Effects of the Antiozonant Ethylenediurea (EDU) on Fraxinus ornus L.: The Role of Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
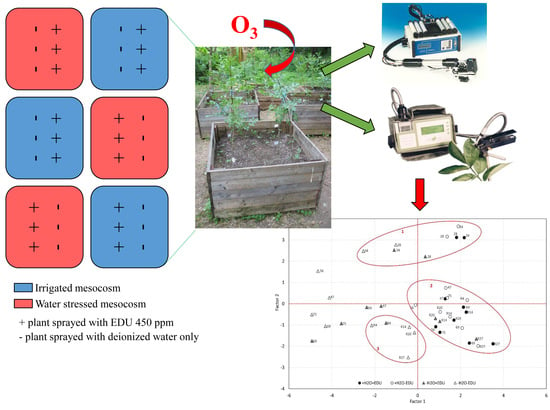
2.2. Experimental Design
2.3. Gas Exchange Measurements
2.4. Chlorophyll “a” Fluorescence Measurements
2.5. Statistical Analyses
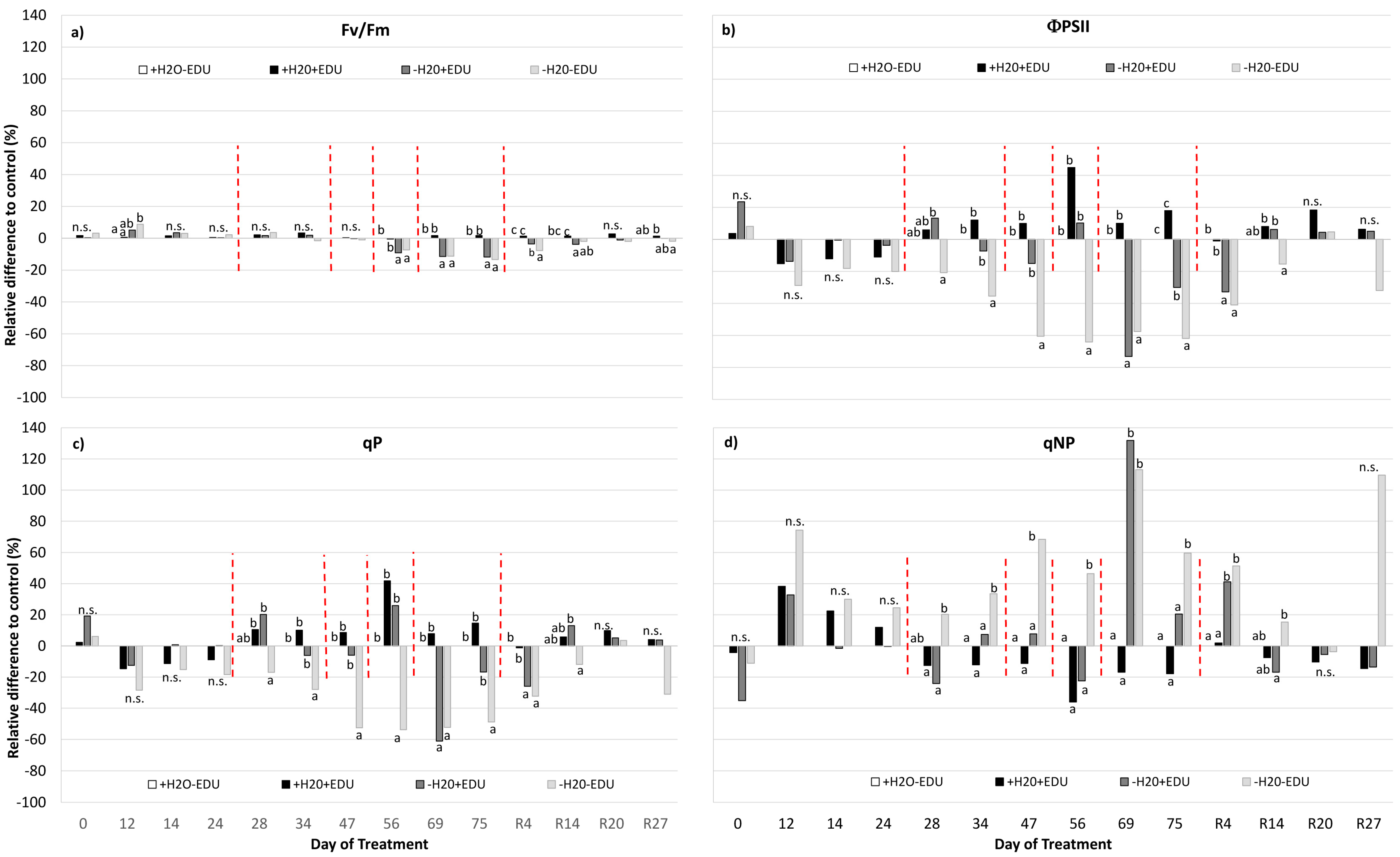
3. Results
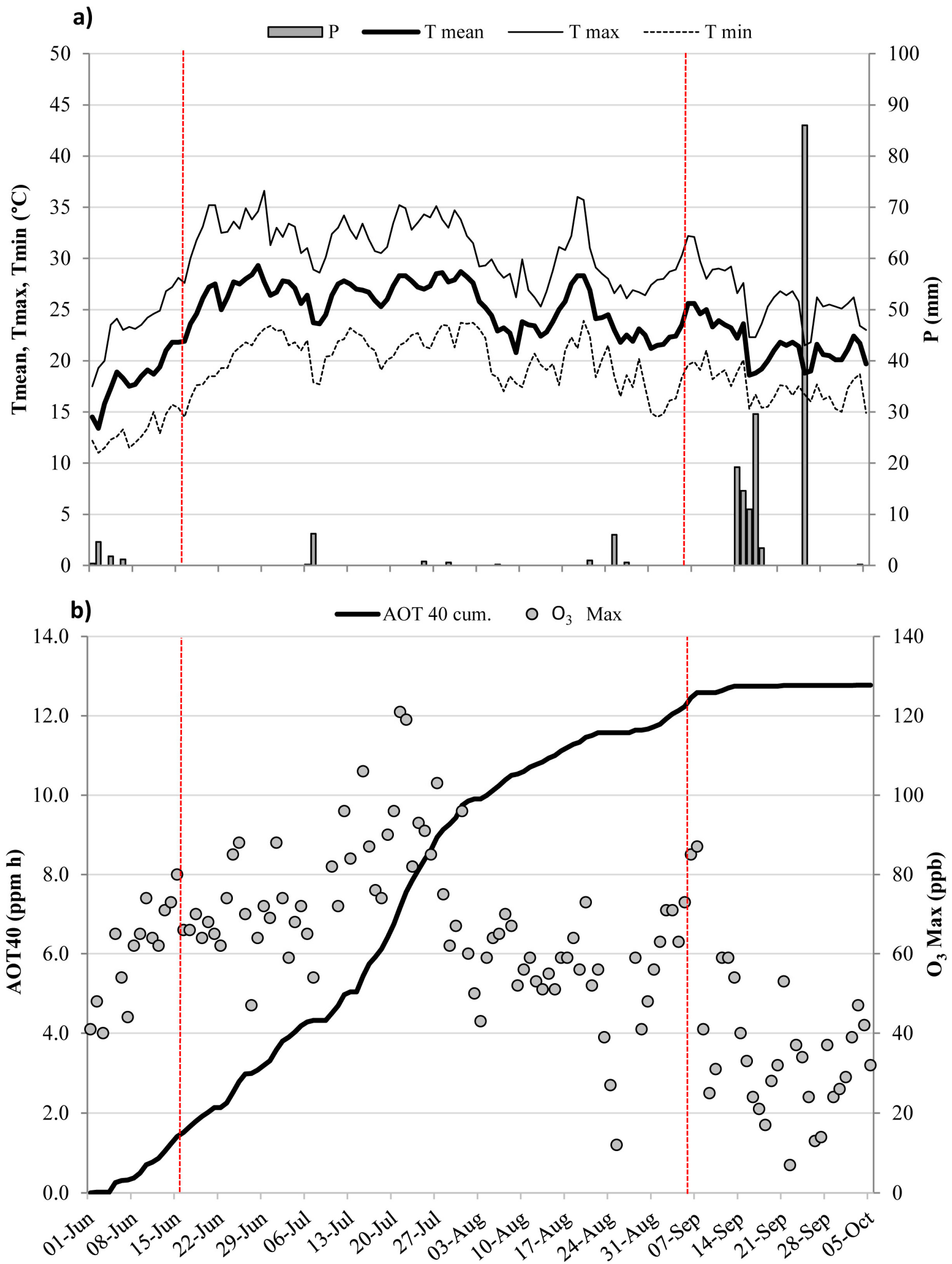
3.1. Meteorological Conditions and Ozone Levels
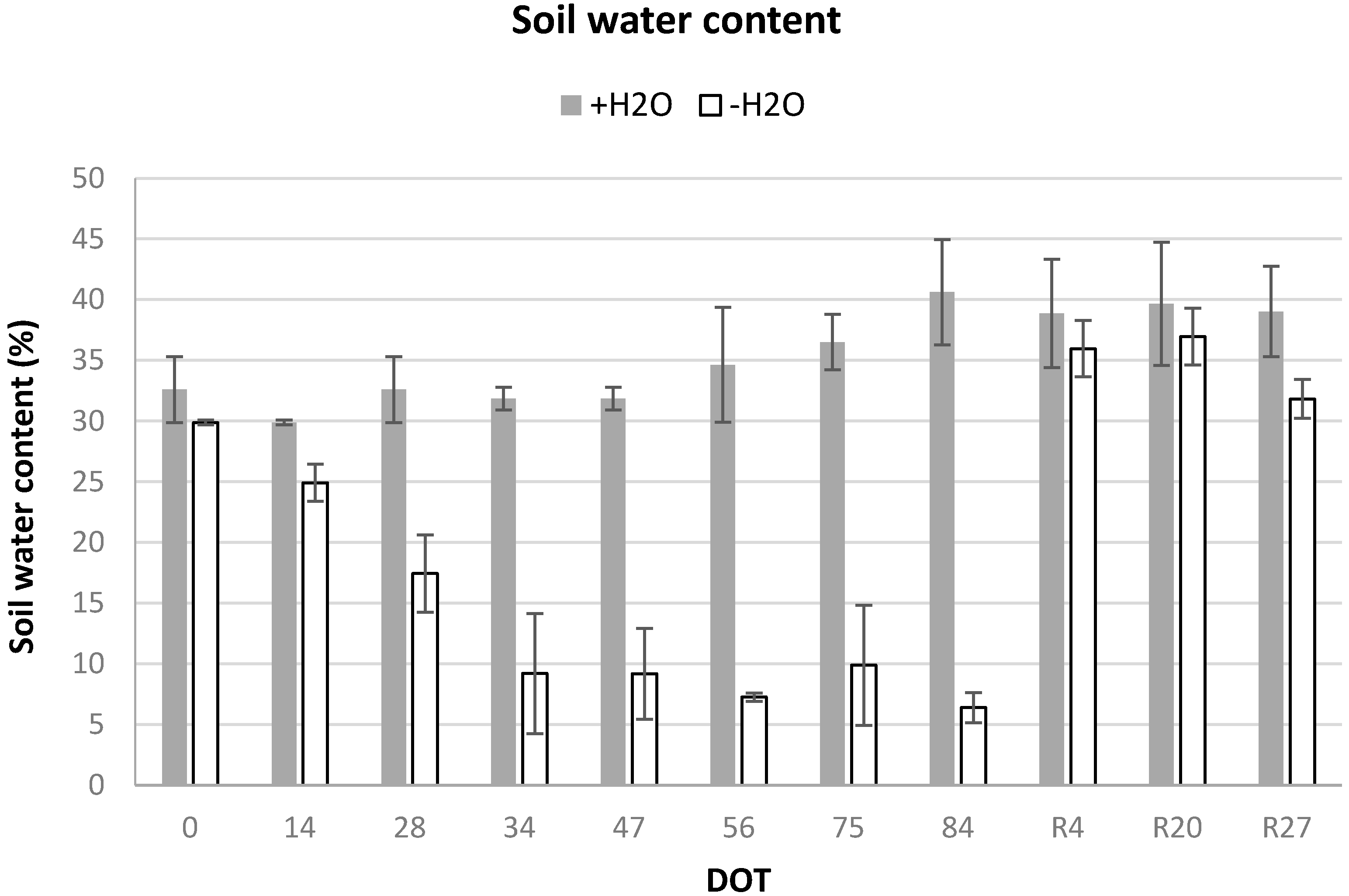
3.2. Soil Water Content
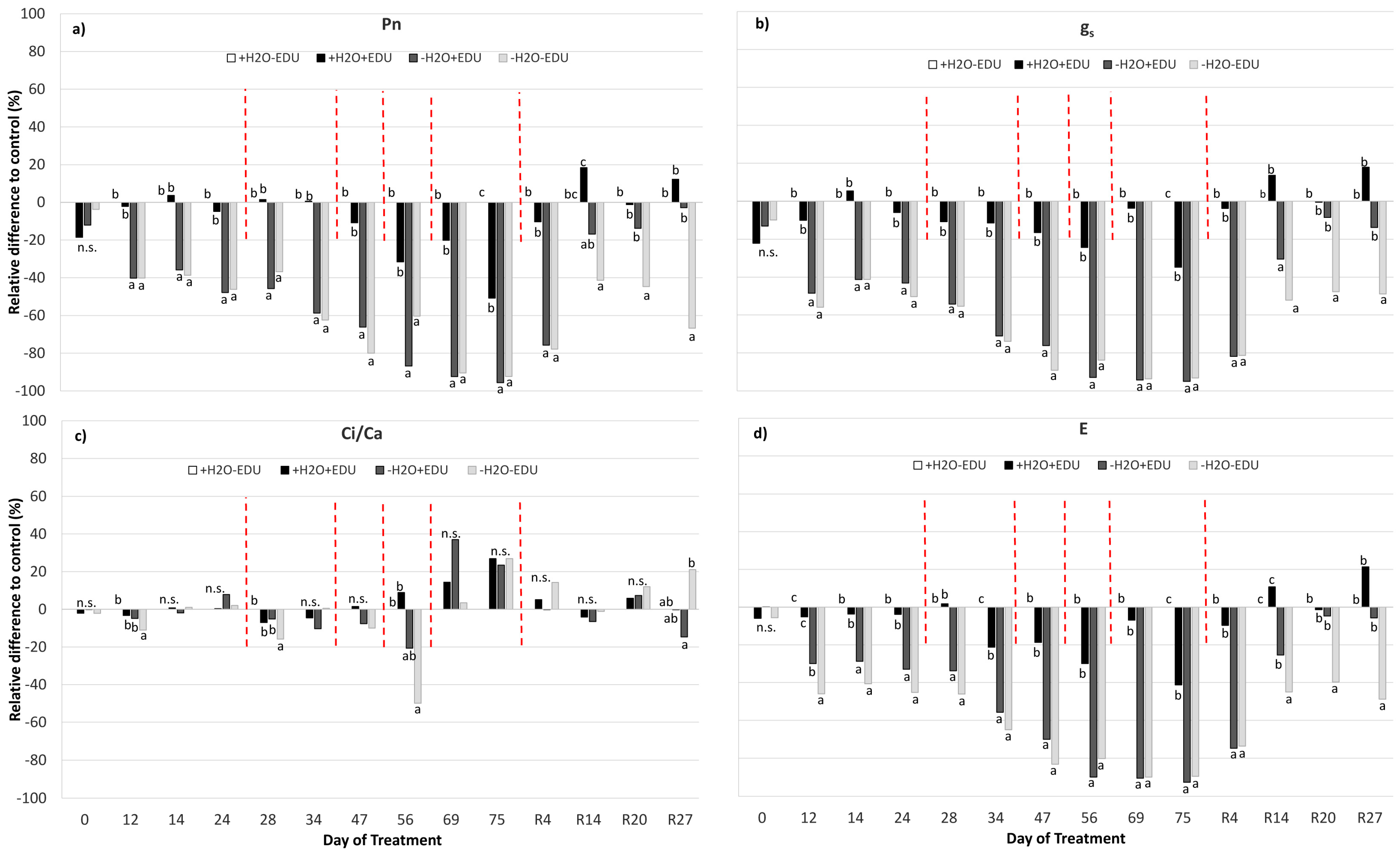
3.3. Ecophysiological Analyses
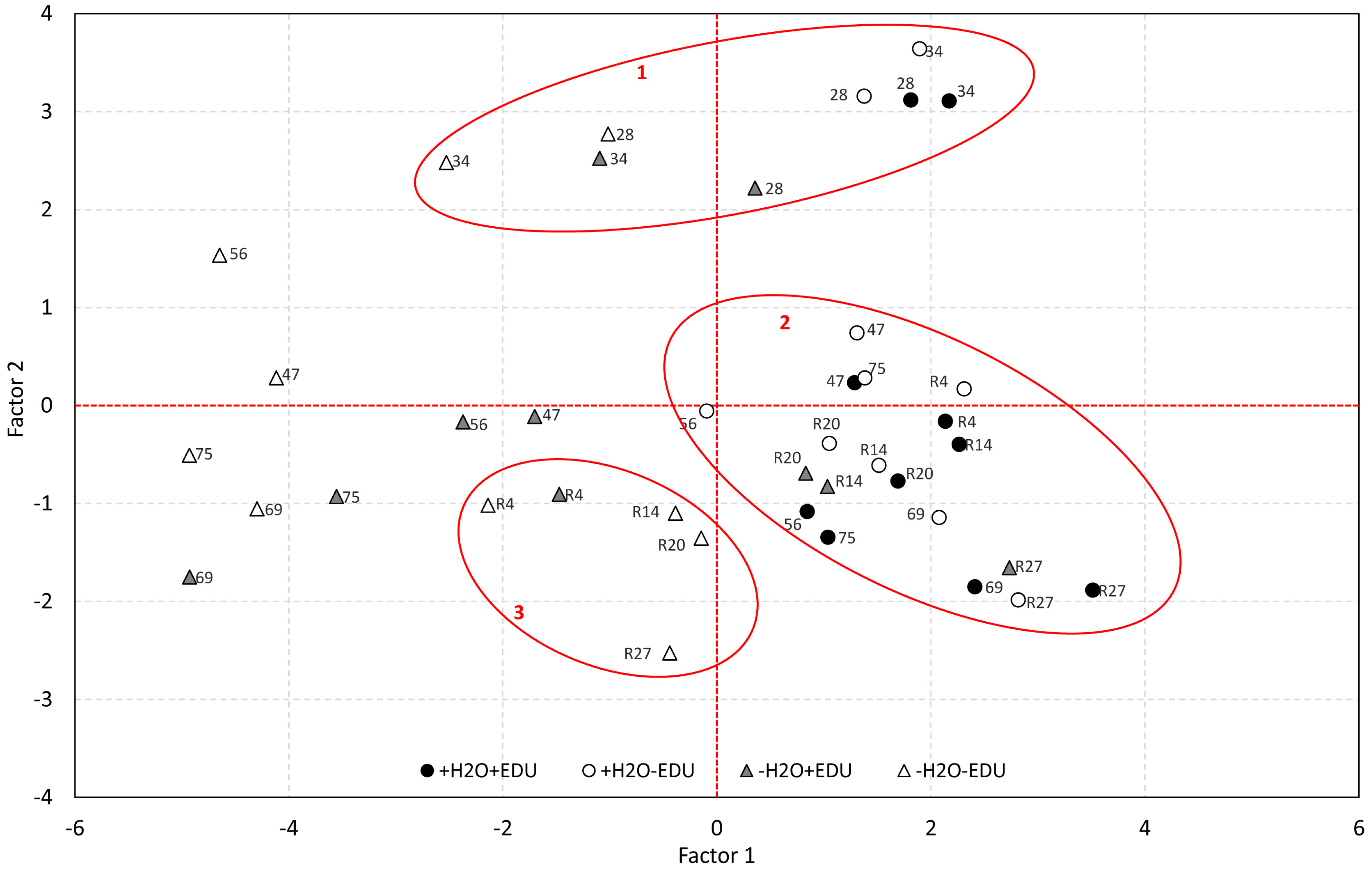
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Arneth, A.; Mills, G.; Solberg, S.; Uddling, J. Ozone—The persistent menace: Interactions with the N cycle and climate change. Curr. Opin. Environ. Sustain. 2014, 9–10, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Karnosky, D.F.; Skelly, J.M.; Percy, K.E.; Chappelka, A.H. Perspectives regarding 50 years of research on effects of tropospheric ozone air pollution on US forests. Environ. Pollut. 2007, 147, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The Effects of Tropospheric Ozone on Net Primary Productivity and Implications for Climate Change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Fagnano, M.; Amoriello, T.; Badiani, M.; Ballarin-Denti, A.; Buffoni, A.; Bussotti, F.; Castagna, A.; Cieslik, S.; Costantini, A.; et al. Measuring, modelling and testing ozone exposure, flux and effects on vegetation in southern European conditions—What does not work? A review from Italy. Environ. Pollut. 2007, 146, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Savi, F.; Muller, J.; Matteucci, G.; Paoletti, E. Simultaneous measurements of above and below canopy ozone fluxes help partitioning ozone deposition between its various sinks in a Mediterranean Oak Forest. Agric. For. Meteorol. 2014, 198–199, 181–191. [Google Scholar] [CrossRef]
- Mereu, S.; Gerosa, G.; Marzuoli, R.; Fusaro, L.; Salvatori, E.; Finco, A.; Spano, D.; Manes, F. Gas exchange and JIP-test parameters of two Mediterranean maquis species are affected by sea spray and ozone interaction. Environ. Exp. Bot. 2011, 73, 80–88. [Google Scholar] [CrossRef]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Fusaro, L.; Salvatori, E.; Manes, F. Effects of nitrogen deposition, drought and their interaction, on functional and structural traits of Fraxinus ornus L. and Quercus ilex L. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 151, 174–189. [Google Scholar] [CrossRef]
- Salvatori, E.; Fusaro, L.; Manes, F. Chlorophyll fluorescence for phenotyping drought stressed trees in a mixed deciduous forest. Ann. Bot. 2016, 6, 39–49. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Maria Fabi, A.; De Santis, F.; Zona, D. Estimates of potential ozone stomatal uptake in mature trees of Quercus ilex in a Mediterranean climate. Environ. Exp. Bot. 2007, 59, 235–241. [Google Scholar] [CrossRef]
- Kühn, A.R.; Grill, S.; Baumgarten, M.; Ankerst, D.P.; Matyssek, R. Daily growth of European beech (Fagus sylvatica L.) on moist sites is affected by short-term drought rather than ozone uptake. Trees 2015, 29, 1501–1519. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; González-Fernández, I.; Calvete, H.; García-Gómez, H.; Bermejo, V. Drought stress does not protect Quercus ilex L. from ozone effects: Results from a comparative study of two subspecies differing in ozone sensitivity. Plant Biol. 2014, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Desotgiu, R.; Camin, F.; Ziller, L.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Severe drought events increase the sensitivity to ozone on poplar clones. Environ. Exp. Bot. 2014, 100, 94–104. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Contran, N.; Manning, W.J.; Ferrara, A.M. Use of the antiozonant ethylenediurea (EDU) in Italy: Verification of the effects of ambient ozone on crop plants and trees and investigation of EDU′s mode of action. Environ. Pollut. 2009, 157, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, S.; Szantoi, Z.; Chen, S.; Wang, X. Protection of plants from ambient ozone by applications of ethylenediurea (EDU): A meta-analytic review. Environ. Pollut. 2010, 158, 3236–3242. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Paoletti, E.; Sandermann, H., Jr.; Ernst, D. Ethylenediurea (EDU): A research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ. Pollut. 2011, 159, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Koike, T.; Watanabe, M.; Hoshika, Y.; Saitanis, C.J. Ethylene-di-urea (EDU), an effective phytoproctectant against O3 deleterious effects and a valuable research tool. J. Agric. Meteorol. 2015, 71, 185–195. [Google Scholar] [CrossRef]
- Carnhan, J.E.; Jenner, E.L.; Wat, E.K.W. Prevention of Ozone Injury to Plants by a New Protectant Chemical. Phytopathology 1978, 68, 1225–1229. [Google Scholar] [CrossRef]
- Manes, F.; Altieri, A.; Tripodo, P.; Booth, C.E.; Unsworth, M.H. Bioindication study of effects of ambient ozone on tobacco and radish plants using a protectant chemical (EDU). Ann. Bot. 1990, 48, 133–149. [Google Scholar]
- Astorino, G.; Margani, I.; Tripodo, P.; Manes, F. The response of Phaseolus vulgaris L. cv. Lit. to different dosages of the anti-ozonant ethylenediurea (EDU) in relation to chronic treatment with ozone. Plant Sci. 1995, 111, 237–248. [Google Scholar] [CrossRef]
- Tiwari, S.; Agrawal, M. Effectiveness of different EDU concentrations in ameliorating ozone stress in carrot plants. Ecotoxicol. Environ. Saf. 2010, 73, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Calatayud, V.; Jiang, L.; Manning, W.J.; Hayes, F.; Tian, Y.; Feng, Z. Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ. Pollut. 2015, 205, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. Ecotoxicol. Environ. Saf. 2017, 142, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Long, R.P.; Davis, D.D. Black cherry growth response to ambient ozone and EDU. Environ. Pollut. 1991, 70, 241–254. [Google Scholar] [CrossRef]
- Ainsworth, N.; Ashmore, M.R. Assessment of ozone effects on beech (Fagus sylvatica) by injection of a protectant chemical. For. Ecol. Manag. 1992, 51, 129–136. [Google Scholar] [CrossRef]
- Contran, N.; Poletti, E.; Manning, W.J.; Tagliaferro, F. Ozone sensitivity and ethylenediurea protection in ash trees assessed by JIP chlorophyll a fluorescence transient analysis. Photosynthetica 2009, 47, 68–78. [Google Scholar] [CrossRef]
- Basahi, J.M.; Ismail, I.M.; Haiba, N.S.; Hassan, I.A.; Lorenzini, G. Assessing ambient ozone injury in olive (Olea europaea L.) plants by using the antioxidant ethylenediurea (EDU) in Saudi Arabia. Environ. Monit. Assess. 2016, 188, 371. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Paoletti, E.; Saitanis, C.J.; Manning, W.J.; Sugai, T.; Koike, T. Impacts of ethylenediurea (EDU) soil drench and foliar spray in Salix sachalinensis protection against O3-induced injury. Sci. Total Environ. 2016, 573, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Paoletti, E.; Saitanis, C.J.; Manning, W.J.; Shi, C.; Koike, T. High doses of ethylene diurea (EDU) are not toxic to willow and act as nitrogen fertilizer. Sci. Total Environ. 2016, 566–567, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Mancino, L.; Federico, R. Translocation and persistence of EDU (ethylenediurea) in plants: The relationship with its role in ozone damage. Environ. Pollut. 1997, 96, 445–448. [Google Scholar] [CrossRef]
- Paoletti, E.; Contran, N.; Manning, W.J.; Castagna, A.; Ranieri, A.; Tagliaferro, F. Protection of ash (Fraxinus excelsior) trees from ozone injury by ethylenediurea (EDU): Roles of biochemical changes and decreased stomatal conductance in enhancement of growth. Environ. Pollut. 2008, 155, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.A. Physiological and biochemical response of potato (Solanum tuberosum L. cv. Kara) to O3 and antioxidant chemicals: Possible roles of antioxidant enzymes. Ann. Appl. Biol. 2006, 148, 197–206. [Google Scholar] [CrossRef]
- Pandey, A.K.; Majumder, B.; Keski-Saari, S.; Kontunen-Soppela, S.; Pandey, V.; Oksanen, E. Differences in responses of two mustard cultivars to ethylenediurea (EDU) at high ambient ozone concentrations in India. Agric. Ecosyst. Environ. 2014, 196, 158–166. [Google Scholar] [CrossRef]
- Pandey, A.K.; Majumder, B.; Keski-Saari, S.; Kontunen-Soppela, S.; Mishra, A.; Sahu, N.; Pandey, V.; Oksanen, E. Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: Results from ethylenediurea studies. Sci. Total Environ. 2015, 532, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Arndal, M.F.; Boesgaard, K.; Michelsen, A.; Bruhn, D.; Schmidt, N.M. Solar UV-B effects on PSII performance in Betula nana are influenced by PAR level and reduced by EDU: Results of a 3-year experiment in the High Arctic. Physiol. Plant. 2012, 145, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yuan, X.; Shang, B.; Manning, W.J.; Yang, A.; Wang, Y.; Feng, Z. Moderate drought did not affect the effectiveness of ethylenediurea (EDU) in protecting Populus cathayana from ambient ozone. Sci. Total Environ. 2016, 569–570, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Contran, N.; Bernasconi, P.; Günthardt-Goerg, M.S.; Vollenweider, P. Erratum to “Structural and physiological responses to ozone in Manna ash (Fraxinus ornus L.) leaves of seedlings and mature trees under controlled and ambient conditions”. Sci. Total Environ. 2010, 408, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Tretiach, M. Photosynthesis and transpiration of evergreen Mediterranean and deciduous trees in an ecotone during a growing season. Acta Oecol. 1993, 14, 341–360. [Google Scholar]
- Mills, G.; Hayes, F.; Simpson, D.; Emberson, L.; Norris, D.; Harmens, H.; Büker, P. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Glob. Change Biol. 2011, 17, 592–613. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Carriero, G.; Emiliani, G.; Giovannelli, A.; Hoshika, Y.; Manning, W.J.; Traversi, M.L.; Paoletti, E. Effects of long-term ambient ozone exposure on biomass and wood traits in poplar treated with ethylenediurea (EDU). Environ. Pollut. 2015, 206, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Pecori, F.; Conese, I.; Bardelli, T.; Marchi, E.; Manning, W.J.; Badea, O.; Paoletti, E. Effects of a three-year exposure to ambient ozone on biomass allocation in poplar using ethylenediurea. Environ. Pollut. 2013, 180, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- ICP Vegetation. Chapter III: Mapping critical levels for vegetation. In Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads and Levels and Air Pollution Effects, Risks and Trends; Umweltbundesamt: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- Tonneijck, A.E.G.; Van Dijk, C.J. Effects of ambient ozone on injury and yield of Phaseolus vulgaris at four rural sites in the Netherlands as assessed by using ethylenediurea (EDU). New Phytol. 1997, 135, 93–100. [Google Scholar] [CrossRef]
- Kostka-Rick, R.; Manning, W.J. Dose-response studies with ethylenediurea (EDU) and radish. Environ. Pollut. 1993, 79, 249–260. [Google Scholar] [CrossRef]
- Mills, G.; Benton, J.; Skärby, L.; Fuhrer, J.; Gimeno, B. ICP Vegetation Experimental Protocol for the ICP-CROPS; ICP-CROPS Coordination Centre, The Nottingham Trent University: Nottingham, UK, 1997; p. 47. [Google Scholar]
- Oksanen, E.; Pandey, V.; Pandey, A.K.; Keski-Saari, S.; Kontunen-Soppela, S.; Sharma, C. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013, 177, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Koike, T.; Saitanis, J.C.; Watanabe, M.; Satoh, F.; Hoshika, Y. Ethylenediurea (EDU) as a protectant of plants against O3. Eurasian J. For. Res. 2015, 18, 37–50. [Google Scholar]
- Tiwari, S. Ethylenediurea as a potential tool in evaluating ozone phytotoxicity: A review study on physiological, biochemical and morphological responses of plants. Environ. Sci. Pollut. Res. 2017, 24, 14019–14039. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Agrawal, M. Protection of Plants against Air Pollutants: Role of Chemical Protectants. J. Environ. Manag. 1993, 37, 163–174. [Google Scholar] [CrossRef]
- Lee, E.H.; Kramer, G.F.; Rowland, R.A.; Agrawal, M. Antioxidants and growth regulators counter the effects of O3 and SO2 in crop plants. Agric. Ecosyst. Environ. 1992, 38, 99–106. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S.; Trifilò, P.; Gullo, M.A.L. Water relations and hydraulic characteristics of three woody species co-occurring in the same habitat. Ann. For. Sci. 2003, 60, 297–305. [Google Scholar] [CrossRef]
- Gortan, E.; Nardini, A.; Gascó, A.; Salleo, S. The hydraulic conductance of Fraxinus ornus leaves is constrained by soil water availability and coordinated with gas exchange rates. Tree Physiol. 2009, 29, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Nali, C.; Paoletti, E.; Marabottini, R.; Della Rocca, G.; Lorenzini, G.; Paolacci, A.R.; Ciaffi, M.; Badiani, M. Ecophysiological and biochemical strategies of response to ozone in Mediterranean evergreen broadleaf species. Atmos. Environ. 2004, 38, 2247–2257. [Google Scholar] [CrossRef]
- Vitale, M.; Salvatori, E.; Loreto, F.; Fares, S.; Manes, F. Physiological responses of Quercus ilex Leaves to Water Stress and Acute Ozone Exposure under Controlled Conditions. Water Air Soil Pollut. 2008, 189, 113–125. [Google Scholar] [CrossRef]
- Pellegrini, E.; Francini, A.; Lorenzini, G.; Nali, C. PSII photochemistry and carboxylation efficiency in Liriodendron tulipifera under ozone exposure. Environ. Exp. Bot. 2011, 70, 217–226. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Agrawal, M. Low-temperature scanning electron microscope studies of stomatal responses in snap bean plants treated with ozone and ethylenediurea. Biotronics. 1999, 28, 45–53. [Google Scholar]
- Singh, S.; Agrawal, S.B.; Agrawal, M. Differential protection of ethylenediurea (EDU) against ambient ozone for five cultivars of tropical wheat. Environ. Pollut. 2009, 157, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Mouzaki-Paxinou, A.-C.; Saitanis, C.J.; Paoletti, E.; Manning, W.J. The first toxicological study of the antiozonant and research tool ethylene diurea (EDU) using a Lemna minor L. bioassay: Hints to its mode of action. Environ. Pollut. 2016, 213, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Castagna, A.; Ederli, L.; Pasqualini, S.; Ranieri, A.; Manning, W.J. Gene expression in snapbeans exposed to ozone and protected by ethylenediurea. Environ. Pollut. 2014, 193, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.M.; Kim, M.S.; Krizek, D.T.; Bajwa, R.K.S. Evaluating UV-B Effects and EDU Protection in Soybean Leaves Using Fluorescence. Photochem. Photobiol. 2005, 81, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
| Type of Measurement | ||||||
|---|---|---|---|---|---|---|
| Date of Sampling (Year 2006) | Day of Treatment (DOT) | EDU Treatment | SWC | Gas Exchange | Chlorophyll Fluorescence | |
| 16 June | Days from the last irrigation (−H2O sets) | 0 | × | × | × | |
| 28 June | 12 | × | × | |||
| 30 June | 14 | × | × | × | ||
| 10 July | 24 | × | × | × | ||
| 14 July | 28 | × | × | × | ||
| 20 July | 34 | × | × | × | ||
| 24 July | 38 | × | ||||
| 2 August | 47 | × | × | × | ||
| 7 August | 52 | × | ||||
| 11 August | 56 | × | × | × | ||
| 22 August | 67 | × | ||||
| 24 August | 69 | × | × | |||
| 30 August | 75 | × | × | × | ||
| 5 September | 83 | × | ||||
| 6 September | 84 | × | ||||
| 12 September | Days from re-watering (−H2O sets) | R4 | × | × | × | |
| 22 September | R14 | × | × | |||
| 28 September | R20 | × | × | × | ||
| 5 October | R27 | × | × | × | ||
| DOT | Pn | gs | E | Ci/Ca | Fv/Fm | ΦPSII | qP | qNP | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Drought | 0.834 | 0.954 | 0.971 | 0.964 | 0.586 | 0.183 | 0.207 | 0.207 |
| EDU | 0.052 | 0.077 | 0.985 | 0.989 | 0.729 | 0.362 | 0.395 | 0.395 | |
| Drought × EDU | 0.431 | 0.186 | 0.338 | 0.373 | 0.180 | 0.575 | 0.550 | 0.550 | |
| 12 | Drought | 0.000 | 0.000 | 0.000 | 0.001 | 0.207 | 0.115 | 0.112 | 0.112 |
| EDU | 0.798 | 0.745 | 0.197 | 0.411 | 0.395 | 0.674 | 0.668 | 0.668 | |
| Drought × EDU | 0.808 | 0.039 | 0.012 | 0.008 | 0.550 | 0.088 | 0.075 | 0.075 | |
| 14 | Drought | 0.000 | 0.000 | 0.000 | 0.782 | 0.022 | 0.645 | 0.823 | 0.823 |
| EDU | 0.581 | 0.679 | 0.491 | 0.706 | 0.399 | 0.695 | 0.733 | 0.733 | |
| Drought × EDU | 0.946 | 0.672 | 0.191 | 0.526 | 0.575 | 0.036 | 0.048 | 0.048 | |
| 24 | Drought | 0.000 | 0.000 | 0.000 | 0.057 | 0.346 | 0.345 | 0.470 | 0.470 |
| EDU | 0.556 | 0.918 | 0.507 | 0.206 | 0.495 | 0.689 | 0.448 | 0.448 | |
| Drought × EDU | 0.774 | 0.269 | 0.205 | 0.267 | 0.265 | 0.043 | 0.033 | 0.033 | |
| 28 | Drought | 0.000 | 0.000 | 0.000 | 0.007 | 0.241 | 0.444 | 0.632 | 0.632 |
| EDU | 0.600 | 0.464 | 0.229 | 0.470 | 0.958 | 0.026 | 0.003 | 0.003 | |
| Drought × EDU | 0.461 | 0.371 | 0.364 | 0.001 | 0.146 | 0.115 | 0.084 | 0.084 | |
| 34 | Drought | 0.000 | 0.000 | 0.000 | 0.682 | 0.252 | 0.001 | 0.004 | 0.004 |
| EDU | 0.742 | 0.481 | 0.269 | 0.234 | 0.013 | 0.013 | 0.033 | 0.033 | |
| Drought × EDU | 0.811 | 0.241 | 0.006 | 0.622 | 0.988 | 0.307 | 0.425 | 0.425 | |
| 47 | Drought | 0.000 | 0.000 | 0.000 | 0.135 | 0.701 | 0.000 | 0.000 | 0.000 |
| EDU | 0.870 | 0.772 | 0.695 | 0.763 | 0.779 | 0.002 | 0.001 | 0.001 | |
| Drought × EDU | 0.177 | 0.017 | 0.028 | 0.944 | 0.927 | 0.041 | 0.020 | 0.020 | |
| 56 | Drought | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.007 | 0.007 |
| EDU | 0.009 | 0.030 | 0.007 | 0.021 | 0.506 | 0.000 | 0.000 | 0.000 | |
| Drought × EDU | 0.814 | 0.316 | 0.164 | 0.213 | 0.699 | 0.282 | 0.132 | 0.132 | |
| 69 | Drought | 0.000 | 0.000 | 0.000 | 0.239 | 0.000 | 0.000 | 0.000 | 0.000 |
| EDU | 0.304 | 0.822 | 0.694 | 0.032 | 0.764 | 0.623 | 0.939 | 0.939 | |
| Drought × EDU | 0.397 | 0.868 | 0.741 | 0.386 | 0.718 | 0.026 | 0.153 | 0.153 | |
| 75 | Drought | 0.000 | 0.000 | 0.000 | 0.212 | 0.000 | 0.000 | 0.000 | 0.000 |
| EDU | 0.001 | 0.023 | 0.013 | 0.211 | 0.585 | 0.016 | 0.018 | 0.018 | |
| Drought × EDU | 0.003 | 0.039 | 0.032 | 0.108 | 0.981 | 0.489 | 0.361 | 0.361 | |
| R4 | Drought | 0.000 | 0.000 | 0.000 | 0.309 | 0.000 | 0.000 | 0.000 | 0.000 |
| EDU | 0.563 | 0.756 | 0.329 | 0.282 | 0.024 | 0.642 | 0.730 | 0.730 | |
| Drought × EDU | 0.383 | 0.818 | 0.434 | 0.028 | 0.238 | 0.550 | 0.611 | 0.611 | |
| R14 | Drought | 0.000 | 0.000 | 0.000 | 0.675 | 0.001 | 0.163 | 0.669 | 0.669 |
| EDU | 0.018 | 0.036 | 0.027 | 0.260 | 0.795 | 0.019 | 0.007 | 0.007 | |
| Drought × EDU | 0.731 | 0.631 | 0.509 | 0.881 | 0.094 | 0.273 | 0.085 | 0.085 | |
| R20 | Drought | 0.034 | 0.029 | 0.030 | 0.255 | 0.062 | 0.601 | 0.940 | 0.940 |
| EDU | 0.261 | 0.125 | 0.087 | 0.919 | 0.245 | 0.312 | 0.439 | 0.439 | |
| Drought × EDU | 0.224 | 0.115 | 0.065 | 0.376 | 0.548 | 0.299 | 0.579 | 0.579 | |
| R27 | Drought | 0.001 | 0.001 | 0.001 | 0.643 | 0.013 | 0.136 | 0.141 | 0.141 |
| EDU | 0.002 | 0.025 | 0.004 | 0.016 | 0.017 | 0.056 | 0.071 | 0.071 | |
| Drought × EDU | 0.029 | 0.453 | 0.300 | 0.018 | 0.677 | 0.167 | 0.148 | 0.148 |
| Factor 1 | Factor 2 | |
|---|---|---|
| E | 0.676808 | −0.652821 |
| gs | 0.855971 | −0.352427 |
| Pn | 0.812304 | −0.428020 |
| Ci/Ca | 0.014049 | 0.571931 |
| SWC | 0.829174 | 0.219180 |
| O3 Max | −0.108951 | −0.902128 |
| AOT40 | 0.118850 | 0.887376 |
| ΦPSII | 0.922908 | 0.103793 |
| qP | 0.897010 | 0.116943 |
| qNP | −0.897010 | −0.116943 |
| Fv/Fm | 0.838633 | 0.194126 |
| % Total variance | 52.24 | 25.16 |
| Cumulative % | 52.24 | 77.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatori, E.; Fusaro, L.; Manes, F. Effects of the Antiozonant Ethylenediurea (EDU) on Fraxinus ornus L.: The Role of Drought. Forests 2017, 8, 320. https://doi.org/10.3390/f8090320
Salvatori E, Fusaro L, Manes F. Effects of the Antiozonant Ethylenediurea (EDU) on Fraxinus ornus L.: The Role of Drought. Forests. 2017; 8(9):320. https://doi.org/10.3390/f8090320
Chicago/Turabian StyleSalvatori, Elisabetta, Lina Fusaro, and Fausto Manes. 2017. "Effects of the Antiozonant Ethylenediurea (EDU) on Fraxinus ornus L.: The Role of Drought" Forests 8, no. 9: 320. https://doi.org/10.3390/f8090320