Natural Regeneration Dynamics of Mora paraensis (Ducke) Ducke in Estuarine Floodplain Forests of the Amazon River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
(nN4 × 10,000/A4)
- DRI = Density of regenerating individuals (ind. ha−1);
- nN = Height level category (see above);
- A = area sampled in each level.
- RR = natural regeneration rate;
- A0 = absolute abundance at the start of the period;
- A1 = absolute abundance at the end of the period.
- ni = number of individuals that were recruited into the study;
- ns = number of individuals that left the study.
M = nm/A0
- R—recruitment rate expressed as a percentage;
- M—mortality rate expressed as a percentage;
- nm—number of individuals that died during the period.
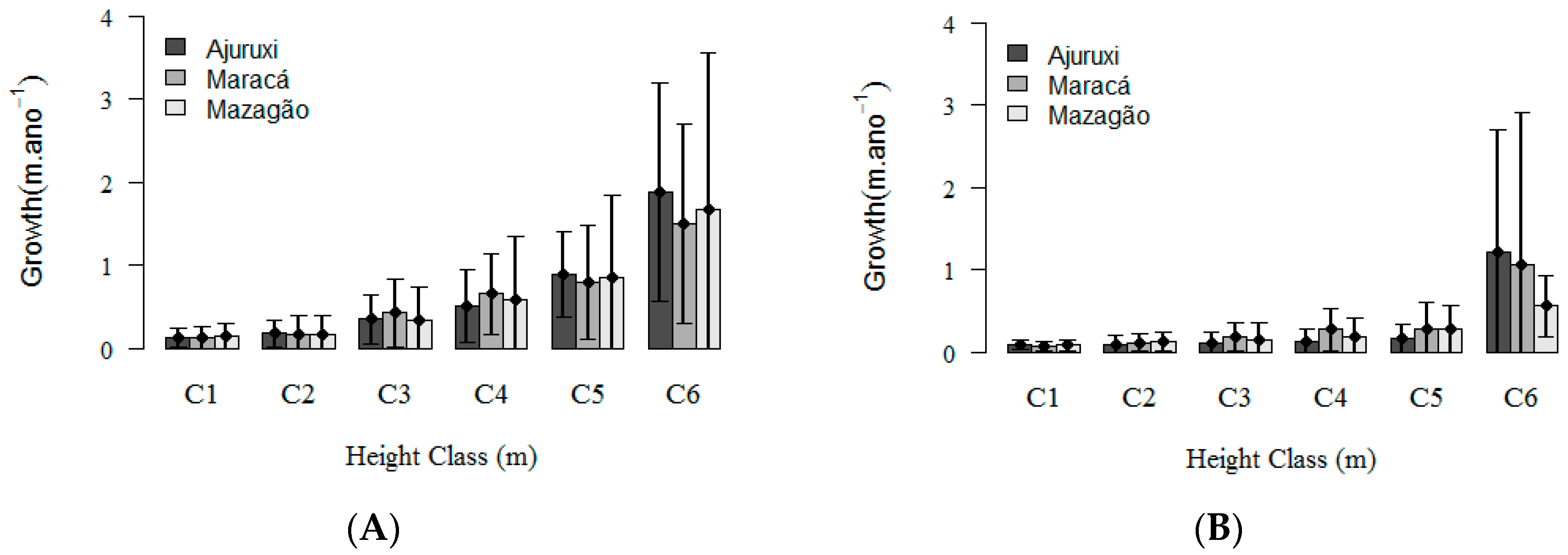
3. Results
3.1. Natural Regeneration Dynamics
3.2. Canopy Openness and Abundance of Regenerating Individuals
4. Discussion
4.1. Regeneration Dynamics of Mora paraensis
4.2. Relationship with Canopy Openness
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Santos, S.R.M.; Miranda, I.S.; Tourinho, M.M. Análise florística e estrutural de sistemas agroflorestais das várzeas do rio Juba, Cametá, Pará. Acta Amazon. 2004, 34, 251–263. [Google Scholar] [CrossRef]
- Almeida, S.S.; Amaral, D.D.; Silva, A.S.L. Análise florística e estrutura de florestas de várzea no estuário amazônico. Acta Amazon. 2004, 34, 513–524. [Google Scholar] [CrossRef]
- Rabelo, F.G.; Zarin, D.J.; Oliveira, F.A.; Jardim, F.C.S. Regeneração natural de florestas estuarinas na região do rio Amazonas-Amapá-Brasil. Rev. Cienc. Agrar. 2000, 2, 129–138. [Google Scholar]
- Carim, M.J.V.; Jardim, M.A.G.; Medeiros, T.D.S. Composição florística e estrutura de floresta de várzea no município de Mazagão, Estado do Pará, Brasil. Sci. For. 2008, 36, 191–201. [Google Scholar]
- Fortini, L.B.; Zarin, D.J. Population dynamics and management of Amazon tidal floodplain forests: Links to the past, present and future. For. Ecol. Manag. 2011, 261, 551–561. [Google Scholar] [CrossRef]
- Queiroz, J.A.L.; Mochiutti, S.; Machado, A.S.; Galvão, F. Composição florística e estrutura de floresta em várzea alta estuarina Amazônica. Rev. Floresta 2005, 35, 41–56. [Google Scholar] [CrossRef]
- Queiroz, J.A.L.; Machado, S.A. Potencial de Utilização Madeireira de Espécies Florestais de Várzea no Município de Mazagão no Estado do Amapá. Rev. Floresta 2007, 37, 293–302. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, E.; Piedade, M.T.F.; Junk, W.J. Habitat specificity, endemism and the neotropical distribution of Amazonian white-water floodplain trees. Ecography 2013, 36, 690–707. [Google Scholar] [CrossRef]
- Jardim, M.A.G.; Vieira, I.C.G. Composição florística e estrutura de uma floresta de várzea do estuário Amazônico, Ilha do Combu, Estado do Pará. Brazil. Bol. Mus. Para. Emílio Goeldi 2001, 17, 333–354. [Google Scholar]
- Rodrigues, L.M.B.; Lira, A.U.S.; Santos, F.A.; Jardim, M.A.G. Composição florística e usos das espécies vegetais de dois ambientes de floresta de várzea. Rev. Bras. Farm. 2006, 87, 45–48. [Google Scholar]
- Lima, R.B.; Aparício, P.S.; Ferreira, R.L.C.; Silva, W.C.; Guedes, M.C.; Oliveira, C.P.; Silva, D.A.S.; Batista, A.P.B. Volumetria e classificação da capacidade produtiva para Mora paraensis (Ducke) no estuário amapaense. Sci. For. 2014, 42, 141–154. [Google Scholar]
- Castilho, N.T.F. Manejo da Regeneração Natural de Pau-Mulato em Floresta de Várzea do Estuário Amazônico. Master’s Thesis, Universidade Federal do Amapá, Macapá, Brazil, 2013. [Google Scholar]
- Lieberman, S.S.; Dock, C.F. Analysis of the leaf litter arthropod fauna of a lowland tropical evergreen forest site (La Selva, Costa Rica). Rev. Biol. Trop. 1982, 30, 27–34. [Google Scholar]
- Hoffmann, J. Assessing the effects of environmental changes in a landscape by means of ecological characteristics of plant species. Landsc. Urban Plan. 1998, 41, 239–248. [Google Scholar] [CrossRef]
- Almeida, D.R.; Cogliatti-Carvalho, L.; Rocha, C.F.D. As bromeliáceas da Mata Atlântica da Ilha Grande, RJ: Composição e diversidade de espécies em três ambientes diferentes. Bromélia 1998, 5, 54–65. [Google Scholar]
- Clark, D.A.; Clark, D.B. Population ecology and microhabitat distribution of Dipteryx panamensis, a neotropical rain forest emergent tree. Biotropica 1987, 19, 236–244. [Google Scholar] [CrossRef]
- Costa, F.R.C.; Magnusson, W.E. Effects of selective logging on the diversity and abundance of flowering and fruiting understory plants on a Central Amazonian Forest. Biotropica 2003, 35, 103–114. [Google Scholar] [CrossRef]
- Viana, A.C.N.; Jardim, F.C.S. Natural regeneration dynamic of Couratari guianesis in a tropical forest selectively logged in Moju, state of Para, Brazil. Rev. Cienc. Agrar. 2013, 56, 112–119. [Google Scholar]
- Gama, J.R.V.; Botelho, S.A.; Bentes-Gama, M.M.; Scolforo, J.R.S. Estrutura e potencial futuro de utilização da regeneração natural de floresta de várzea alta no município de Afuá, Estado do Pará, Brasil. Cienc. Florest. 2003, 13, 71–82. [Google Scholar] [CrossRef]
- Hüller, A.; Rauber, A.; Wolski, M.S.; Almeida, N.L.; De Wolski, S.R.S. Regeneração natural do componente arbóreo e arbustivo do parque natural municipal de Santo Ângelo-RS. Rev. Soc. Bras. Arborização Urbana 2011, 6, 25–35. [Google Scholar]
- Klauberg, C.; Vidal, E.; Silva, C.A.; Hudak, A.T.; Oliveira, M.; Higuchi, P. Short-Term Effects of Reduced-Impact Logging on Copaifera spp. (Fabaceae) Regeneration in Eastern Amazon. Forests 2017, 8, 257. [Google Scholar] [CrossRef]
- Leal Filho, N.; Sena, J.S.; Santos, G.R. Variações espaço-temporais no estoque de sementes do solo na floresta amazônica. Acta Amazon. 2013, 43, 305–314. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T. Plant life in the floodplain with special reference to herbaceous plants. In The Central Amazon Floodplain; Junk, W.J., Ed.; Springer: New York, NY, USA, 1997; pp. 147–185. [Google Scholar]
- Wittmann, F.; Junk, W. Sapling communities in Amazonian white-water forests. J. Biogeogr. 2003, 30, 1533–1544. [Google Scholar] [CrossRef]
- Cunha, A.C.; Mustin, K.; Santos, E.S.; Santos, E.W.G.; Guedes, M.C.; Cunha, H.F.A.; Rosman, P.C.C.; Sternberg, L.S.L. Hydrodynamics and seed dispersal in the lower Amazon. Freshw. Biol. 2017, 62, 1721–1729. [Google Scholar] [CrossRef]
- Salomão, P.; Terezo, E.F.M.; Jardim, M.A.G. Manejo Florestal nas Várzeas: Oportunidades e Desafios; MPEG: Belém, Portugal, 2007; 210p. [Google Scholar]
- Ward, N.D.; Keil, R.G.; Medeiros, P.M.; Brito, D.C.; Cunha, A.C.; Dittmar, T.; Richey, J.E. Degradation of terrestrially derived macromolecules in the Amazon River. Nat. Geosci. 2013, 6, 530–533. [Google Scholar] [CrossRef]
- Pinto, E.R. Solos, Hidrologia e Estrutura Populacional de Pracuubeiras em Florestas de Várzea do Estuário Amazônico. Master’s Thesis, Universidade Federal do Amapá, Macapá, Brazil, 2014. [Google Scholar]
- Instituto Nacional de Meteorologia. Ministério da Agricultura, Pecuária e Abastecimento. 2015. Available online: http://www.inmet.gov.br (accessed on 14 November 2017).
- Fortini, L.B.; Carter, D.R. The economic viability of smallholder timber production under expanding acai palm production in the Amazon Estuary. J. For. Econ. 2014, 20, 223–235. [Google Scholar] [CrossRef]
- Pinedo-Vasquez, M. Changing in soil formation and vegetation on silt bars and backslopes of levees following intensive production of rice and jute. In Várzea: Diversity, Development, and Conservation of Amazonias Whitewater Floodplains; Padoch, C., Ayres, J., Pinedo-Vasquez, M., Henderson, A., Eds.; The New York Botanical Garden Press: Bronx, NY, USA, 1999; pp. 301–311. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Manual Técnico da Vegetação Brasileira; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012; 275p.
- Rich, P.M. Characterizing Plant Canopies with Hemispherical Photographs. Remote Sens. Rev. 1990, 5, 13–29. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer. Imaging Software to Extract Canopy Structure and Gal Light Transmission Indices from True-colour Fisheye Photographs, User’s Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada; The Institute of Ecosystem Studies: Millbrook, AL, USA, 1999. [Google Scholar]
- Volpato, M.M.L. Regeneração Natural em Uma Floresta Secundária no Domínio de Mata Atlântica: Uma Análise Fitossociológica. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1994. [Google Scholar]
- Mory, A.M.; Jardim, F.C.S. Comportamento de Eschweilera odora (Poepp.) Miers. (Matamatá-amarelo) em diferentes níveis de desbaste por anelamento. Rev. Cienc. Agrar. 2001, 36, 29–53. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 1.0.143 R; Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 10 October 2017).
- De Mendiburu, F. Package Agricolae. R Package, Version 1.2-1; Universidad Nacional Agraria: Lima, Peru, 2015; Available online: http://cran.rproject.org/web/packages/agricolae (accessed on 10 October 2017).
- Souza, A.L.; Soares, C.P.B. Florestas Nativas: Estrutura, Dinâmica e Manejo; UFV: Viçosa, Brazil, 2013; 322p. [Google Scholar]
- Batista, A.P.B.; Rodal, M.J.N.; Silva, J.A.A.; Silva, A.C.B.L.; Alves Junior, F.T.; Mello, J.M. Dynamics and Prediction of Diametric Structure in Two Atlantic Forest Fragments in Northeastern Brazil. Rev. Árvore 2016, 40, 307–317. [Google Scholar] [CrossRef]
- Rabelo, F.G.; Zarin, D.J.; Oliveira, F.A.; Jardim, F.C.S. Regeneração natural de florestas estuarinas na região do rio Amazonas Amapá-Brasil. Rev. Cienc. Agrar. 2000, 34, 129–137. [Google Scholar]
- Vasconcelos, L.M.R. Avaliação da Dinâmica Populacional de Rinorea guianensis Aublet (Acariquarana) Violaceae, em Uma Floresta Tropical Primária Explorada Seletivamente, Moju—PA. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2004. [Google Scholar]
- Maciel, M.N.M.; Watzlawick, L.F.; Schoeninger, E.R.; Yamaji, F.M. Efeito da radiação solar na dinâmica de uma floresta. Rev. Cienc. Exatas Nat. 2002, 4, 101–114. [Google Scholar]
- Jardim, F.C.; Da, S.; Hosokawa, R.T. Estrutura da floresta equatorial úmida da Estação Experimental de Silvicultura Tropical do INPA. Acta Amazon. 1987, 16/17, 411–508. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Vogt, N.D.; Pinedo-Vasquez, M.; Brondízio, E.S.; Rabelo, F.G.; Fernandes, K.; Almeida, O.; Rivero, S.; Deadman, P.J.; Dou, J. Local Ecological Knowledge and Incremental Adaptation to Changing Flood Patterns in the Amazon Delta. Sustain. Sci. 2016, 11, 624–643. [Google Scholar] [CrossRef]
- Marengo, J.A.; Alves, L.M.; Alvala, R.C.S.; Cunha, A.P.; Brito, S.; Moraes, O.L.L. Climatic characteristics of the 2010–2016 drought in the semiarid Northeast Brazil region. Ann. Braz. Acad. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2017, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gomes Sobrinho, T.R. Estrutura, Funcionamento e Evolução das Paisagens Cobertas por Savanas na Reserva Extrativista do Rio Cajari, Amazônia Oriental, Brasil. Ph.D. Thesis, Pontífica Universidade Católica de Minas Gerais, Belo Horizonte, Brazil, 2017. [Google Scholar]
- Wright, S.J.; Muller-Landau, H.C.; Condit, R.; Hubbell, S.P. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology 2003, 84, 3174–3185. [Google Scholar] [CrossRef]
- Kubitzki, K.; Ziburski, A. Seed Dispersal in Floodplain Forest of Amazonian. Biotropica 1994, 26, 30–43. [Google Scholar] [CrossRef]
- Oliveira-Wittmann, A.; Piedade, M.T.F.; Parolin, P.; Wittmann, F. Germination in four low-várzea tree species of Central Amazonia. Aquat. Bot. 2007, 86, 197–203. [Google Scholar] [CrossRef]
- Vasconcelos, S.S.; Higuchi, N.; Oliveira, M.V.N. Projeção da distribuição diamétrica de uma floresta explorada seletivamente na Amazônia Ocidental. Acta Amazon. 2009, 39, 71–80. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Chambers, J.Q.; Silva, A.R.; Lima, A.J.N.; Carneiro, V.M.C.; Santos, J.; Higuchi, N. Projeção da dinâmica da floresta natural de Terra-firme, região de Manaus-AM, com o uso da cadeia de transição probabilística de Markov. Acta Amazon. 2007, 37, 377–384. [Google Scholar] [CrossRef]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1987, 75, 81–86. [Google Scholar] [CrossRef]
- Finegan, B.; Camacho, M. Stand dynamics in a logged and silviculturally treated Costa Rica rain Forest, 1988–1996. For. Ecol. Manag. 1999, 121, 177–189. [Google Scholar] [CrossRef]
- Jardim, F.C.S. Natural regeneration in tropical forests. Rev. Cienc. Agrar. 2015, 58, 105–113. [Google Scholar]
- d’Oliveira, M.V.N. Sustainable Forest Management for Small Farmers in Acre State in the Brazilian Amazon. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2000. [Google Scholar]
- D’Oliveira, M.V.N. Regeneração florestal em clareiras artificiais após dois anos de abertura de dossel no Estado do Acre: Amazônia Ocidental. In Embrapa Acre: Ciência e Tecnologia Para o Desenvolvimento Sustentável do Sudoeste da Amazônia; Gonçaalvez, R.C., de Oliveira, L.C., Eds.; Embrapa Acre: Rio Branco, Brazil, 2009; pp. 68–96. [Google Scholar]
- Dupuy, J.M.; Chazdon, R.L. Effects of vegetation cover on seedling and sapling dynamics in secondary tropical wet forests in Costa Rica. J. Trop. Ecol. 2006, 22, 65–76. [Google Scholar] [CrossRef]
- Dupuy, J.M.; Chazdon, R.L. Interacting effects of canopy gap, understorey vegetation and leaf litter on tree seedling recruitment and composition in tropical secondary forests. For. Ecol. Manag. 2008, 255, 3716–3725. [Google Scholar] [CrossRef]
- D’Oliveira, M.V.N.; Braz, E.; d’Oliveira, M.V.N.; Ribas, L.A. Forest regeneration in artificial gaps twelve years after canopy opening in Acre State Western Amazon. For. Ecol. Manag. 2011, 261, 1722–1731. [Google Scholar] [CrossRef]
- Lowman, M.D.; Wittman, P.K. Forest Canopies: Methods, Hypotheses and Future directions. Annu. Rev. Ecol. Syst. 1996, 27, 55–81. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Herz, H.M. Evaluation of Different Methods to Estimate Understory Light Conditions in Tropical Forests. J. Trop. Ecol. 2001, 17, 207–224. [Google Scholar] [CrossRef]


| Parameters | Mazagão | Maracá | Ajuruxi |
|---|---|---|---|
| Regenerating individuals 2012 (ind. ha−1) | 2248 (±831) ns | 1676 (±667) ns | 2472 (±951) ns |
| Regenerating individuals 2013 (ind. ha−1) | 2693 (±1107) ns | 1996 (±653) ns | 2983 (±1275) ns |
| Regenerating individuals 2015 (ind. ha−1) | 1543 (±554) ns | 937 (±255) ns | 1194 (±452) ns |
| Regeneration rate 2012–2013 (%) | 7.9 ns | 10.4 ns | 8.3 ns |
| Regeneration rate 2013–2015 (%) | −22.0 ** a | −29.0 ** ab | −40.3 ** b |
| Mortality rate 2012–2013 (%) | 24.7 *** a | 43.4 *** b | 18.0 *** c |
| Mortality rate 2013–2015 (%) | 51.8 ns | 53.6 ns | 60.3 ns |
| Recruitment rate 2012–2013 (%) | 41.9 * ab | 66.6 * a | 31.4 * b |
| Recruitment rate 2013–2015 (%) | 17.4 ns | 8.7 ns | 4.8 ns |
| Height Class | C1 | C2 | C3 | C4 | C5 | C6 | M |
|---|---|---|---|---|---|---|---|
| R 2012–2013 | 0.687 | 0.191 | 0.054 | 0.029 | 0.018 | 0.019 | 0 |
| R 2013–2015 | 0.622 | 0.228 | 0.070 | 0.033 | 0.023 | 0.0202 | 0 |
| C1 2012–2013 | 0.580 | 0.067 | 0.014 | 0.004 | 0.003 | 0.008 | 0.320 |
| C1 2013–2015 | 0.363 | 0.103 | 0.004 | 0 | 0 | 0 | 0.527 |
| C2 2012–2013 | 0 | 0.600 | 0.157 | 0.035 | 0.005 | 0 | 0.201 |
| C2 2013–2015 | 0 | 0.504 | 0.073 | 0.002 | 0.002 | 0 | 0.416 |
| C3 2012–2013 | 0 | 0 | 0.609 | 0.143 | 0.028 | 0.028 | 0.189 |
| C3 2013–2015 | 0 | 0 | 0.405 | 0.092 | 0 | 0 | 0.502 |
| C4 2012–2013 | 0 | 0 | 0 | 0.544 | 0.17 | 0.071 | 0.205 |
| C4 2013–2015 | 0 | 0 | 0 | 0.365 | 0.087 | 0.015 | 0.531 |
| C5 2012–2013 | 0 | 0 | 0 | 0 | 0.5 | 0.195 | 0.304 |
| C5 2013–2015 | 0 | 0 | 0 | 0 | 0.194 | 0.138 | 0.666 |
| C6 2012–2013 | 0 | 0 | 0 | 0 | 0 | 0.666 | 0.333 |
| C6 2013–2015 | 0 | 0 | 0 | 0 | 0 | 0.16 | 0.840 |
| Height Class | C1 | C2 | C3 | C4 | C5 | C6 | M |
|---|---|---|---|---|---|---|---|
| R 2012–2013 | 0.732 | 0.148 | 0.043 | 0.023 | 0.016 | 0.035 | 0 |
| R 2013–2015 | 0.468 | 0.270 | 0.072 | 0.125 | 0 | 0.062 | 0 |
| C1 2012–2013 | 0.420 | 0.039 | 0.002 | 0 | 0 | 0 | 0.537 |
| C1 2013–2015 | 0.355 | 0.089 | 0.001 | 0 | 0 | 0.004 | 0.548 |
| C2 2012–2013 | 0 | 0.482 | 0.122 | 0.013 | 0 | 0 | 0.381 |
| C2 2013–2015 | 0 | 0.477 | 0.080 | 0.008 | 0 | 0.008 | 0.424 |
| C3 2012–2013 | 0 | 0 | 0.554 | 0.168 | 0.048 | 0.012 | 0.216 |
| C3 2013–2015 | 0 | 0 | 0.336 | 0.136 | 0.018 | 0.009 | 0.500 |
| C4 2012–2013 | 0 | 0 | 0 | 0.360 | 0.120 | 0.18 | 0.340 |
| C4 2013–2015 | 0 | 0 | 0 | 0.192 | 0.052 | 0.017 | 0.736 |
| C5 2012–2013 | 0 | 0 | 0 | 0 | 0.347 | 0.217 | 0.434 |
| C5 2013–2015 | 0 | 0 | 0 | 0 | 0.250 | 0.107 | 0.642 |
| C6 2012–2013 | 0 | 0 | 0 | 0 | 0 | 0.535 | 0.464 |
| C6 2013–2015 | 0 | 0 | 0 | 0 | 0 | 0.333 | 0.666 |
| Height Class | C1 | C2 | C3 | C4 | C5 | C6 | M |
|---|---|---|---|---|---|---|---|
| R 2012–2013 | 0.527 | 0.249 | 0.115 | 0.046 | 0.028 | 0.032 | 0 |
| R 2013–2015 | 0.284 | 0.438 | 0.107 | 0.123 | 0.023 | 0.023 | 0 |
| C1 2012–2013 | 0.622 | 0.099 | 0.004 | 0.004 | 0 | 0.004 | 0.265 |
| C1 2013–2015 | 0.298 | 0.126 | 0.001 | 0 | 0 | 0.009 | 0.564 |
| C2 2012–2013 | 0 | 0.626 | 0.204 | 0.004 | 0.002 | 0.001 | 0.160 |
| C2 2013–2015 | 0 | 0.386 | 0.082 | 0.001 | 0 | 0.003 | 0.526 |
| C3 2012–2013 | 0 | 0 | 0.640 | 0.198 | 0.020 | 0.002 | 0.139 |
| C3 2013–2015 | 0 | 0 | 0.315 | 0.056 | 0 | 0.001 | 0.626 |
| C4 2012–2013 | 0 | 0 | 0 | 0.464 | 0.269 | 0.107 | 0.158 |
| C4 2013–2015 | 0 | 0 | 0 | 0.244 | 0.027 | 0.017 | 0.710 |
| C5 2012–2013 | 0 | 0 | 0 | 0 | 0.321 | 0.344 | 0.333 |
| C5 2013–2015 | 0 | 0 | 0 | 0 | 0.193 | 0.075 | 0.731 |
| C6 2012–2013 | 0 | 0 | 0 | 0 | 0 | 0.463 | 0.536 |
| C6 2013–2015 | 0 | 0 | 0 | 0 | 0 | 0.137 | 0.862 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, Z.P.; Guedes, M.C.; Batista, A.P.B.; Silva, D.A.S.d. Natural Regeneration Dynamics of Mora paraensis (Ducke) Ducke in Estuarine Floodplain Forests of the Amazon River. Forests 2018, 9, 54. https://doi.org/10.3390/f9020054
Miranda ZP, Guedes MC, Batista APB, Silva DASd. Natural Regeneration Dynamics of Mora paraensis (Ducke) Ducke in Estuarine Floodplain Forests of the Amazon River. Forests. 2018; 9(2):54. https://doi.org/10.3390/f9020054
Chicago/Turabian StyleMiranda, Zenaide Palheta, Marcelino Carneiro Guedes, Anderson Pedro Bernardina Batista, and Diego Armando Silva da Silva. 2018. "Natural Regeneration Dynamics of Mora paraensis (Ducke) Ducke in Estuarine Floodplain Forests of the Amazon River" Forests 9, no. 2: 54. https://doi.org/10.3390/f9020054





