Ash Dieback on Sample Points of the National Forest Inventory in South-Western Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inventory Plots
2.2. Data Collection
2.3. Data Analyses
3. Results
3.1. Change in the Number of Ash Trees over Time
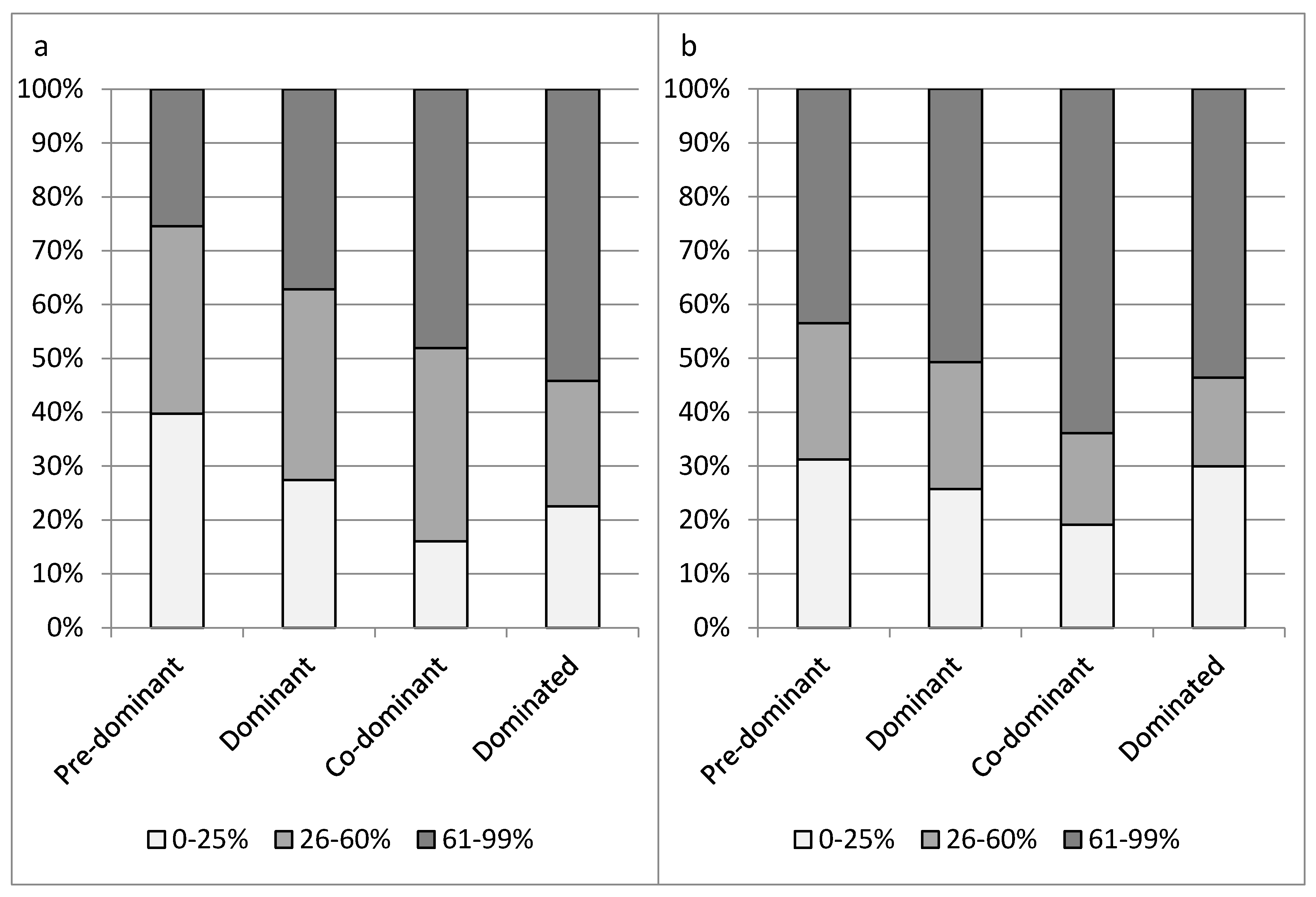
3.2. Proportions of Trees in Different Health Conditions
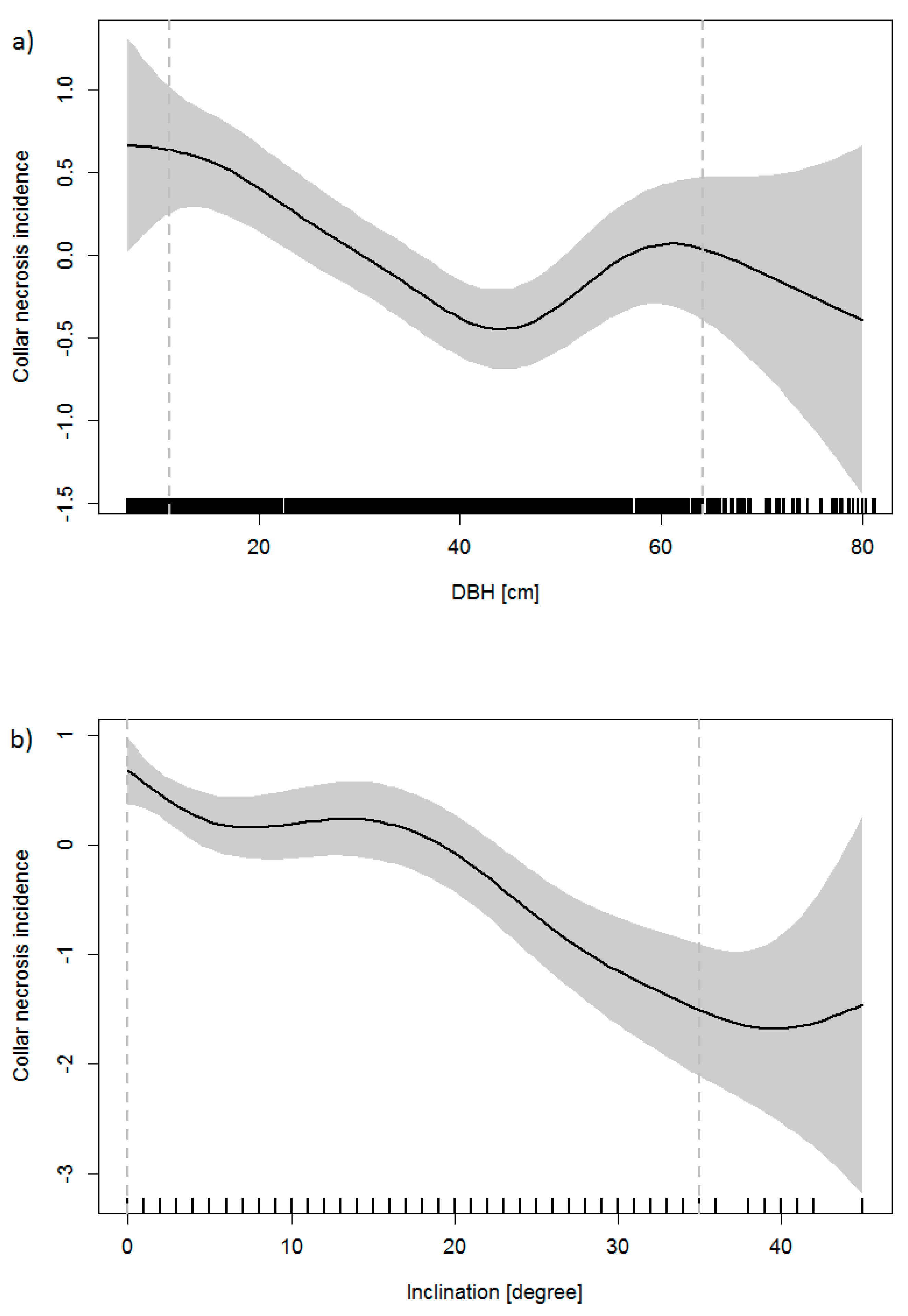
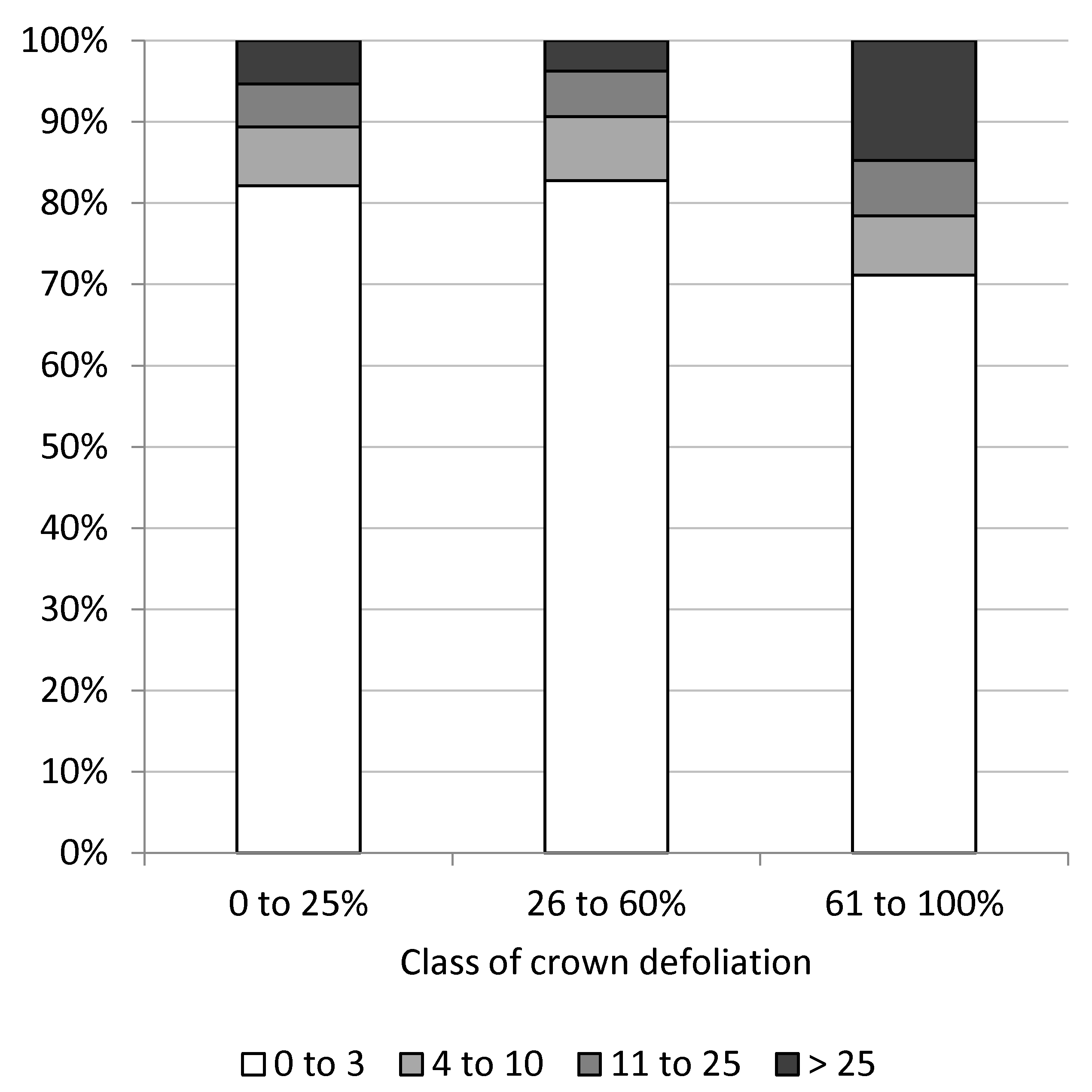
3.3. Factors That May Influence Symptom Prevalence or Severity
3.3.1. Severity of Crown Symptoms
3.3.2. Prevalence of Collar Necroses
3.4. Tree Growth
3.5. Epicormic Shoots at Trunks
3.6. Regeneration (DBH (Diameter at Breast Height) < 7 cm)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Drenkhan, R.; Sander, H.; Hanso, M. Introduction of Mandshurian ash (Fraxinus mandshurica Rupr.) to Estonia: Is it related to the current epidemic on European ash (F. excelsior L.)? Eur. J. For. Res. 2014, 133, 769–781. [Google Scholar] [CrossRef]
- Timmermann, V.; Børja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash dieback: Pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway. EPPO Bull. 2011, 41, 14–20. [Google Scholar] [CrossRef]
- Dal Maso, E. Risk of natural spread of Hymenoscyphus fraxineus with environmental niche modelling and ensemble forecasting technique. For. Res 2014, 3. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Wilhelm, G.J.; Thomsen, I.M.; Metzler, B.; Kirisits, T.; Havrdová, L.; Enderle, R.; Dobrowolska, D.; Cleary, M.; Clark, J. Silvicultural strategies for Fraxinus excelsior in response to dieback caused by Hymenoscyphus fraxineus. Forestry 2017, 90, 455–472. [Google Scholar] [CrossRef]
- Hemery, G.E. Forest management and silvicultural responses to projected climate change impacts on European broadleaved trees and forests. Int. For. Rev. 2008, 10, 591–607. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A review of European ash (Fraxinus excelsior L.): Implications for silviculture. Forestry 2011, 84, 133–148. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback—A conservation biology challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Beaton, J.K.; Bellamy, P.E.; Broome, A.; Chetcuti, J.; Eaton, S.; Ellis, C.J.; Gimona, A.; Harmer, R.; Hester, A.J.; et al. Ash dieback in the UK: A review of the ecological and conservation implications and potential management options. Biol. Conserv. 2014, 175, 95–109. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hewison, R.L.; Hester, A.J.; Broome, A.; Kirby, K.J. Potential impacts of the loss of Fraxinus excelsior (Oleaceae) due to ash dieback on woodland vegetation in Great Britain. New J. Bot. 2016, 6, 2–15. [Google Scholar] [CrossRef]
- Cleary, M.R.; Daniel, G.; Stenlid, J. Light and scanning electron microscopy studies of the early infection stages of Hymenoscyphus pseudoalbidus on Fraxinus excelsior. Plant Pathol. 2013, 62, 1294–1301. [Google Scholar] [CrossRef]
- Schumacher, J. The general situation regarding ash dieback in Germany and investigations concerning the invasion and distribution strategies of Chalara fraxinea in woody tissue. EPPO Bull. 2011, 41, 7–10. [Google Scholar] [CrossRef]
- Haňáčková, Z.; Koukol, O.; Čmoková, A.; Zahradník, D.; Havrdová, L.; Cleary, M. Direct evidence of Hymenoscyphus fraxineus infection pathway through the petiole-shoot junction. For. Path. 2017, 7, e12370. [Google Scholar] [CrossRef]
- Enderle, R.; Nakou, A.; Thomas, K.; Metzler, B. Susceptibility of autochthonous German Fraxinus excelsior clones to Hymenoscyphus pseudoalbidus is genetically determined. Ann. For. Sci. 2015, 72, 183–193. [Google Scholar] [CrossRef]
- Husson, C.; Caël, O.; Grandjean, J.P.; Nageleisen, L.M.; Marçais, B. Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol. 2012, 61, 889–895. [Google Scholar] [CrossRef]
- Enderle, R.; Peters, F.; Nakou, A.; Metzler, B. Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior. Eur. J. For. Res. 2013, 132, 865–876. [Google Scholar] [CrossRef]
- Chandelier, A.; Gerarts, F.; San Martin, G.; Herman, M.; Delahaye, L.; Hantula, J. Temporal evolution of collar lesions associated with ash dieback and the occurrence of Armillaria in Belgian forests. For. Path. 2016, 46, 289–297. [Google Scholar] [CrossRef]
- Muñoz, F.; Marçais, B.; Dufour, J.; Dowkiw, A. Rising out of the ashes: Additive genetic variation for crown and collar resistance to Hymenoscyphus fraxineus in Fraxinus excelsior. Phytopathology 2016, 106, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Marçais, B.; Husson, C.; Godart, L.; Caël, O. Influence of site and stand factors on Hymenoscyphus fraxineus -induced basal lesions. Plant Pathol. 2016, 65, 1452–1461. [Google Scholar] [CrossRef]
- Langer, G. Collar rots in forests of Northwest Germany affected by ash dieback. Balt. For. 2017, 23, 4–19. [Google Scholar]
- Enderle, R.; Sander, F.; Metzler, B. Temporal development of collar necroses and butt rot in association with ash dieback. iForest 2017, 10, 529–536. [Google Scholar] [CrossRef]
- Metzler, B.; Baumann, M.; Baier, U.; Heydeck, P.; Bressem, U.; Lenz, H.D. Bundesweite Zusammenstellung: Handlungsempfehlungen beim Eschentriebsterben. AFZ-Der Wald 2013, 68, 17–20. [Google Scholar]
- McKinney, L.V.; Nielsen, L.R.; Collinge, D.B.; Thomsen, I.M.; Hansen, J.K.; Kjær, E.D. The ash dieback crisis: Genetic variation in resistance can prove a long-term solution. Plant Pathol. 2014, 63, 485–499. [Google Scholar] [CrossRef]
- Havrdová, L.; Zahradník, D.; Romportl, D.; Pešková, V.; Černý, K. Environmental and silvicultural characteristics influencing the extent of ash dieback in forest stands. Balt. For. 2017, 23, 168–182. [Google Scholar]
- Enderle, R.; Fussi, B.; Lenz, H.D.; Langer, G.; Nagel, R.; Metzler, B. Ash dieback in Germany: Research on disease development, resistance and management options. In Dieback of European Ash (Fraxinus spp.)—Consequences and Guidelines for Sustainable Management; Vasaitis, R., Enderle, R., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017; pp. 89–105. [Google Scholar]
- Enderle, R.; Kändler, K.; Metzler, B. Eschentriebsterben. In Waldzustandsbericht 2015 für Baden-Württemberg; Forstliche Versuchs- und Forschungsantstalt Baden-Württemberg, Ed.; Forstliche Versuchs- und Forschungsantstalt Baden-Württemberg: Freiburg, Germany, 2015; pp. 46–54. [Google Scholar]
- Kändler, G. The design of the second German national forest inventory. In Proceedings of the Eighth Annual Forest Inventory and Analysis Symposium, Monterey, CA, USA, 16–19 October 2006; McRoberts, R.E., Reams, G.A., van Deusen, P.C., McWilliams, W.H., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2008; pp. 19–24. [Google Scholar]
- Kraft, G. Beiträge zur Lehre von den Durchforstungen, Schlagstellungen und Lichtungshieben; Klindworth: Hannover, Germany, 1884. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Cochran, W.G. Sampling Techniques, 3rd ed.; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Hauptman, T.; Piškur, B.; Groot, M.; de Ogris, N.; Ferlan, M.; Jurc, D. Temperature effect on Chalara fraxinea: Heat treatment of saplings as a possible disease control method. For. Path. 2013, 58, 360–370. [Google Scholar] [CrossRef]
- Nothdurft, A.; Wolf, T.; Ringeler, A.; Böhner, J.; Saborowski, J. Spatio-temporal prediction of site index based on forest inventories and climate change scenarios. For. Ecol. Manag. 2012, 279, 97–111. [Google Scholar] [CrossRef]
- Dietrich, H.; Wolf, T.; Kawohl, T.; Wehberg, J.; Kändler, G.; Mette, T.; Röder, A.; Böhner, J. Temporal and spatial high-resolution climate data from 1961–2100 for the German National Forest Inventory (NFI). Ann. For. Sci. Under review.
- Wood, S. Generalized Additive Models: An Introduction with R; CRC Press: Hoboken, NY, USA, 2006. [Google Scholar]
- Pušpure, I.; Matisons, R.; Laiviņš, M.; Gaitnieks, T.; Jansons, J. Natural regeneration of common ash in young stands in Latvia. Balt. For. 2017, 23, 209–217. [Google Scholar]
- Enderle, R.; Bußkamp, J.; Metzler, B. Growth performance of dense natural regeneration of Fraxinus excelsior under attack of the ash dieback agent Hymenoscyphus fraxineus. Balt. For. 2017, 23, 218–228. [Google Scholar]
- Giongo, S.; Oliveira Longa, C.M.; Dal Maso, E.; Montecchio, L.; Maresi, G. Evaluating the impact of Hymenoscyphus fraxineus in Trentino (Alps, Northern Italy): First investigations. iForest 2017, 10, 871–878. [Google Scholar] [CrossRef]



| Symptom Severity | Number of Trees (%) | Occupying Area (%) | Volume (%) |
|---|---|---|---|
| Crown defoliation | |||
| 0–25% | 26.6 ± 2.7 | 21.6 ± 1.7 | 17.2 ± 1.3 |
| 26–60% | 34.0 ± 2.5 | 42.4 ± 1.6 | 44.3 ± 1.6 |
| 61–99% | 39.3 ± 3.0 | 36.0 ± 2.0 | 38.6 ± 2.0 |
| Portion of epicormic shoots | |||
| 0–25% | 25.2 ± 3.1 | 16.3 ± 1.7 | 12.9 ± 1.4 |
| 26–60% | 20.6 ± 1.9 | 23.9 ± 1.5 | 24.1 ± 1.6 |
| 61–99% | 54.1 ± 3.5 | 59.8 ± 2.3 | 63.0 ± 2.3 |
| Collar necrosis prevalence | |||
| Necrosis present | 23.6 ± 3.1 | 21.6 ± 1.9 | 17.1 ± 1.5 |
| Necrosis absent | 66.1 ± 3.3 | 70.0 ± 2.2 | 73.5 ± 2.1 |
| Unreliable | 5.1 ± 1.3 | 6.2 ± 0.9 | 7.2 ± 1.2 |
| Not available | 5.1 ± 1.4 | 2.2 ± 0.6 | 2.2 ± 0.8 |
| In sound condition 1 | 13.8 ± 2.1 | 8.6 ± 1.2 | 6.8 ± 1.0 |
| NFI 2012 | Loss | Inventory 2015 | |||||
|---|---|---|---|---|---|---|---|
| Tree Size | Total | 2012–2015 | Total | Healthy | <50% of Crown | >50% of Crown | Dead |
| 50–130 cm | 965.8 | 312.9 (32.4) | 652.9 | 306.9 (47.0) | 79.7 (12.2) | 94.8 (14.5) | 171.5 (26.3) |
| >130 cm and DBH < 7 cm | 579.2 | 182.0 (31.4) | 397.1 | 28.6 (7.2) | 73.7 (18.6) | 90.3 (22.7) | 204.6 (51.5) |
| Sum | 1544.9 | 494.9 (32.0) | 1050.0 | 335.5 (31.9) | 153.4 (14.6) | 185.0 (17.6) | 376.1 (35.8) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enderle, R.; Metzler, B.; Riemer, U.; Kändler, G. Ash Dieback on Sample Points of the National Forest Inventory in South-Western Germany. Forests 2018, 9, 25. https://doi.org/10.3390/f9010025
Enderle R, Metzler B, Riemer U, Kändler G. Ash Dieback on Sample Points of the National Forest Inventory in South-Western Germany. Forests. 2018; 9(1):25. https://doi.org/10.3390/f9010025
Chicago/Turabian StyleEnderle, Rasmus, Berthold Metzler, Uli Riemer, and Gerald Kändler. 2018. "Ash Dieback on Sample Points of the National Forest Inventory in South-Western Germany" Forests 9, no. 1: 25. https://doi.org/10.3390/f9010025




