Allometric Equations for Estimating Compartment Biomass and Stem Volume in Mature Hybrid Poplars: General or Site-Specific?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Soil Characteristics
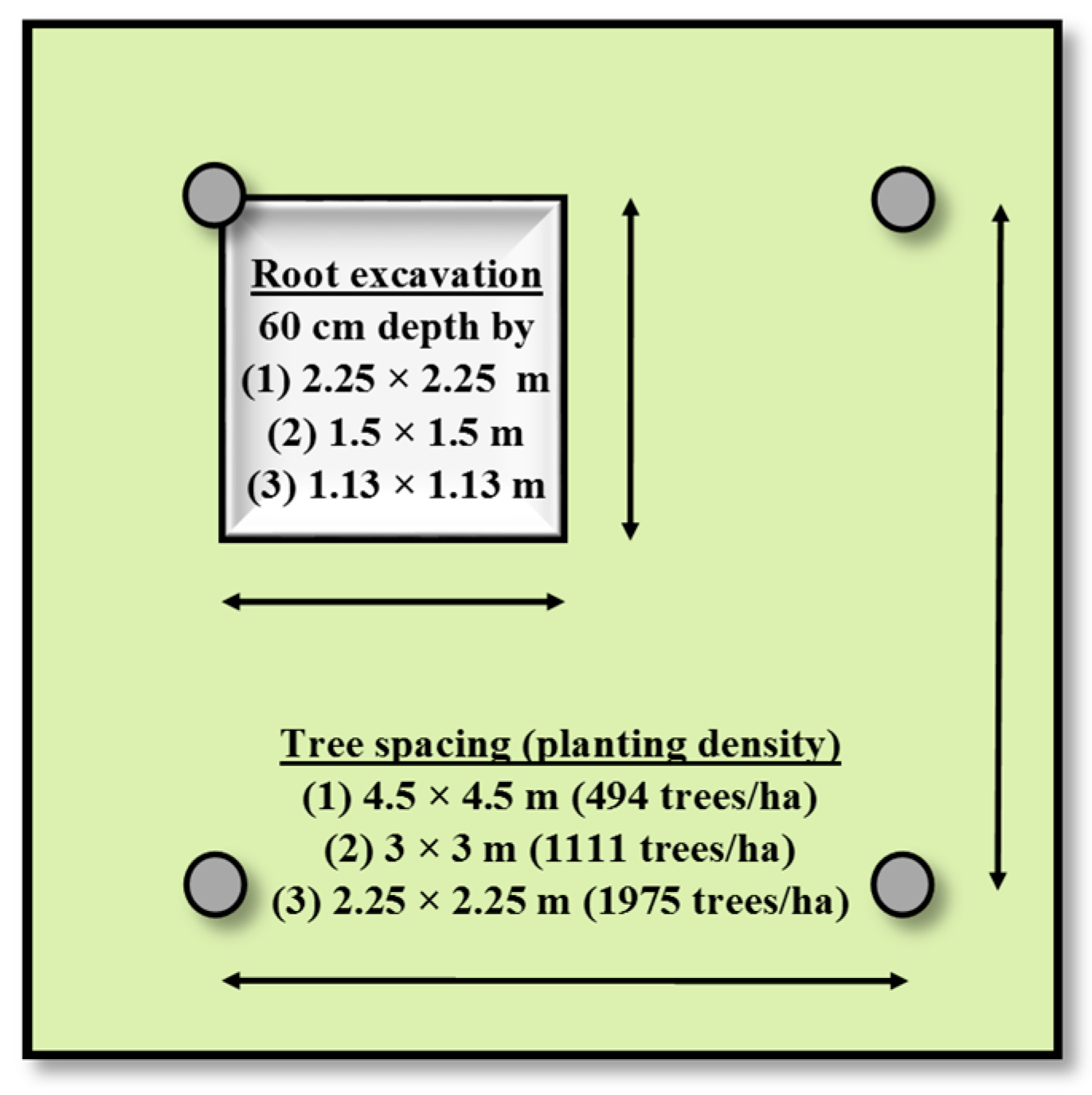
2.3. Destructive Sampling for Aboveground Biomass Compartments and Stem Volume
2.4. Coarse Root Biomass Sampling
2.5. Aboveground Biomass Properties
2.6. Allometric Equations
2.7. Statistical Analysis
3. Results and Discussion
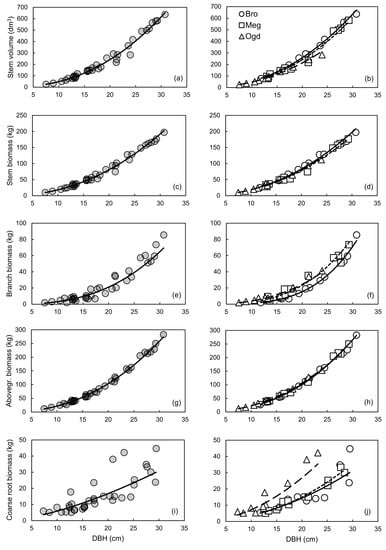
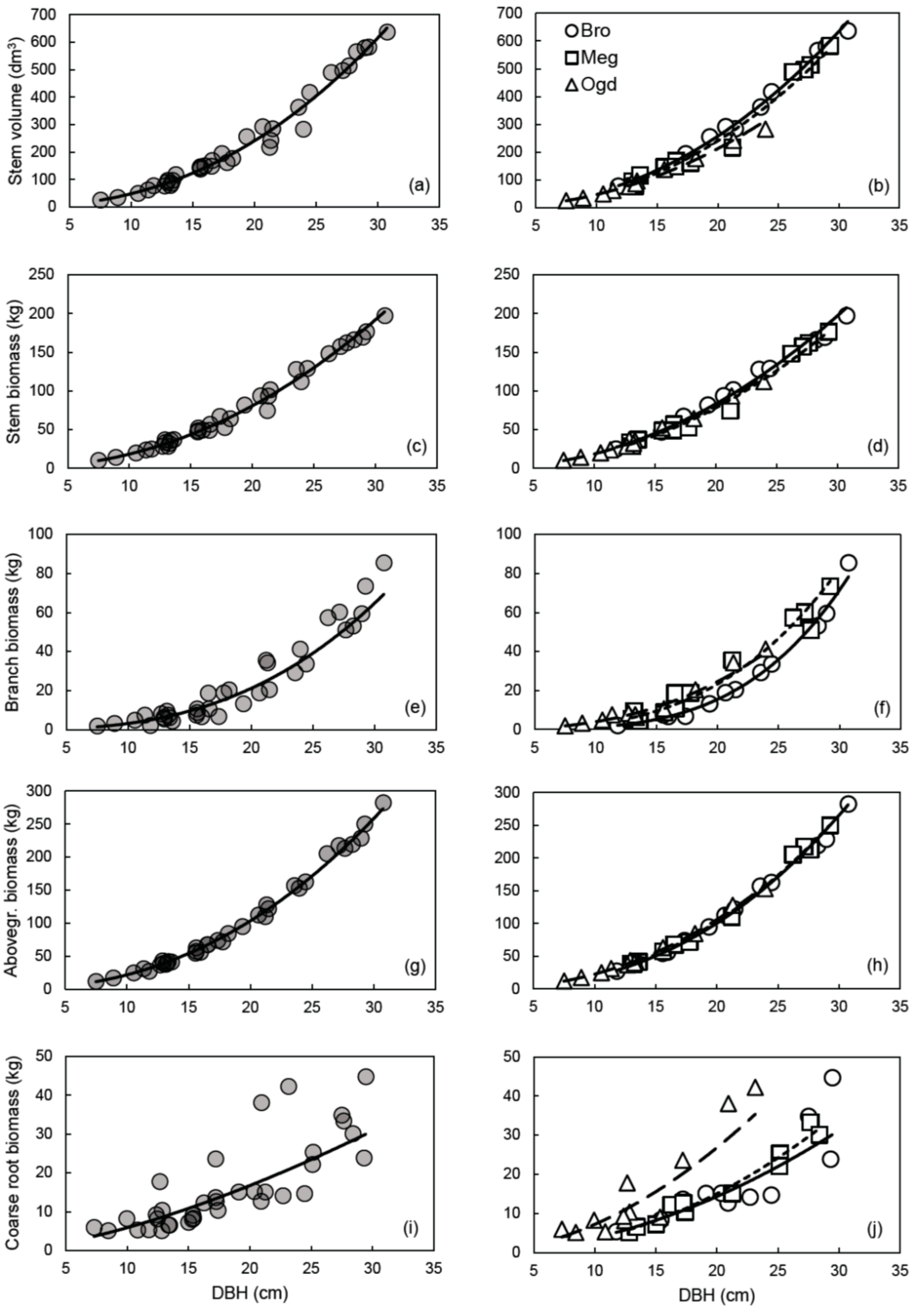
3.1. Equations for Compartment Biomass and Stem Volume Estimations, General or Site-Specific?
3.2. High Plasticity in Coarse Root Biomass Allocation: The Effect of Soil Fertility
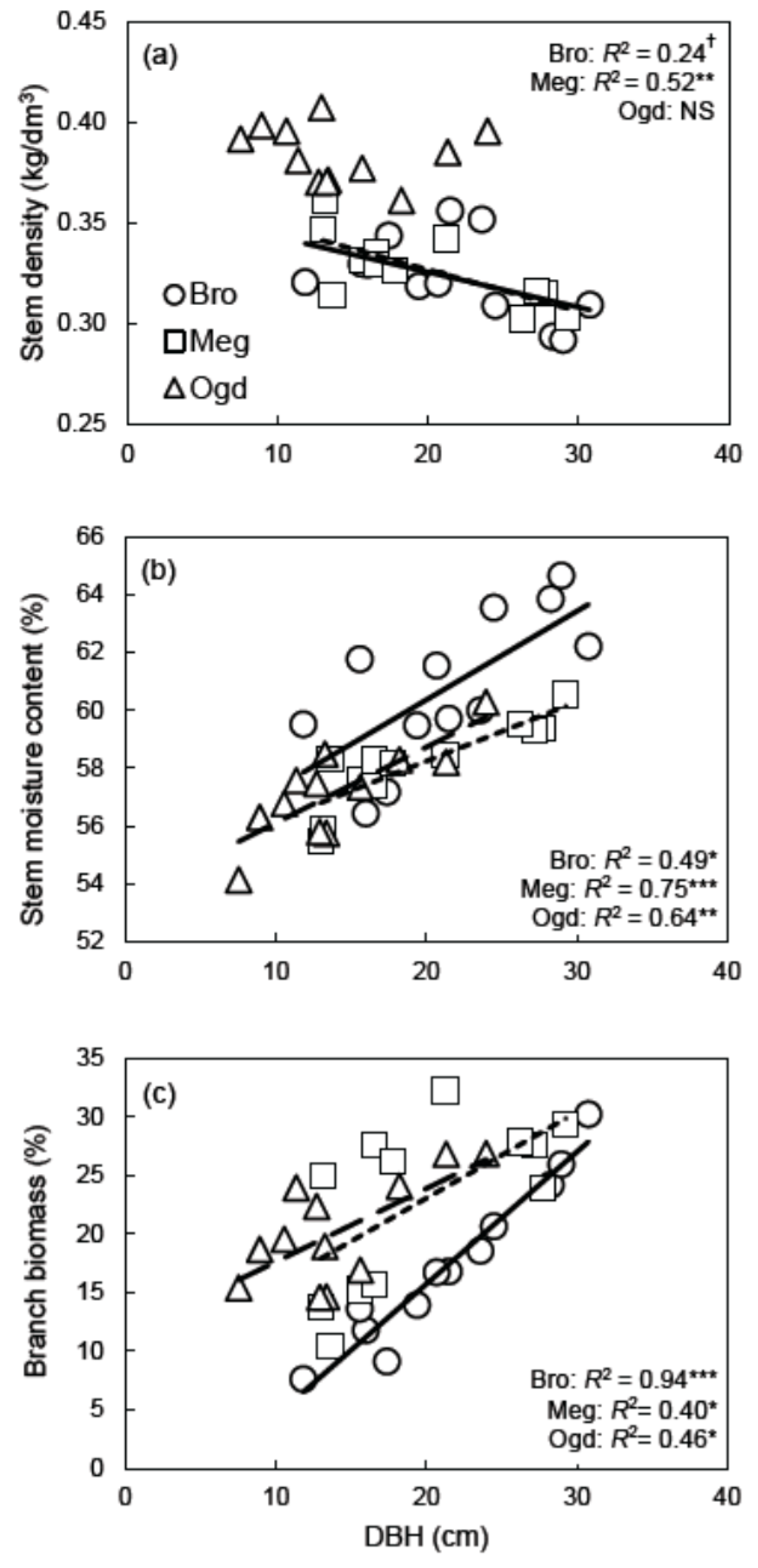
3.3. Plasticity in Architecture and Wood Density Leads to Non-Plastic Allocation to Aboveground Woody Biomass
3.4. Biomass and Wood Quality Indicators
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Can hybrid poplar plantations accelerate the restoration of forest understory attributes on abandoned fields? For. Ecol. Manag. 2013, 287, 77–89. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Biomass carbon, nitrogen and phosphorus stocks in hybrid poplar buffers, herbaceous buffers and natural woodlots in the riparian zone on agricultural land. J. Environ. Manag. 2015, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Biomass and volume yield in mature hybrid poplar plantations on temperate abandoned farmland. Forests 2014, 5, 3107–3130. [Google Scholar] [CrossRef]
- Heilman, P.E.; Stettler, R.F. Nutritional concerns in selection of black cottonwood and hybrid clones for short rotation. Can. J. For. Res. 1986, 16, 860–863. [Google Scholar] [CrossRef]
- Johansson, T.; Hjelm, B. Stump and root biomass of poplar stands. Forests 2012, 3, 166–178. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Eisenbies, M.H.; Vance, E.D.; Aust, W.M.; Seiler, J.R. Intensive utilization of harvest residues in southern pine plantations: Quantities available and implications for nutrient budgets and sustainable site productivity. BioEnergy Res. 2009, 2, 90–98. [Google Scholar] [CrossRef]
- West, P. Tree and Forest Measurement; Springer: Berlin/Heidelberg, Germany, 2009; p. 190. [Google Scholar]
- Taylor, B.N.; Beidler, K.V.; Cooper, E.R.; Strand, A.E.; Pritchard, S.G. Sampling volume in root studies: The pitfalls of under-sampling exposed using accumulation curves. Ecol. Lett. 2013, 16, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Zabek, L.M.; Prescott, C.E. Biomass equations and carbon content of aboveground leafless biomass of hybrid poplar in Coastal British Columbia. For. Ecol. Manag. 2006, 223, 291–302. [Google Scholar] [CrossRef]
- Stark, H.; Nothdurft, A.; Bauhus, J. Allometries for widely spaced Populus ssp. and Betula ssp. in nurse crop systems. Forests 2013, 4, 1003. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Plastic allometry in coarse root biomass of mature hybrid poplar plantations. BioEnergy Res. 2015, 8, 1691–1704. [Google Scholar] [CrossRef]
- Taeroe, A.; Nord-Larsen, T.; Stupak, I.; Raulund-Rasmussen, K. Allometric biomass, biomass expansion factor and wood density models for the OP42 hybrid poplar in southern Scandinavia. BioEnergy Res. 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Dillen, S.Y.; Marron, N.; Bastien, C.; Ricciotti, L.; Salani, F.; Sabatti, M.; Pinel, M.P.C.; Rae, A.M.; Taylor, G.; Ceulemans, R. Effects of environment and progeny on biomass estimations of five hybrid poplar families grown at three contrasting sites across Europe. For. Ecol. Manag. 2007, 252, 12–23. [Google Scholar] [CrossRef]
- Lupi, C.; Larocque, G.; DesRochers, A.; Labrecque, M.; Mosseler, A.; Major, J.; Beaulieu, J.; Tremblay, F.; Gordon, A.M.; Thomas, B.R.; et al. Evaluating sampling designs and deriving biomass equations for young plantations of poplar and willow clones. Biomass Bioenergy 2015, 83, 196–205. [Google Scholar] [CrossRef]
- Oliveira, N.; Rodríguez-Soalleiro, R.; Pérez-Cruzado, C.; Cañellas, I.; Sixto, H. On the genetic affinity of individual tree biomass allometry in poplar short rotation coppice. BioEnergy Res. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Rensema, T.R. Clonal differences in biomass characteristics, coppice ability, and biomass prediction equations among four Populus clones grown in eastern North Dakota. Can. J. For. Res. 1992, 22, 348–354. [Google Scholar] [CrossRef]
- Sileshi, G.W. A critical review of forest biomass estimation models, common mistakes and corrective measures. For. Ecol. Manag. 2014, 329, 237–254. [Google Scholar] [CrossRef]
- Wu, R.; Ma, C.-X.; Lou, X.-Y.; Casella, G. Molecular dissection of allometry, ontogeny, and plasticity: A genomic view of developmental biology. BioSC 2003, 53, 1041–1047. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Schall, P.; Lödige, C.; Beck, M.; Ammer, C. Biomass allocation to roots and shoots is more sensitive to shade and drought in European beech than in Norway spruce seedlings. For. Ecol. Manag. 2012, 266, 246–253. [Google Scholar] [CrossRef]
- Forrester, D.I.; Tachauer, I.H.H.; Annighoefer, P.; Barbeito, I.; Pretzsch, H.; Ruiz-Peinado, R.; Stark, H.; Vacchiano, G.; Zlatanov, T.; Chakraborty, T.; et al. Generalized biomass and leaf area allometric equations for European tree species incorporating stand structure, tree age and climate. For. Ecol. Manag. 2017, 396, 160–175. [Google Scholar] [CrossRef]
- António, N.; Tomé, M.; Tomé, J.; Soares, P.; Fontes, L. Effect of tree, stand, and site variables on the allometry of Eucalyptus globulus tree biomass. Can. J. For. Res. 2007, 37, 895–906. [Google Scholar] [CrossRef]
- Johansson, T. Biomass production and allometric above- and below-ground relations for young birch stands planted at four spacings on abandoned farmland. For. Int. J. For. Res. 2007, 80, 41–52. [Google Scholar] [CrossRef]
- Telenius, B.; Verwijst, T. The influence of allometric variation, vertical biomass distribution and sampling procedure on biomass estimates in commercial short-rotation forests. Bioresour. Technol. 1995, 51, 247–253. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.E.; Labrecque, M.; Larocque, G.R. Allometric relationships in coppice biomass production for two North American willows (Salix spp.) across three different sites. For. Ecol. Manag. 2014, 320, 190–196. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Weih, M. Nitrogen storage and seasonal nitrogen cycling in Populus: Bridging molecular physiology and ecophysiology. New Phytol. 2005, 167, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, K.; Rytter, L. The influence of soil conditions, with focus on soil acidity, on the establishment of poplar (Populus spp.). New For. 2016, 47, 731–750. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource limitation in plants—An economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Wu, R.; Stettler, R.F. Quantitative genetics of growth and development in Populus. III. Phenotypic plasticity of crown structure and function. Heredity 1998, 81, 299–310. [Google Scholar] [CrossRef]
- Verwijst, T.; Telenius, B. Biomass estimation procedures in short rotation forestry. For. Ecol. Manag. 1999, 121, 137–146. [Google Scholar] [CrossRef]
- Beaudoin, M.; Hernández, R.E.; Koubaa, A.; Poliquin, J. Interclonal, intraclonal and within-tree variation in wood density of poplar hybrid clones. Wood Fiber Sci. 1992, 24, 147–153. [Google Scholar]
- DeBell, D.S.; Singleton, R.; Harrington, C.A.; Gartner, B.L. Wood density and fiber length in young populus stems: Relation to clone, age, growth rate, and pruning. Wood Fiber Sci. 2002, 34, 529–539. [Google Scholar]
- Tharakan, P.J.; Volk, T.A.; Abrahamson, L.P.; White, E.H. Energy feedstock characteristics of willow and hybrid poplar clones at harvest age. Biomass Bioenergy 2003, 25, 571–580. [Google Scholar] [CrossRef]
- Pliura, A.; Zhang, S.Y.; MacKay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. For. Ecol. Manag. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yu, Q.; Chauret, G.; Koubaa, A. Selection for both growth and wood properties in hybrid poplar clones. For. Sci. 2003, 49, 901–908. [Google Scholar]
- Huxley, J.S.; Teissier, G. Terminology of relative growth. Nature 1936, 137, 780–781. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Timothy, A.M.; Davis, J.M. Shot-term physiological and developmental responses to nitrogen availability in hybrid poplar. New Phytol. 2005, 167, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, A.; Saucier, J.-P. Paysages Régionaux du Québec Méridional; Les publications du Québec: Ste-Foy, QC, Canada, 1998; p. 213. [Google Scholar]
- Périnet, P.; Gagnon, H.; Morin, S. Liste des Clones Recommandés de Peuplier Hybride par Sous-Région Écologique au Québec (Mise à Jour Octobre 2010); Direction de la Recherche Forestière, MRN: Québec, QC, Canada, 2010; p. 1. [Google Scholar]
- Eckenwalder, J.E. Descriptions of clonal characteristics. In Poplar Culture in North America; Dickmann, D.I., Isenbrands, J.G., Eds.; NRC Research Press, National Research Council of Canada: Ottawa, ON, Canada, 2001; Part B, Chapter 13; pp. 331–382. [Google Scholar]
- Truax, B.; Gagnon, D.; Fortier, J.; Lambert, F. Yield in 8 year-old hybrid poplar plantations on abandoned farmland along climatic and soil fertility gradients. For. Ecol. Manag. 2012, 267, 228–239. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Biomass and volume yield after 6 years in multiclonal hybrid poplar riparian buffer strips. Biomass Bioenergy 2010, 34, 1028–1040. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Mature hybrid poplar riparian buffers along farm streams produce high yields in response to soil fertility assessed using three methods. Sustainability 2013, 5, 1893–1916. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Linking biomass productivity to genotype-specific nutrient cycling strategies in mature hybrid poplars planted along an environmental gradient. BioEnergy Res. 2017, 10, 876–890. [Google Scholar] [CrossRef]
- Conseil des Productions Végétales du Québec. Méthodes D’analyse des Sols, des Fumiers et des Tissus Végétaux; Gouvernement du Quebec, Ministeres des Communications: Québec, QC, Canada, 1988.
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Centre de Référence en Agriculture et Agroalimentaire du Québec. Guide de Référence en Fertilisation, 1st ed.; CRAAQ: Ste-Foy, QC, Canada, 2003; p. 40. [Google Scholar]
- Tran, T.S.; Simard, R.R. Mehlich III-Extractable elements. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers and CRC Press: Boca Raton, FL, USA, 1993; pp. 43–49. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis. Method 984.27: Calcium, Copper, Iron, Magnesium, Manganese, Phosphorus, Potassium, Sodium and Zinc in Infant Formula—Inductively Coupled Plasma Emission Spectroscopic, 16th ed.; AOAC International: Rockville, MD, USA, 1999; p. 1200. [Google Scholar]
- Perron, J.-Y. Inventaire forestier. In Manuel de Foresterie; Ordre des Ingénieurs Forestiers du Québec, Ed.; Les Presses de l’Université Laval: Ste-Foy, QC, Canada, 1996; pp. 390–473. [Google Scholar]
- Ajit; Das, D.K.; Chaturvedi, O.P.; Jabeen, N.; Dhyani, S.K. Predictive models for dry weight estimation of above and below ground biomass components of Populus deltoides in India: Development and comparative diagnosis. Biomass Bioenergy 2011, 35, 1145–1152. [Google Scholar]
- Fang, S.; Xue, J.; Tang, L. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Heilman, P.E.; Ekuan, G.; Fogle, D. Above- and below-ground biomass and fine roots of 4-year-old hybrids of Populus trichocarpa × Populus deltoides and parental species in short-rotation culture. Can. J. For. Res. 1994, 24, 1186–1192. [Google Scholar] [CrossRef]
- Picard, N.; Saint-André, L.; Henry, M. Manual for Building Tree Volume and Biomass Allometric Equations: From Field Measurement to Prediction; Food and Agricultural, Organization of the United Nations: Rome, Italy; Centre de Coopération Internationale en Recherche Agronomique pour le Développement: Montpellier, France, 2012; p. 215. [Google Scholar]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar]
- Chojnacky, D.C.; Heath, L.S.; Jenkins, J.C. Updated generalized biomass equations for North American tree species. Forestry 2014, 87, 129–151. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. National-scale biomass estimators for United States tree species. For. Sci. 2003, 49, 12–35. [Google Scholar]
- Ter-Mikaelian, M.T.; Korzukhin, M.D. Biomass equations for sixty-five North American tree species. For. Ecol. Manag. 1997, 97, 1–24. [Google Scholar] [CrossRef]
- Ben Brahim, M.; Gavaland, A.; Cabanettes, A. Generalized allometric regression to estimate biomass of Populus in short-rotation coppice. Scand. J. For. Res. 2000, 15, 171–176. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associated, Inc.: Sunderland, MA, USA, 2004; p. 510. [Google Scholar]
- Taskinen, S.; Warton, D.I. Robust estimation and inference for bivariate line-fitting in allometry. Biom. J. 2011, 53, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.J. Robust regression: Asymptotics, conjectures and Monte Carlo. Ann. Stat. 1973, 1, 799–821. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Stone, M. Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. Ser. B Methodol. 1974, 36, 111–147. [Google Scholar]
- Arlot, S.; Celisse, A. A survey of cross-validation procedures for model selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Petersen, R.G. Design and Analysis of Experiments; Marcel-Dekker: New York, NY, USA, 1985; p. 429. [Google Scholar]
- Day, R.W.; Quinn, G.P. Comparisons of treatments after an analysis of variance in ecology. Ecol. Monogr. 1989, 59, 433–463. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Yin, T.M.; DiFazio, S.P.; Tschaplinski, T.J.; Gunter, L.E.; Davis, M.F.; Tuskan, G.A. Phenotypic variation in growth and biomass distribution for two advanced-generation pedigrees of hybrid poplar. Can. J. For. Res. 2005, 35, 1779–1789. [Google Scholar] [CrossRef]
- Bradshaw, H.D.; Stettler, R.F. Molecular genetics of growth and development in Populus. IV. Mapping QTLs with large effects on growth, form, and phenology traits in a forest tree. Genetics 1995, 139, 963. [Google Scholar] [PubMed]
- Wu, R.; Ma, C.-X.; Littell, R.C.; Casella, G. A statistical model for the genetic origin of allometric scaling laws in biology. J. Theor. Biol. 2002, 219, 121–135. [Google Scholar] [CrossRef]
- Pretzsch, H.; Uhl, E.; Biber, P.; Schütze, G.; Coates, K.D. Change of allometry between coarse root and shoot of Lodgepole pine (Pinus contorta Dougl. ex. Loud) along a stress gradient in the sub-boreal forest zone of British Columbia. Scand. J. For. Res. 2012, 27, 532–544. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed]
- Kranjcec, J.; Mahoney, J.M.; Rood, S.B. The responses of three riparian cottonwood species to water table decline. For. Ecol. Manag. 1998, 110, 77–87. [Google Scholar] [CrossRef]
- Dickmann, D.I.; Kuzovkina, Y.A. Poplars and willows of the world, with emphasis on silviculturally important species. In Poplars and Willows: Tree for the Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CAB International and FAO: Rome, Italy, 2015; pp. 8–91. [Google Scholar]
- Rood, S.B.; Bigelow, S.G.; Hall, A.A. Root architecture of riparian trees: River cut-banks provide natural hydraulic excavation, revealing that cottonwoods are facultative phreatophytes. Trees 2011, 25, 907–917. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Hamelin, C.; Gagnon, D.; Truax, B. Aboveground biomass of glossy buckthorn is similar in open and understory environments but architectural strategy differs. Forests 2015, 6, 1083–1093. [Google Scholar] [CrossRef]
- Peterson, E.B.; Peterson, N.M. Ecology, Management, and Use of Aspen and Balsam Poplar in the Prairie Provinces; Forestry Canada, Northwest Region, Northern Forestry Centre: Edmonton, AB, Canada, 1992; p. 252.
- Hacke, U.G.; Plavcová, L.; Almeida-Rodriguez, A.; King-Jones, S.; Zhou, W.; Cooke, J.E.K. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol. 2010, 30, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Rosell, J.A. Vessel diameter-stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol. 2013, 197, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Kauter, D.; Lewandowski, I.; Claupein, W. Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use: A review of the physiological basis and management influences. Biomass Bioenergy 2003, 24, 411–427. [Google Scholar] [CrossRef]
- Balatinecz, J.; Mertens, P.; Boever, L.; de Yukun, H.; Jin, J.; van Acker, J. Properties, processing and utilization. In Poplar and Willows: Tree for the Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CAB International and FAO: Rome, Italy, 2014; Chapter 10; pp. 527–561. [Google Scholar]
- Côté, M.-A.; Gilbert, D.; Nadeau, S. Characterizing the profiles, motivations and behaviour of Quebec’s forest owners. For. Policy Econ. 2015, 59, 83–90. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Potential for hybrid poplar riparian buffers to provide ecosystem services in three watersheds with contrasting agricultural land use. Forests 2016, 7, 37. [Google Scholar] [CrossRef]
- Truax, B.; Gagnon, D.; Lambert, F.; Fortier, J. Multiple-use zoning model for private forest owners in agricultural landscapes: A case study. Forests 2015, 6, 3614–3664. [Google Scholar] [CrossRef]
- Kuljich, S.; Cáceres, C.B.; Hernández, R.E. Steam-bending properties of seven poplar hybrid clones. Int. J. Mater. Form. 2015, 8, 67–72. [Google Scholar] [CrossRef]
- Huda, A.A.S.M.; Koubaa, A.; Cloutier, A.; Hernández, R.E.; Fortin, Y. Variation of the physical and mechanical properties of hybrid poplar clones. Bioresource 2014, 9, 1456–1471. [Google Scholar] [CrossRef]
- Hernández, R.E.; Constantineau, S.; Fortin, Y. Wood machining properties of poplar hybrid clones from different sites following various drying treatments. Wood Fiber Sci. 2011, 43, 394–411. [Google Scholar]
| Site a | Elev. (m) | MAT b (°C) | MAP b (mm) | pH | OM (%) | P (mg/L) | K (mg/L) | Ca (mg/L) | Mg (mg/L) | Base Sat. (%) | CEC (meq/100 g) | Clay (%) | Silt (%) | Sand (%) | Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bro | 165 | 5.6 | 1146 | 5.27 a | 7.60 a | 44.3 b | 24.1 a | 772 a | 42.3 ab | 31.0 a | 13.9 a | 16.2 a | 58.5 a | 25.3 b | Silty loam |
| Meg | 470 | 4.2 | 1048 | 5.00 b | 6.70 ab | 68.4 a | 33.7 a | 619 a | 53.0 a | 24.5 b | 15.0 a | 13.1 a | 41.4 b | 45.5 a | Loam |
| Ogd | 265 | 5.3 | 1264 | 5.17 ab | 6.02 b | 5.1 c | 29.4 a | 219 b | 29.5 b | 14.8 c | 10.2 b | 14.6 a | 43.2 b | 42.2 a | Loam |
| SE | - | - | - | 0.05 | 0.32 | 4.8 | 2.9 | 45 | 5.8 | 1.5 | 0.9 | 1.6 | 1.3 | 1.2 | - |
| p< | - | - | - | 0.01 | 0.05 | 0.001 | NS | 0.001 | 0.1 | 0.001 | 0.01 | NS | 0.001 | 0.001 | - |
| Tree Compartments (Response Variables) | Site | DBH | Site × DBH |
|---|---|---|---|
| Stem volume | 0.01 | 0.001 | NS |
| Stem biomass | NS | 0.001 | NS |
| Branch biomass | 0.001 | 0.001 | 0.01 |
| Aboveground woody biomass | 0.01 | 0.001 | 0.05 |
| Coarse root biomass | 0.001 | 0.001 | NS |
| Tree Compartment | Model | n | DBH Range (cm) | Parameter | R2 | p < W b | n of Outliers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ln a | b | |||||||||||||
| Estimate | SE | PRSE a (%) | p < | Estimate | SE | PRSE (%) | p < | |||||||
| Stem | General | 36 | 7.5–30.8 | −1.44 | 0.14 | 9.6 | 0.001 | 2.31 | 0.05 | 2.1 | 0.001 | 0.986 | 0.12 | 2/36 |
| volume | Brompton | 12 | 11.8–30.8 | −1.11 | 0.14 | 12.6 | 0.001 | 2.22 | 0.05 | 2.1 | 0.001 | 0.996 | 0.36 | 0 |
| (dm3) | Mégantic | 12 | 13.0–29.3 | −1.32 | 0.36 | 27.2 | 0.01 | 2.27 | 0.12 | 5.3 | 0.001 | 0.972 | 0.24 | 0 |
| Ogden | 12 | 7.5–24.0 | −1.09 | 0.16 | 14.4 | 0.001 | 2.15 | 0.06 | 2.8 | 0.001 | 0.992 | 0.58 | 0 | |
| Stem | General | 36 | 7.5–30.8 | −2.01 | 0.11 | 5.3 | 0.001 | 2.14 | 0.04 | 1.7 | 0.001 | 0.990 | 0.37 | 2/36 |
| biomass | Brompton | 12 | 11.8–30.8 | −1.94 | 0.20 | 10.5 | 0.001 | 2.13 | 0.07 | 3.1 | 0.001 | 0.990 | 0.15 | 0 |
| (kg) | Mégantic | 12 | 13.0–29.3 | −2.07 | 0.28 | 13.7 | 0.001 | 2.15 | 0.10 | 4.5 | 0.001 | 0.980 | 0.047 | 0 |
| Ogden | 12 | 7.5–24.0 | −1.97 | 0.16 | 8.1 | 0.001 | 2.12 | 0.06 | 2.9 | 0.001 | 0.992 | 0.12 | 1/12 | |
| Branch | General | 36 | 7.5–30.8 | −5.22 | 0.46 | 8.8 | 0.001 | 2.76 | 0.16 | 5.7 | 0.001 | 0.899 | 0.048 | 2/36 |
| biomass | Brompton | 12 | 11.8–30.8 | −8.57 | 0.40 | 4.7 | 0.001 | 3.78 | 0.13 | 3.5 | 0.001 | 0.988 | 0.11 | 1/12 |
| (kg) | Mégantic | 12 | 13.0–29.3 | −6.19 | 0.80 | 13.0 | 0.001 | 3.12 | 0.27 | 8.7 | 0.001 | 0.929 | 0.89 | 0 |
| Ogden | 12 | 7.5–24.0 | −4.68 | 0.43 | 9.1 | 0.001 | 2.63 | 0.16 | 6.2 | 0.001 | 0.963 | 0.83 | 0 | |
| Aboveground | General | 36 | 7.5–30.8 | −2.14 | 0.08 | 3.6 | 0.001 | 2.26 | 0.03 | 1.2 | 0.001 | 0.995 | 0.89 | 2/36 |
| woody biomass | Brompton | 12 | 11.8–30.8 | −2.58 | 0.13 | 5.0 | 0.001 | 2.40 | 0.04 | 1.8 | 0.001 | 0.997 | 0.52 | 0 |
| (kg) | Mégantic | 12 | 13.0–29.3 | −2.38 | 0.13 | 5.6 | 0.001 | 2.34 | 0.05 | 1.9 | 0.001 | 0.996 | 0.62 | 0 |
| Ogden | 12 | 7.5–24.0 | −2.03 | 0.10 | 5.1 | 0.001 | 2.23 | 0.04 | 1.8 | 0.001 | 0.997 | 0.87 | 0 | |
| Coarse root | General | 36 | 7.3–29.5 | −1.69 | 0.47 | 28.2 | 0.01 | 1.50 | 0.17 | 11.1 | 0.001 | 0.706 | 0.019 | 3/36 |
| biomass | Brompton | 12 | 11.8–29.5 | −3.10 | 0.78 | 25.3 | 0.01 | 1.92 | 0.26 | 13.5 | 0.001 | 0.846 | 0.91 | 0 |
| (kg) | Mégantic | 12 | 12.8–28.4 | −3.66 | 0.37 | 10.1 | 0.001 | 2.13 | 0.13 | 5.9 | 0.001 | 0.966 | 0.19 | 1/12 |
| Ogden | 12 | 7.3–23.2 | −2.38 | 0.78 | 32.9 | 0.05 | 1.89 | 0.30 | 16.0 | 0.001 | 0.795 | 0.77 | 0 | |
| Site | Model | MAPE (%) | ||||
|---|---|---|---|---|---|---|
| Stem Volume | Stem Biomass | Branch Biomass | Aboveground Biomass | Coarse Root Biomass | ||
| Brompton | General | 1.2 | 1.2 | 20.4 | 1.0 | 10.3 |
| Site-specific | 0.6 | 1.2 | 3.3 | 0.7 | 6.6 | |
| Mégantic | General | 1.8 | 1.8 | 10.4 | 0.9 | 9.9 |
| Site-specific | 1.9 | 2.0 | 9.0 | 0.8 | 4.0 | |
| Ogden | General | 1.7 | 1.4 | 13.8 | 1.2 | 14.3 |
| Site-specific | 1.1 | 1.4 | 6.9 | 0.9 | 11.9 | |
| Model | Stem Volume | Stem Biomass | Branch Biomass | Aboveground Biomass | Coarse Root Biomass |
|---|---|---|---|---|---|
| General | −57.8 | −76.3 | 28.4 | −100.5 | 32.4 |
| Brompton | −34.1 | −25.2 | −8.9 | −36.2 | 7.2 |
| Mégantic | −9.2 | −14.8 | 10.2 | −32.9 | −9.3 |
| Ogden | −23.9 | −23.5 | 0.2 | −33.7 | 15.4 |
| Model | Stem Volume | Stem Biomass | Branch Biomass | Aboveground Biomass | Coarse Root Biomass |
|---|---|---|---|---|---|
| General | 0.984 | 0.988 | 0.889 | 0.995 | 0.656 |
| Brompton | 0.994 | 0.984 | 0.982 | 0.995 | 0.796 |
| Mégantic | 0.961 | 0.971 | 0.892 | 0.995 | 0.947 |
| Ogden | 0.987 | 0.990 | 0.954 | 0.996 | 0.721 |
| Tree Characteristics | DBH (cm) | General | Brompton | Variation (%) | Mégantic | Variation (%) | Ogden | Variation (%) |
|---|---|---|---|---|---|---|---|---|
| Volume (dm3) | 10 | 48.6 | 55.1 | −13.4 | 49.9 | −2.6 | 47.9 | +1.5 |
| 15 | 124.1 | 135.8 | −9.5 | 125.3 | −0.9 | 114.7 | +7.6 | |
| 20 | 241.3 | 257.6 | −6.7 | 240.8 | +0.2 | 213.0 | +11.7 | |
| 25 | 404.2 | 423.1 | −4.7 | 399.8 | +1.1 | 344.3 | +14.8 | |
| 30 | 616.1 | 634.8 | −3.0 | 604.9 | +1.8 | 509.8 | +17.3 | |
| Stem biomass (kg) | 10 | 18.4 | 19.1 | −3.9 | 17.7 | +3.9 | 18.5 | −0.8 |
| 15 | 43.7 | 45.2 | −3.4 | 42.2 | +3.6 | 43.9 | −0.3 | |
| 20 | 80.8 | 83.3 | −3.1 | 78.1 | +3.3 | 80.8 | − | |
| 25 | 130.2 | 133.9 | −2.8 | 126.1 | +3.1 | 129.8 | +0.3 | |
| 30 | 192.2 | 197.2 | −2.6 | 186.5 | +2.9 | 191.3 | +0.5 | |
| Branch biomass (kg) | 10 | 3.1 | 1.1 | +63.9 | 2.7 | +14.1 | 4.0 | −27.1 |
| 15 | 9.6 | 5.2 | +45.5 | 9.5 | +0.7 | 11.5 | −20.5 | |
| 20 | 21.2 | 15.5 | +27.0 | 23.3 | −10.1 | 24.6 | −16.1 | |
| 25 | 39.2 | 35.9 | +8.4 | 46.7 | −19.2 | 44.2 | −12.8 | |
| 30 | 64.8 | 71.5 | −10.2 | 82.5 | −27.3 | 71.4 | −10.1 | |
| Aboveground woody biomass (kg) a | 10 | 21.6 | 19.0 | +11.9 | 20.3 | +5.9 | 22.5 | −4.5 |
| 15 | 54.0 | 50.3 | +6.8 | 52.5 | +2.8 | 55.7 | −3.3 | |
| 20 | 103.4 | 100.3 | +3.0 | 102.9 | +0.5 | 106.0 | −2.5 | |
| 25 | 171.3 | 171.4 | −0.1 | 173.4 | −1.2 | 174.4 | −1.8 | |
| 30 | 258.7 | 265.6 | −2.7 | 265.7 | −2.7 | 262.1 | −1.3 | |
| Coarse root biomass (kg) | 10 | 5.9 | 3.8 | +35.9 | 3.4 | +41.7 | 7.2 | −22.2 |
| 15 | 10.9 | 8.2 | +24.1 | 8.2 | +24.9 | 15.6 | −43.1 | |
| 20 | 16.7 | 14.3 | +14.4 | 15.0 | +10.2 | 26.8 | −60.1 | |
| 25 | 23.4 | 22.0 | +6.0 | 24.2 | −3.2 | 40.9 | −74.6 | |
| 30 | 30.8 | 31.2 | −1.4 | 35.6 | −15.6 | 57.8 | −87.5 | |
| Total biomass (kg) b | 10 | 27.5 | 22.8 | +17.1 | 23.8 | +13.6 | 29.8 | −8.3 |
| 15 | 64.8 | 58.6 | +9.7 | 60.6 | +6.5 | 71.3 | −10.0 | |
| 20 | 120.2 | 114.7 | +4.6 | 117.9 | +1.9 | 132.8 | −10.5 | |
| 25 | 194.7 | 193.5 | +0.7 | 197.6 | −1.5 | 215.3 | −10.6 | |
| 30 | 289.5 | 296.8 | −2.5 | 301.4 | −4.1 | 319.9 | −10.5 | |
| Shoot:root ratio c | 10 | 3.7 | 5.0 | −37.5 | 5.9 | −61.5 | 3.1 | +14.5 |
| 15 | 5.0 | 6.1 | −22.8 | 6.4 | −29.5 | 3.6 | +27.8 | |
| 20 | 6.2 | 7.0 | −13.4 | 6.8 | −10.7 | 4.0 | +36.0 | |
| 25 | 7.3 | 7.8 | −6.5 | 7.2 | +1.9 | 4.3 | +41.7 | |
| 30 | 8.4 | 8.5 | −1.2 | 7.5 | +11.2 | 4.5 | +46.0 |
| DBH (cm) | Aboveground Woody Biomass (kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| General Model | Brompton Model | Mégantic Model | Ogden Model | |||||
| Stem + Branch | Abovegr. | Stem + Branch | Abovegr. | Stem + Branch | Abovegr. | Stem + Branch | Abovegr. | |
| 10 | 21.5 | 21.6 | 20.2 | 19.0 | 20.3 | 20.3 | 22.5 | 22.5 |
| 15 | 53.3 | 54.0 | 50.4 | 50.3 | 51.6 | 52.5 | 55.4 | 55.7 |
| 20 | 102.0 | 103.4 | 98.8 | 100.3 | 101.4 | 102.9 | 105.4 | 106.0 |
| 25 | 169.4 | 171.3 | 169.8 | 171.4 | 172.8 | 173.4 | 174.0 | 174.4 |
| 30 | 257.0 | 258.7 | 268.7 | 265.6 | 269.0 | 265.7 | 262.6 | 262.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Allometric Equations for Estimating Compartment Biomass and Stem Volume in Mature Hybrid Poplars: General or Site-Specific? Forests 2017, 8, 309. https://doi.org/10.3390/f8090309
Fortier J, Truax B, Gagnon D, Lambert F. Allometric Equations for Estimating Compartment Biomass and Stem Volume in Mature Hybrid Poplars: General or Site-Specific? Forests. 2017; 8(9):309. https://doi.org/10.3390/f8090309
Chicago/Turabian StyleFortier, Julien, Benoit Truax, Daniel Gagnon, and France Lambert. 2017. "Allometric Equations for Estimating Compartment Biomass and Stem Volume in Mature Hybrid Poplars: General or Site-Specific?" Forests 8, no. 9: 309. https://doi.org/10.3390/f8090309