricultura y jardinería, Tomo II, Fascículo 1: regiones fitogeográficas Argentinas; ACME: Buenos Aires, Argentina, 1994; p. 85. [Google Scholar]
- Antuña, J.C.; Rossanigo, C.; Arano, A.; Caldera, J. Análisis de la Actividad Ganadera Bovina de Carne por Estratos de Productores y Composición del Stock. Años 2008 y 2009. Provincia de Tucumán. Red de Información Agropecuaria Nacional Ganadero–Instituto Nacional de Tecnología Agropecuaria, Buenos Aires, Argentina. Available online: http://www.inta.gov.ar/info/rian/2010/Pais_por_provincias.pdf (accessed on 10 August 2017).
- Steinfeld, H.; Mäki-Hokkonen, J. A classification of livestock production systems. World Anim. Rev. 1995, 83–94. Available online: http://www.fao.org/ag/aga/agap/frg/FEEDback/War/V8180b/v8180b0y.htm#TopOfPage (accessed on 17 August 2017).
- Pimentel, D.; Burgess, M. Soil erosion threatens food production. Agriculture 2013, 3, 443–463. [Google Scholar] [CrossRef]
- Running, S.W.; Justice, C.O.; Salomonson, V.; Hall, D.; Barker, J.; Kaufmann, Y.J.; Strahler, A.H.; Huete, A.R.; Muller, J.P.; Vanderbilt, V.; et al. Terrestrial remote sensing science and algorithms planned for EOS/MODIS. Int. J. Remote Sens. 1994, 15, 3587–3620. [Google Scholar] [CrossRef]
- Gasparri, N.I.; Baldi, G. Regional patterns and controls of biomass in semiarid woodlands: Lessons from the Northern Argentina Dry Chaco. Reg. Environ. Chang. 2013, 13, 1131–1144. [Google Scholar] [CrossRef]
- Secretaria de Ambiente y Desarrollo Sustentable (SAyDS). Bases para una Agenda Ambiental Nacional. Politica Ambiental Sostenible para El Crecimiento y La Equidad. 2004. Available online: http://publicaciones.ops.org.ar/publicaciones/saludAmbiental/RM/cdsMCS/05/pub_msan/base_agenda.pdf (accessed on 17 August 2017).
- Brown, S.; Lugo, A.E. Biomass of tropical forests: A new estimate based on forest volumes. Science 1984, 223, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Huespe, J.; Suppo, H.; Tabernig, D. Estudios de Batimetría 2009. Embalse El Cadillal. INCOCIV S.R.L. Consultora. 2009, p. 53. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwjrio3O1t3VAhVQhrwKHRHRCAkQFgglMAA&url=http%3A%2F%2Fhidrotuc.homelinux.net%2Fcomprasweb%2Ftucuman%2Fadjuntos%2FRM%25203697%2FEl%2520Cadillal%2F2009%2520Cadillal%2FInforme%2520El%2520Cadillal%2520rev3.pdf&usg=AFQjCNFB-0m4cZOn-X62g_PRel24osxMoA (accessed on 17 August 2017).
- Renard, K.G.; Freimund, J.R. Using monthly precipitation data to estimate the R-factor in the revised USLE. J. Hydrol. 1994, 157, 287–306. [Google Scholar] [CrossRef]
- Millward, A.A.; Mersey, J.E. Adapting the RUSLE to model soil erosion potential in a mountainous tropical watershed. Catena 1999, 38, 109–129. [Google Scholar] [CrossRef]
- Volante, J.N.; Noe, Y.E.; Gonzalez, A.C. Isohietas Anuales del Noroeste Argentino. Instituto Nacional de Tecnología Agropecuaria, Artículo de Divulgación. 2012. Available online: http://inta.gob.ar/documentos/isohietas-anuales-del-noroeste-argentino-0 (accessed on 17 August 2017).
- Atlas de Suelos de la República Argentina. Available online: https://searchworks.stanford.edu/view/669553 (accessed on 17 August 2017).
- Wischmeier, W.H.; Smith, D.D. Predicting Rainfall Erosion Losses—A Guide to Conservation Planning; Science and Education Administration, U.S. Department of Agriculture: Washington, DC, USA, 1978.
- Jarvis, A.; Reuter, H.I.; Nelson, A. Hole-filled SRTM for the globe Version 4. The CGIAR-CSI SRTM 90 m Database. 2008. Available online: http://srtm.csi.cgiar.org (accessed on 12 August 2012).
- Mitasova, H.; Hofierka, J.; Zlocha, M.; Iverson, L.R. Modelling topographic potential for erosion and deposition using GIS. Int. J. Geogr. Inf. Syst. 1996, 10, 629–641. [Google Scholar] [CrossRef]
- Sayago, J.M. Aspectos Metodológicos del Inventario de la Erosión Hidrica Mediante Technicas de Perceptión Remota en la Región Sub-Tropical del Noroeste Argentino; International Institute for Aerospace Survey and Earth Sciences: Enschede, The Netherlands, 1985. [Google Scholar]
- Mármol, L.A. Introducción al Manejo de Cuencas Hidrográficas y Corrección de Torrentes, 1st ed.; Universidad Nacional de Salta: Salta, Argentina, 2006; p. 287. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical ecology: Second English edition. In Developments in Environmental Modelling; Elsevier Science: Amsterdam, The Netherlands, 1998; Volume 20. [Google Scholar]
- Ralph, C.J.; Geupel, G.R.; Pyle, P.; Martin, T.E.; DeSante, D.F.; Milá, B. Manual de Métodos de Campo Para el Monitoreo de Aves Terrestres; U.S. Department of Agriculture: Albany, KY, USA, 1996; p. 46.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Community Ecol. Package 2013, 2. Available online: cran.ism.ac.jp/web/packages/vegan/vegan.pdf (accessed on 17 August 2017).
- Rempel, R.S.; Kaukinen, D.; Carr, A.P. Patch Analyst and Patch Grid; Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research: Thunder Bay, ON, Canada, 2012.
- Grau, H.R.; Paolini, L.; Malizia, A.; Carilla, J. Distribución, estructura y dinámica de los bosques de la Sierra de San Javier. In Ecologıa de una Interfase Natural-Urbana: La Sierra de San Javier y el Gran San Miguel de Tucumán; EDUNT: Tucumán, Argentina, 2010; pp. 33–48. [Google Scholar]
- Rudel, T.K.; Bates, D.; Machinguiashi, R. A tropical forest transition? Agricultural change, out-migration, and secondary forests in the Ecuadorian Amazon. Ann. Assoc. Am. Geogr. 2002, 92, 87–102. [Google Scholar] [CrossRef]
- Lambin, E.F.; Meyfroidt, P. Land use transitions: Socio-ecological feedback versus socio-economic change. Land Use Policy 2010, 27, 108–118. [Google Scholar] [CrossRef]
- Nanni, A.S. Dissimilar responses of the Gray brocket deer (Mazama gouazoubira), Crab-eating fox (Cerdocyon thous) and Pampas fox (Lycalopex gymnocercus) to livestock frequency in subtropical forests of NW Argentina. Mamm. Biol.-Z. Säugetierkunde 2015, 80, 260–264. [Google Scholar] [CrossRef]
- Loveridge, A.J.; Hemson, G.; Davidson, Z.; Macdonald, D.W. African lions on the edge: Reserve boundaries as ‘attractive sinks’. In Biology and Conservation of Wild Felids; Macdonald, D.W., Loveridge, A.J., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 283–304. [Google Scholar]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large-and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Wilson, J.B.; King, W.M. Human-mediated vegetation switches as processes in landscape ecology. Landsc. Ecol. 1995, 10, 191–196. [Google Scholar] [CrossRef]
- Grau, H.R. Equilibrios alternativos mediados por decisiones humanas: Controles de la estabilidad y la eficiencia del uso y cobertura del territorio en América Latina. In Naturaleza y Sociedad. Perspectivas Socioecológicas sobre Cambios Globales en América Latina; Postigo, J.C., Young, K.R., Eds.; Instituto de Estudios Peruanos: Lima, Peru, 2016; pp. 171–192. [Google Scholar]
- Liu, Y.Y.; Van Dijk, A.I.; De Jeu, R.A.; Canadell, J.G.; McCabe, M.F.; Evans, J.P.; Wang, G. Recent reversal in loss of global terrestrial biomass. Nat. Clim. Chang. 2015, 5, 470–474. [Google Scholar] [CrossRef]
- Michelena, R.G.; Irurtia, C.B. Physical conservationist diagnosis and degradation processes in the Choromoro river basin, Tucuman, Argentina [poster]. In Proceedings of the Reunion Internacional Sobre Procesos de Erosion en Tierras Altas Pendientes: Evaluacion y Modelaje, Merida (Venezuela), Mexico, 16–20 May 1993. [Google Scholar]
- Grau, H.R.; Aide, T.M. Globalization and land use transitions in Latin America. Ecol. Soc. 2008, 13, 16. [Google Scholar] [CrossRef]
- Wise, M.; Calvin, K.; Thomson, A.; Clarke, L.; Bond-Lamberty, B.; Sands, R.; Smith, S.J.; Janetos, A.; Edmonds, J. Implications of limiting CO2 concentrations for land use and energy. Science 2009, 324, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Burney, J.A.; Davis, S.J.; Lobell, D.B. Greenhouse gas mitigation by agricultural intensification. Proc. Natl. Acad. Sci. USA 2010, 107, 12052–12057. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Brosi, B.; Daily, G.C.; Ehrlich, P.R.; Goldman, R.; Goldstein, J.; Lindenmayer, D.B.; Manning, A.D.; Mooney, H.A.; Pejchar, L.; et al. Should agricultural policies encourage land sparing or wildlife-friendly farming? Front. Ecol. Environ. 2008, 6, 380–385. [Google Scholar] [CrossRef]
- Ceddia, M.G.; Gunter, U.; Corriveau-Bourque, A. Land tenure and agricultural expansion in Latin America: The role of Indigenous Peoples’ and local communities’ forest rights. Glob. Environ. Chang. 2015, 35, 316–322. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kastner, T.; Rivas, M.J.I.; Koch, W.; Nonhebel, S. Global changes in diets and the consequences for land requirements for food. Proc. Natl. Acad. Sci. USA 2012, 109, 6868–6872. [Google Scholar] [CrossRef] [PubMed]
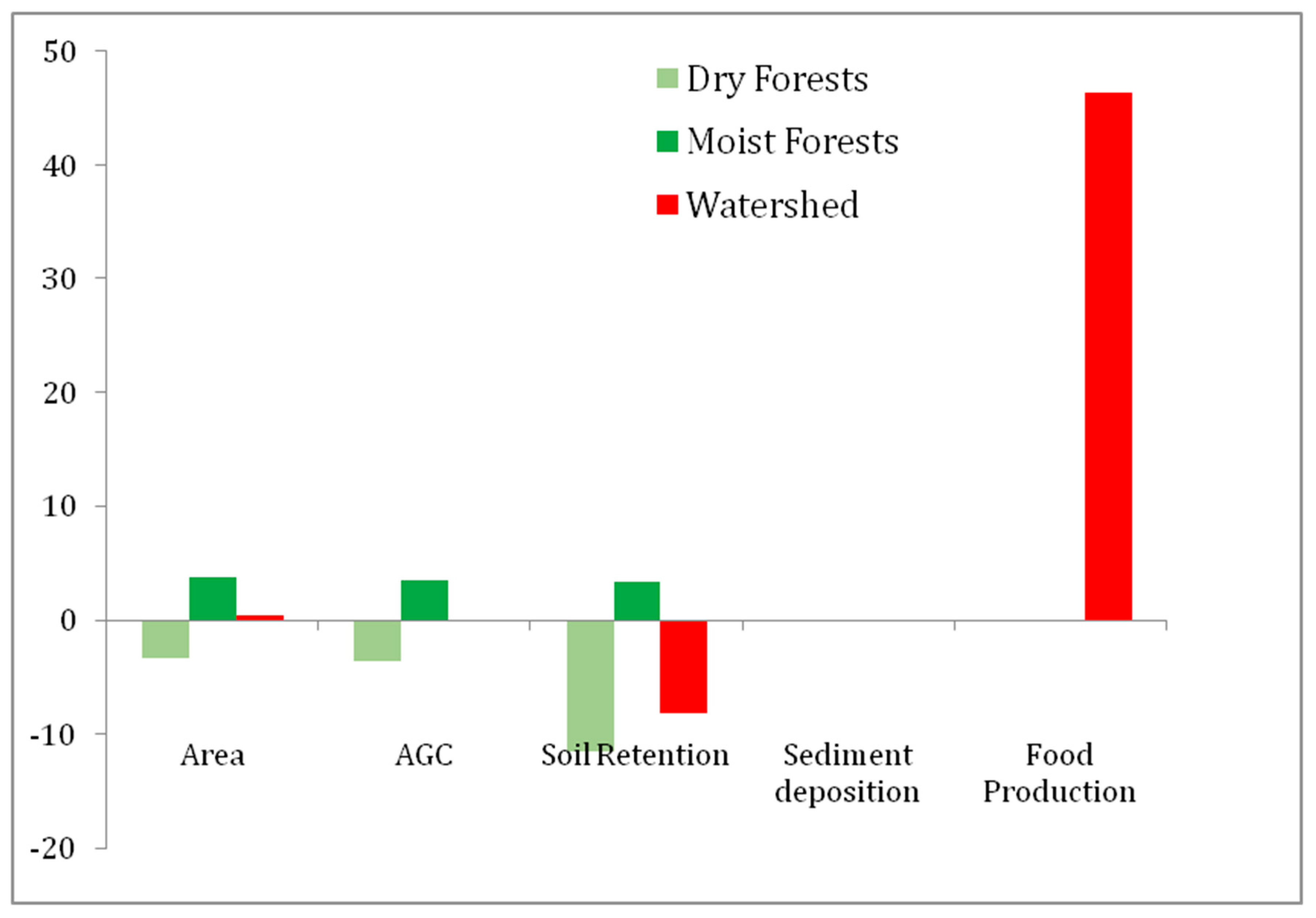
| Year | Dry F (ha) | Moist F (ha) | Total F (ha) | Food Prod. (N° Cattle Heads) | AGC (Tn/ha) | Soil Retention (Tn/ha) | Sed. Dep (Hm3) |
|---|---|---|---|---|---|---|---|
| 1986 | 103,345 | 117,580 | 220,925 | 31,029 | 20,048,413 | −2,576,610 | 77.71 |
| 2006 | 95,989 | 125,684 | 214,651 | 45,407 | 20,022,435 | −2,805,943 | 77.91 |
| Change | −7356 | 8104 | 748 | 14,378 | −25,979 | −229,333 | −0.19 |
| % Change | −7.66 | 6.4 | 0.34 | 46.34 | −0.13 | −8.90 | −0.2 |
| Bird Richness | Mammal Richness | Bird Similarity | Mammal Similarity | |
|---|---|---|---|---|
| DF-MF | 0.76 | 0.10 | 0.670 ** | 0.35 * |
| DF-MonF | 0.001 | 0.82 | 0.99 ** | 0.70 * |
| MF-MonF | 0.009 | 0.23 | 0.90 ** | 0.62 * |
| HS Proxies | NMDS1 | NMDS2 | R2 | NMDS1 | NMDS2 | R2 |
|---|---|---|---|---|---|---|
| Altitude | 0.99 | −0.09 | 0.86 *** | 0.99 | −0.12 | 0.25 * |
| F Prop | −0.99 | 0.99 | 0.50 *** | 0.73 | 0.67 | 0.35 ** |
| AGC | 0.97 | 0.23 | 0.02 | −0.92 | 0.37 | 0.29 * |
| Livestock F | - | - | - | 0.48 | 0.87 | 0.30 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanni, A.S.; Grau, H.R. Land-Use Redistribution Compensated for Ecosystem Service Losses Derived from Agriculture Expansion, with Mixed Effects on Biodiversity in a NW Argentina Watershed. Forests 2017, 8, 303. https://doi.org/10.3390/f8080303
Nanni AS, Grau HR. Land-Use Redistribution Compensated for Ecosystem Service Losses Derived from Agriculture Expansion, with Mixed Effects on Biodiversity in a NW Argentina Watershed. Forests. 2017; 8(8):303. https://doi.org/10.3390/f8080303
Chicago/Turabian StyleNanni, Ana Sofía, and Héctor Ricardo Grau. 2017. "Land-Use Redistribution Compensated for Ecosystem Service Losses Derived from Agriculture Expansion, with Mixed Effects on Biodiversity in a NW Argentina Watershed" Forests 8, no. 8: 303. https://doi.org/10.3390/f8080303




