Updating Bark Proportions for the Estimation of Tropical Timber Volumes by Indigenous Community-Based Forest Enterprises in Quintana Roo, Mexico
Abstract
:1. Introduction
2. Materials and Methods
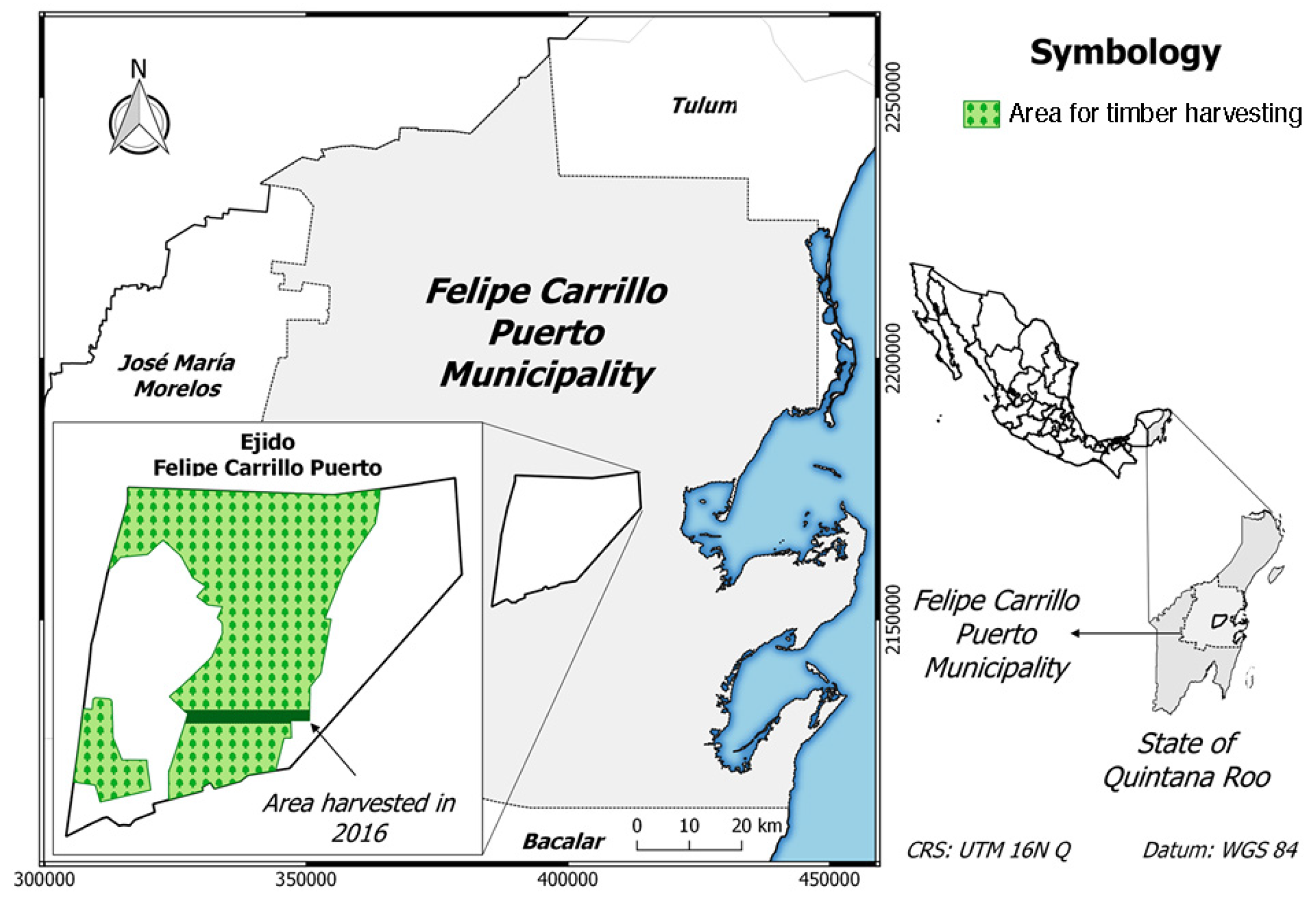
2.1. Study Area
2.2. Measurements and Calculations
2.3. Statistical Analyses
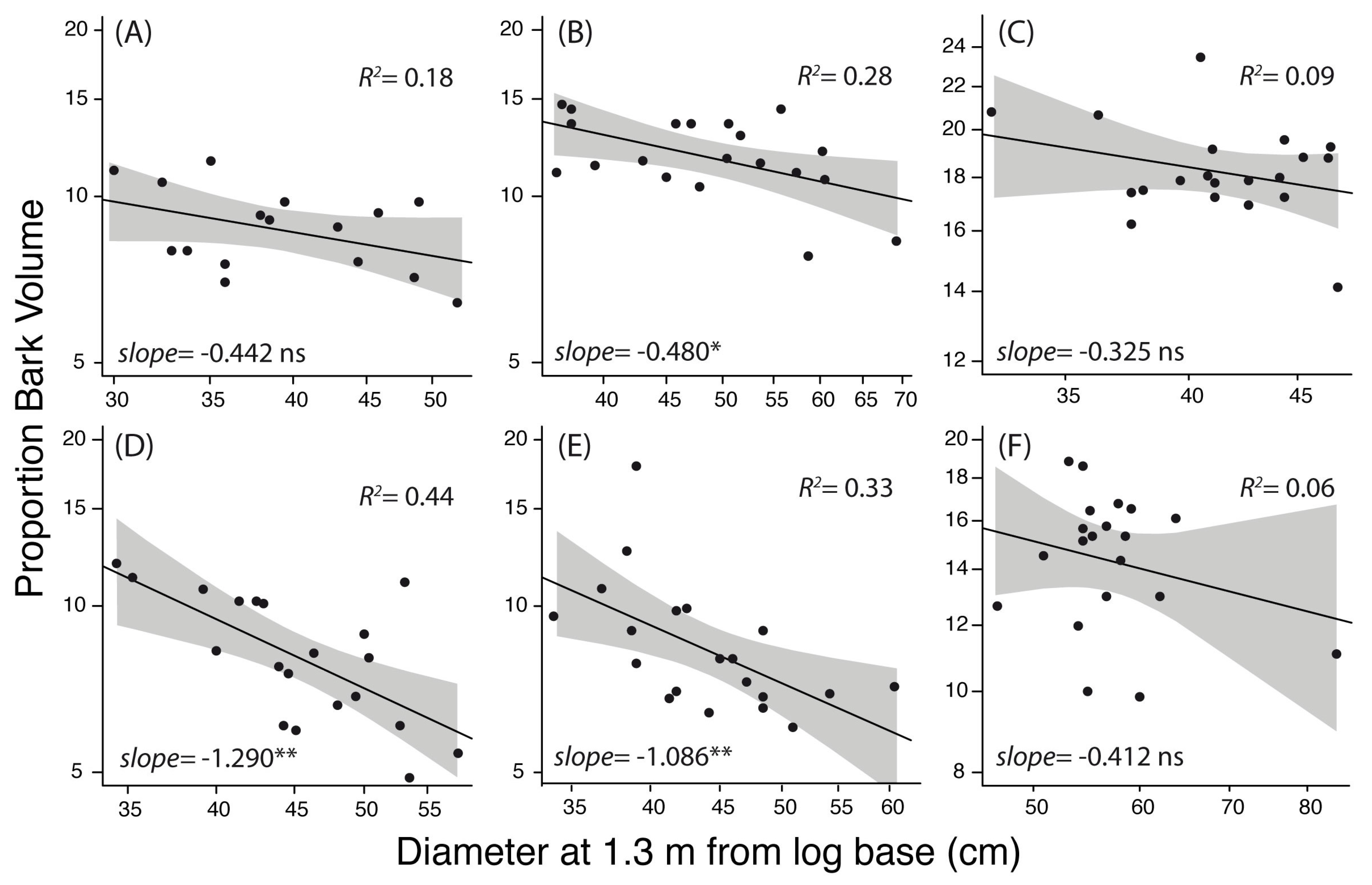
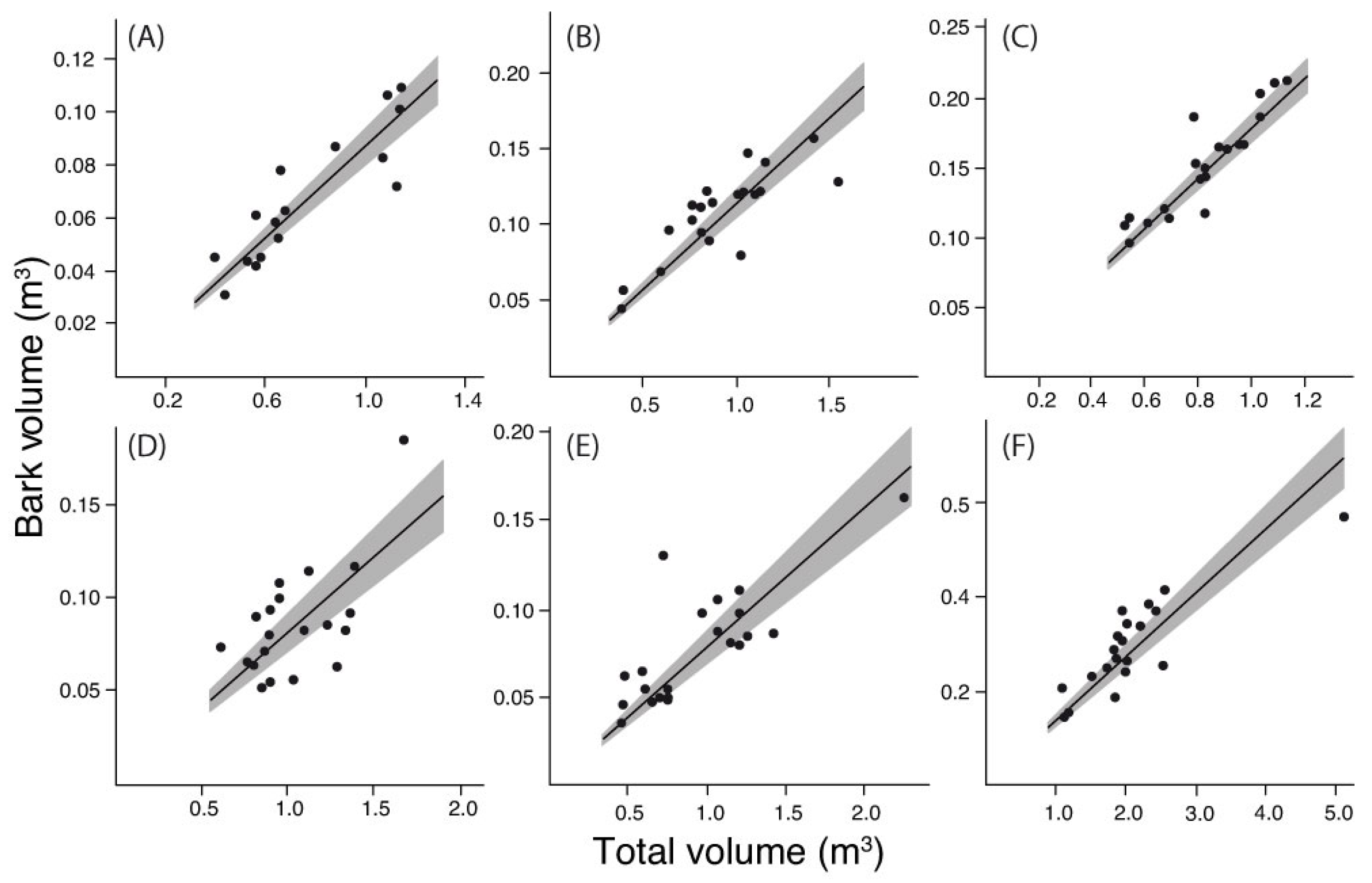
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nasi, R.; Wunder, S.; Campos, A.J.J. Forest Ecosystem Services: Can They Pay Our Way out of Deforestation? Forestry Roundtable; CIFOR: UNFFⅡ, San José, Costa Rica, 2002. [Google Scholar]
- Mukul, S.A.; Sohel, M.S.I.; Herbohn, J.; Inostroza, L.; König, H. Integrating ecosystem services supply potential from future land-use scenarios in protected area management: A Bangladesh case study. Ecosyst. Serv. 2017, 26, 355–364. [Google Scholar] [CrossRef]
- Ellis, E.A.; Porter-Bolland, L. Is community-based forest management more effective than protected areas?: A comparison of land use/land cover change in two neighboring study areas of the Central Yucatan Peninsula, Mexico. For. Ecol. Manag. 2008, 256, 1971–1983. [Google Scholar] [CrossRef]
- Burivalova, Z.; Şekercioğlu, Ç.H.; Koh, L.P. Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 2014, 24, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.A.; Byers, B.; Nassikas, A.A. A role for tropical forests in stabilizing atmospheric CO2. Nat. Clim. Chang. 2015, 5, 1022–1023. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Broadbent, E.N.; Rozendaal, D.M.A.; Bongers, F.; Zambrano, A.M.A.; Aide, T.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; et al. Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2016, 2, e1501639. [Google Scholar] [CrossRef] [PubMed]
- Vira, B.; Agarwal, B.; Jamnadass, R.H.; Kleinschmit, D.; McMullin, S.; Mansourian, S.; Neufeldt, H.; Parrotta, J.A.; Sunderland, T.C.H.; Wildburger, C. Forests, trees and landscapes for food security and nutrition. In Forests and Food: Addressing Hunger and Nutrition Across Sustainable Landscapes; Open Book Publishers: Cambridge, UK, 2015. [Google Scholar]
- Palacio-Prieto, J.L.; Bocco, G.; Velázquez, A.; François, J.; Takaki, F.; Victoria, A.; Luna, L.; Gómez, G.; García, J.L.; Palma, M.; et al. La condición actual de los recursos forestales en México: Resultados del Inventario Forestal Nacional 2000. Investig. Geogr. 2000, 43, 183–203. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2015; UN Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Pacheco, P.; Barry, D.; Cronkleton, P.; Larson, A.M. The recognition of forest rights in Latin America: Progress and shortcomings of forest tenure reforms. Soc. Nat. Resour. 2012, 25, 556–571. [Google Scholar] [CrossRef]
- Bray, D.B.; Merino-Pérez, L.; Negreros-Castillo, P.; Segura-Warnholtz, G.; Torres-Rojo, J.M.; Vester, H.F. Mexico’s community-managed forests as a global model for sustainable landscapes. Conserv. Biol. 2003, 17, 672–677. [Google Scholar] [CrossRef]
- INEGI. Conjunto de Datos Vectoriales de la Carta de Uso del Suelo y Vegetación, Escala 1:250,000; Serie V (Continuo Nacional); Instituto Nacional de Estadística y Geografía (INEGI): Aguascalientes, México, 2011.
- Urquiza-Haas, T.; Dolman, P.M.; Peres, C.A. Regional scale variation in forest structure and biomass in the Yucatan Peninsula, Mexico: Effects of forest disturbance. For. Ecol. Manag. 2007, 247, 80–90. [Google Scholar] [CrossRef]
- Ellis, E.A.; Romero Montero, J.A.; Hernández Gómez, I.U. Deforestation Processes in the State of Quintana Roo, Mexico. Trop. Conserv. Sci. 2017, 10. [Google Scholar] [CrossRef]
- Ellis, E.A.; Kainer, K.A.; Sierra-Huelsz, J.A.; Negreros-Castillo, P.; Rodriguez-Ward, D.; DiGiano, M. Endurance and Adaptation of Community Forest Management in Quintana Roo, Mexico. Forests 2015, 6, 4295–4327. [Google Scholar] [CrossRef]
- Porter-Bolland, L.; Ellis, E.A.; Guariguata, M.R.; Ruiz-Mallén, I.; Negrete-Yankelevich, S.; Reyes-García, V. Community managed forests and forest protected areas: An assessment of their conservation effectiveness across the tropics. For. Ecol. Manag. 2012, 268, 6–17. [Google Scholar] [CrossRef]
- Olguín-Álvarez, M.; Wayson, C.; Fellows, M.; Birdsey, R.; Smyth, C.; Magnan, M.; Dugan, A.; Mascorro, V.; Alanis, A.; Serrano, E.; et al. Applying a systems approach to assess carbon emission reductions from climate change mitigation in Mexico’s forest sector. Environ. Res. Lett. Under review.
- López Pérez, L.; Keenan, R. Ecosystem Services and Community Forest Management: The Case of Quintana Roo. Master’s Thesis, Master of Forest Ecosystem Science, University of Melbourne, Melbourne, Australia, 2015. [Google Scholar]
- Galletti, H.A. La selva maya en Quintana Roo (1983–1996) trece años de conservación y desarrollo comunal. In La Selva Maya, Conservación y Desarrollo; Primack, R.B., Ed.; Siglo XXI Editores: Mexico City, Mexico, 1999; pp. 53–73. [Google Scholar]
- Kiernan, M.J.; Freese, C.H. Mexico’s Plan Piloto Forestal: The search for balance between socioeconomic and ecological sustainability. In Harvesting Wild Species: Implications for Biodiversity Conservation; Freese, C.H., Ed.; The John Hopkins University Press: Baltimore, MD, USA, 1997; pp. 93–131. [Google Scholar]
- Godas, M.D.; Valenzuela, E.; Cisneros, H.; de los Ángeles Mejía, J.; Terrón, I. Sustentabilidad y Ecoturismo en Tres Garantías, Quintana Roo; Universidad Nacional Autónoma de México: Ciudad de México, México, 2005; ISBN 978-9-70-321838-7. [Google Scholar]
- Flachsenberg, H.; Galletti, H. El manejo forestal de la selva en Quintana Roo, México. In La Selva Maya, Conservación y Desarrollo; Primack, R.B., Bray, D., Galletti, H.A., Ponciano, I., Eds.; Siglo XXI Editores: Mexico City, Mexico, 1999; pp. 74–97. [Google Scholar]
- Rzedowski, J. Vegetación de México; 1a Edición Digital; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, México, 2006.
- Arreola, J.A.; Be Cituk, E.; Argüelles, L.F.; García, Z.H.M.; García, F.; Palafox, C.; Sánchez, E.F. Plan de Manejo, Ejido Felipe Carrillo Puerto, Quintana Roo 2012–2023; Ejido Felipe Carrillo Puerto, CONAFOR-PROCYMAF-II, PNUD-COMPACT, Maya Natura Tropical AC, Tropica Rural Latinoamericana AC; U’Yo’ol Che AC: Felipe Carrillo Puerto, Quintana Roo, Mexico, 2011; p. 146.
- Snook, L.K.; Negreros-Castillo, P. Regenerating mahogany (Swietenia macrophylla King) on clearings in Mexico’s Maya forest: The effects of clearing method and cleaning on seedling survival and growth. For. Ecol. Manag. 2004, 189, 143–160. [Google Scholar] [CrossRef]
- Racelis, A.E.; Barsimantov, J.A. The management of small diameter, lesser-known hardwood species as polewood in forest communities of central Quintana Roo, Mexico. J. Sustain. For. 2008, 27, 122–144. [Google Scholar] [CrossRef]
- Forster, R.; Albrecht, H.; Belisle, M.; Caballero, A.; Galletti, H.; Lacayo, O.; Ortiz, S.; Robinson, D. Forest Communities and the Marketing of Lesser-Used Tropical Hardwoods in Mesoamerica; Editorial Ducere: Mexico City, Mexico, 2003; ISBN 968-7864-47-8. [Google Scholar]
- Vovides, A.P.; Luna, V.; Medina, G. Relación de algunas plantas y hongos mexicanos raros, amenazados o en peligro de extinción y sugerencias para su conservación. Acta Bot. Mex. 1997, 1–42. [Google Scholar] [CrossRef]
- Wehenkel, C.; Cruz-Cobos, F.; Carrillo, A.; Lujan-Soto, J.E. Estimating bark volumes for 16 native tree species on the Sierra Madre Occidental, Mexico. Scand. J. For. Res. 2012, 27, 578–585. [Google Scholar] [CrossRef]
- Meyer, H.A. Bark volume determination in trees. J. For. 1946, 44, 1067–1070. [Google Scholar]
- Cubbage, F.W.; Davis, R.R.; Paredes, D.R.; Mollenhauer, R.; Elsin, Y.K.; Frey, G.E.; Hernández, I.A.G.; Hurtado, H.A.; Cruz, A.M.S.; Salas, D.N.C. Community Forestry Enterprises in Mexico: Sustainability and Competitiveness. J. Sustain. For. 2015, 34, 623–650. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Anuario Estadístico de la Producción Forestal 2013; SEMARNAT: Mexico City, Mexico, 2014.
- Sánchez-Sánchez, O.; Islebe, G.A. Tropical forest communities in southeastern Mexico. Plant Ecol. 2002, 158, 183–200. [Google Scholar] [CrossRef]
- Márdero, S.; Nickl, E.; Schmook, B.; Schneider, L.; Rogan, J.; Christman, Z.; Lawrence, D. Sequías en el sur de la península de Yucatán: Análisis de la variabilidad anual y estacional de la precipitación. Investig. Geogr. 2012, 78, 19–33. [Google Scholar] [CrossRef]
- Navarro-Martínez, A.; Durán-García, R.; Méndez-González, M. El impacto del huracán Dean sobre la estructura y composición arbórea de un bosque manejado en Quintana Roo, México. Madera Bosques 2012, 18, 57–76. [Google Scholar] [CrossRef]
- Mascorro, V.S.; Coops, N.C.; Kurz, W.A.; Olguín, M. Attributing changes in land cover using independent disturbance datasets: A case study of the Yucatan Peninsula, Mexico. Reg. Environ. Chang. 2016, 16, 213–228. [Google Scholar] [CrossRef]
- Ford, A.; Nigh, R. Origins of the Maya Forest Garden: Maya Resource Management. J. Ethnobiol. 2009, 29, 213–236. [Google Scholar] [CrossRef]
- Chatters, J.C.; Kennett, D.J.; Asmerom, Y.; Kemp, B.M.; Polyak, V.; Blank, A.N.; Beddows, P.A.; Reinhardt, E.; Arroyo-Cabrales, J.; Bolnick, D.A.; et al. Late Pleistocene Human Skeleton and mtDNA Link Paleoamericans and Modern Native Americans. Science 2014, 344, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Pennington, T.; Sarukhán, J. Árboles Tropicales de México. Manual para la Identificación de las Principales Especies; Universidad Nacional Autónoma de México, Fondo de Cultura Económica: Ciudad de México, México, 2005. [Google Scholar]
- Vozzo, J.A. Tropical Tree Seed Manual; Agriculture Handbook 721; USDA Forest Service: Washington, DC, USA, 2002.
- Missouri Botanical Garden Tropicos. Available online: http://www.tropicos.org/ (accessed on 12 November 2016).
- Huet, S.; Bouvier, A.; Poursat, M.-A.; Jolivet, E. Statistical Tools for Nonlinear Regression: A Practical Guide with S-PLUS and R Examples; Springer: New York, NY, USA, 2006. [Google Scholar]
- Husch, B.; Beers, T.W.; Kershaw, J.A. Forest Mensuration; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 978-0-47-101850-6. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing v. 3.3.1: Vienna, Austria, 2016. [Google Scholar]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- Greenwell, B.M. Schubert Kabban investr: An R package for inverse estimation. R J. 2014, 6, 90–100. [Google Scholar]
- Perez, D.; Kanninen, M.; Matamoros, F.; Fonseca, W.; Chaves, E. Heartwood, sapwood and bark contents of Bombacopsis quinata in Costa Rica. J. Trop. For. Sci. 2004, 318–327. [Google Scholar]
- Ogunwusi, A.A. Heartwood, Sapwood and Bark Proportions in Five Lesser Used Tropical Hardwood Species Growing in Nigeria. J. Biol. Agric. Healthc. 2013, 3, 80–83. [Google Scholar]
- Su, Y.; Fu, Y.; Liao, K.; Wei, A.; Xu, T.; Feng, Y.; Deng, H. Bark and Heartwood Percentage and Density of Paramichelia baillonii. J. Northwest For. Univ. 2012, 2, 51. [Google Scholar]
- Stayton, C.L.; Hoffman, M. Estimating Sugar Maple Bark Thickness and Volume; Research Paper NC-38; U.S. Deptartment of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1970; Volume 8.
- Kozak, A.; Yang, R.C. Equations for estimating bark volume and thickness of commercial trees in British Columbia. For. Chron. 1981, 57, 112–115. [Google Scholar] [CrossRef]
- Rosell, J.A. Bark thickness across the angiosperms: More than just fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Laughlin, D.C.; Lawes, M.J.; Holdaway, R.J.; Wilmshurst, J.M.; Wright, M.; Curran, T.J.; Bellingham, P.J.; McGlone, M.S. Functional and environmental determinants of bark thickness in fire-free temperate rain forest communities. Am. J. Bot. 2015, 102, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.F.; Navarro, M.A. Fichas Ecologicas: Arboles Maderables de Quintana Roo; Fondo Mixto de Fomento a la Investigación Científica y Tecnológica CONACYT-Gobierno del Estado de Quintana Roo: Chetumal, Quintana Roo, México, 2007.
- Campos, B.; Simei, M.; Jose Jimenez-Osornio, J.; Barrientos, M. Dasometric analysis of siricote (Cordia dodecandra A. DC.) plantations under three types of management at Xmatkuil Yucatan. Madera Bosques 2015, 21, 47–54. [Google Scholar]
- Sánchez, G.; Arreola, J.A.; López, D.; Maldonado, V.; Olguín, M.; Wayson, C.; Carrillo, O.; Puc, R. Sitio de Monitoreo Intensivo del Carbono en Quintana Roo. Available online: pmcarbono.org/pmc/descargas/proyectos/Documentos_Red_Mex-SMIC/Estudio_de_caso_SMIC_Quintana_Roo.pdf (accessed on 10 September 2017).
- Mize, C.; Negreros-Castillo, P. Stand and species growth of a tropical forest in Quintana Roo, Mexico. J. Sustain. For. 2007, 23, 83–95. [Google Scholar] [CrossRef]
- Atha, D.E.; Romero, L.; Forrest, T. Bark volume determination of Bursera simaruba in Belize. Caribb. J. Sci. 2005, 41, 843–848. [Google Scholar]
- Bonyad, A.E.; Sima, A.; Bakhshandeh, A.; Dadras, H. Evaluation of non-destructive Meyer method for determination of bark volume of beech (Fagus orientalis Lipsky) in different geographical aspects. Casp. J. Environ. Sci. 2012, 10, 67–73. [Google Scholar]
- Marshall, H.D.; Murphy, G.E.; Lachenbruch, B. Effects of bark thickness estimates on optimal log merchandising. For. Prod. J. 2006, 56, 87–92. [Google Scholar]
- Comisión Nacional Forestal (CONAFOR). Sistema Nacional de Información Forestal; CONAFOR: Jalisco, Méico, 2016.
- Bautista-Hernández, J.; Torres-Pérez, J. Valoración económica del almacenamiento de carbono del bosque tropical del ejido Noh Bec, Quintana Roo, México. Rev. Chapingo Ser. Cienc. For. Ambiente 2003, 9, 69–75. [Google Scholar]
- Bray, D.B.; Ellis, E.A.; Armijo-Canto, N.; Beck, C.T. The institutional drivers of sustainable landscapes: A case study of the “Mayan Zone”in Quintana Roo, Mexico. Land Use Policy 2004, 21, 333–346. [Google Scholar] [CrossRef]
- Givnish, T.J.; Wong, S.C.; Stuart-Williams, H.; Holloway-Phillips, M.; Farquhar, G.D. Determinants of maximum tree height in Eucalyptus species along a rainfall gradient in Victoria, Australia. Ecology 2014, 95, 2991–3007. [Google Scholar] [CrossRef]
- Negreros-Castillo, P.; Cámara-Cabrales, L.; Devall, M.; Fajvan, M.A.; Mendoza Briseño, M.A.; Mize, C.W.; Navarro-Martínez, A. Silvicultura de las Selvas de Caoba en Quintana Roo, México. Criterios y Recomendaciones; CFAN, CONAFOR: Felipe Carrillo Puerto, México, 2014.
- Secretaría de Medio Ambiente y Recursos Naturales & Instituto Nacional de Ecología y Cambio Climático (SEMARNAT-INECC). Mexico’s Climate Change Mid-Century Strategy; S SEMARNAT-INECC: Mexico City, Mexico, 2016.
- Tolentino, O.; García-Frapolli, E.; Porter-Bolland, L.; Ruíz-Mallén, I.; Reyes-Garcia, V.; Sánchez-Gonzalez, M.-C.; López-Méndez, M.-E. Triggering community conservation through the trade of carbon offsets: The case of the ejido Felipe Carrillo Puerto, Mexico. J. Environ. Dev. 2015, 24, 187–210. [Google Scholar] [CrossRef]
- Sánchez, G.; Arreola, J.A.; López, D.; Maldonado, C.V.; Olguín, M.; Wayson, O.; Carrillo, O.; Puc, R. Reporte Técnico para el Proyecto Fortalecimiento REDD+ y Cooperación Sur-Sur; CONAFOR: Felipe Carrillo Puerto, Quintana Roo, México, 2016; p. 133.

| Species | Common Name | Family | Distribution | Size 1 |
|---|---|---|---|---|
| Cordia dodecandra A.DC. | Siricote | Boraginaceae | Mexico (Yucatan Peninsula, Chiapas, Veracruz), Guatemala, Belize, Honduras, Cuba 2 | H: up to 30 m, DBH: 70 cm |
| Lysiloma latisiliquum (L.) Benth. | Tzalam | Fabaceae | Mexico (Yucatan Peninsula, Chiapas, Tabasco), Florida, Bahamas, Caribbean, Belize, Guatemala 3 | H: up to 20 m, DBH: 70 cm |
| Manilkara zapota (L.) P. Royen | Chicozapote | Sapotaceae | Mexico (Yucatan Peninsula, Gulf of Mexico, Chiapas, Oaxaca), Central America to Costa Rica 2 | H: up to 40 m, DBH: 150 cm |
| Metopium brownei (Jacq.) Urb. | Chechén | Anacardiaceae | Mexico (Yucatan Peninsula, Gulf of Mexico, Chiapas), Caribbean, Belize, Guatemala, Honduras 3 | H: up to 25 m, DBH: 60 cm |
| Swartzia cubensis (Britton and Wilson) Standl. | Katalox | Fabaceae | Mexico (Yucatan Peninsula, Chiapas, Tabasco), Central America to Costa Rica, Caribbean 3 | H: 40 m, DBH: 150 cm |
| Swietenia macrophylla King | Caoba, big-leaf mahogany | Meliaceae | Mexico (Yucatan Peninsula, Gulf of Mexico, Chiapas), Central America to northern South America 2 | H: up to 70 m, DBH: up to 350 cm |
| Species | n | Db (cm) 1 | Log Length (m) 1 | Bark Thickness (mm) 1 |
|---|---|---|---|---|
| Cordia dodecandra | 16 | 39.7 ± 1.67 | 7.5 ± 0.51 | 8.0 ± 0.29 |
| Lysiloma latisiliquum | 20 | 49.3 ± 2.07 | 5.5 ± 0.43 | 14.2 ± 0.58 |
| Manilkara zapota | 20 | 41.5 ± 0.86 | 6.8 ± 0.22 | 18.7 ± 0.45 |
| Metopium brownei | 20 | 45.8 ± 1.38 | 7.3 ± 0.43 | 8.9 ± 0.41 |
| Swartzia cubensis | 20 | 44.4 ± 1.43 | 6.6 ± 0.42 | 9.0 ± 0.49 |
| Swietenia macrophylla | 20 | 57.6 ± 1.64 | 9.9 ± 0.50 | 19.0 ± 0.72 |
| Species | β3 (95% C.I.) | Rpred-obs | RMSE |
|---|---|---|---|
| Cordia dodecandra | 0.295 (0.282, 0.307) *** | 0.999 | 0.0109 |
| Lysiloma latisiliquum | 0.337 (0.323, 0.351) *** | 0.998 | 0.0187 |
| Manilkara zapota | 0.427 (0.417, 0.437) *** | 0.995 | 0.0148 |
| Metopium brownei | 0.285 (0.268, 0.303) *** | 0.996 | 0.0227 |
| Swartzia cubensis | 0.280 (0.263, 0.298) *** | 0.999 | 0.0212 |
| Swietenia macrophylla | 0.370 (0.353, 0.387) *** | 0.998 | 0.0583 |
| Species | F (95% C.I.) | Rpred-obs | RMSE | PBV (95% C.I.) |
|---|---|---|---|---|
| Cordia dodecandra | 0.9556 (0.9517, 0.9594) *** | 0.891 | 0.0109 | 0.0870 (0.0796, 0.0943) |
| Lysiloma latisiliquum | 0.9416 (0.9366, 0.9465) *** | 0.822 | 0.0187 | 0.1134 (0.1041, 0.1228) |
| Manilkara zapota | 0.9041 (0.8995, 0.9088) *** | 0.908 | 0.0148 | 0.1826 (0.1741, 0.1909) |
| Metopium brownei | 0.9584 (0.9532, 0.9637) *** | 0.638 | 0.0227 | 0.0815 (0.0713, 0.0914) |
| Swartzia cubensis | 0.9599 (0.9549, 0.9650) *** | 0.789 | 0.0212 | 0.0786 (0.0688, 0.0882) |
| Swietenia macrophylla | 0.9289 (0.9222, 0.9357) *** | 0.859 | 0.0583 | 0.1372 (0.1245, 0.1496) |
| Species | Updated PBV | Current PBV | WV Difference (%) | Logged Volume 2010–2016 (m3) | Mean Total Volume per Log (m3) | Number of Felled Trees | ||
|---|---|---|---|---|---|---|---|---|
| Updated PBV | Current PBV | Difference | ||||||
| Cordia dodecandra | 0.0870 | 0.1000 | −1.42 | 33.124 | 0.7662 | 47.3 | 48.0 | −0.7 |
| Lysiloma latisiliquum | 0.1134 | 0.1000 | 1.51 | 883.429 | 0.9171 | 1086.5 | 1070.3 | +16.2 |
| Manilkara zapota | 0.1826 | 0.1000 | 10.11 | 1597.742 | 0.8284 | 2359.6 | 2143.0 | +216.6 |
| Metopium brownei | 0.0815 | 0.1000 | −2.01 | 1465.826 | 1.0546 | 1513.3 | 1544.4 | −31.1 |
| Swartzia cubensis | 0.0786 | 0.1000 | −2.32 | 68.481 | 0.9582 | 77.6 | 79.4 | −1.8 |
| Swietenia macrophylla | 0.1372 | 0.1400 | −0.33 | 443.604 | 2.0702 | 248.4 | 249.2 | −0.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosell, J.A.; Wehenkel, C.; Pérez-Martínez, A.; Arreola Palacios, J.A.; García-Jácome, S.P.; Olguín, M. Updating Bark Proportions for the Estimation of Tropical Timber Volumes by Indigenous Community-Based Forest Enterprises in Quintana Roo, Mexico. Forests 2017, 8, 338. https://doi.org/10.3390/f8090338
Rosell JA, Wehenkel C, Pérez-Martínez A, Arreola Palacios JA, García-Jácome SP, Olguín M. Updating Bark Proportions for the Estimation of Tropical Timber Volumes by Indigenous Community-Based Forest Enterprises in Quintana Roo, Mexico. Forests. 2017; 8(9):338. https://doi.org/10.3390/f8090338
Chicago/Turabian StyleRosell, Julieta A., Christian Wehenkel, Abraham Pérez-Martínez, José Antonio Arreola Palacios, Sandra P. García-Jácome, and Marcela Olguín. 2017. "Updating Bark Proportions for the Estimation of Tropical Timber Volumes by Indigenous Community-Based Forest Enterprises in Quintana Roo, Mexico" Forests 8, no. 9: 338. https://doi.org/10.3390/f8090338





