Moderate Disturbance Has Similar Effects on Production Regardless of Site Quality and Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Litter Trap Leaf Area Index
2.3. Wood Net Primary Production, Wood Mass, and Stem Density
2.4. Post-Recovery Canopy Structure
2.5. Post-Recovery Canopy Light Interception
2.6. Statistical Analyses
3. Results
3.1. Long-Term Leaf Area Index and Wood Net Primary Production Response to Disturbance
3.2. Stand Structure Before and After Disturbance
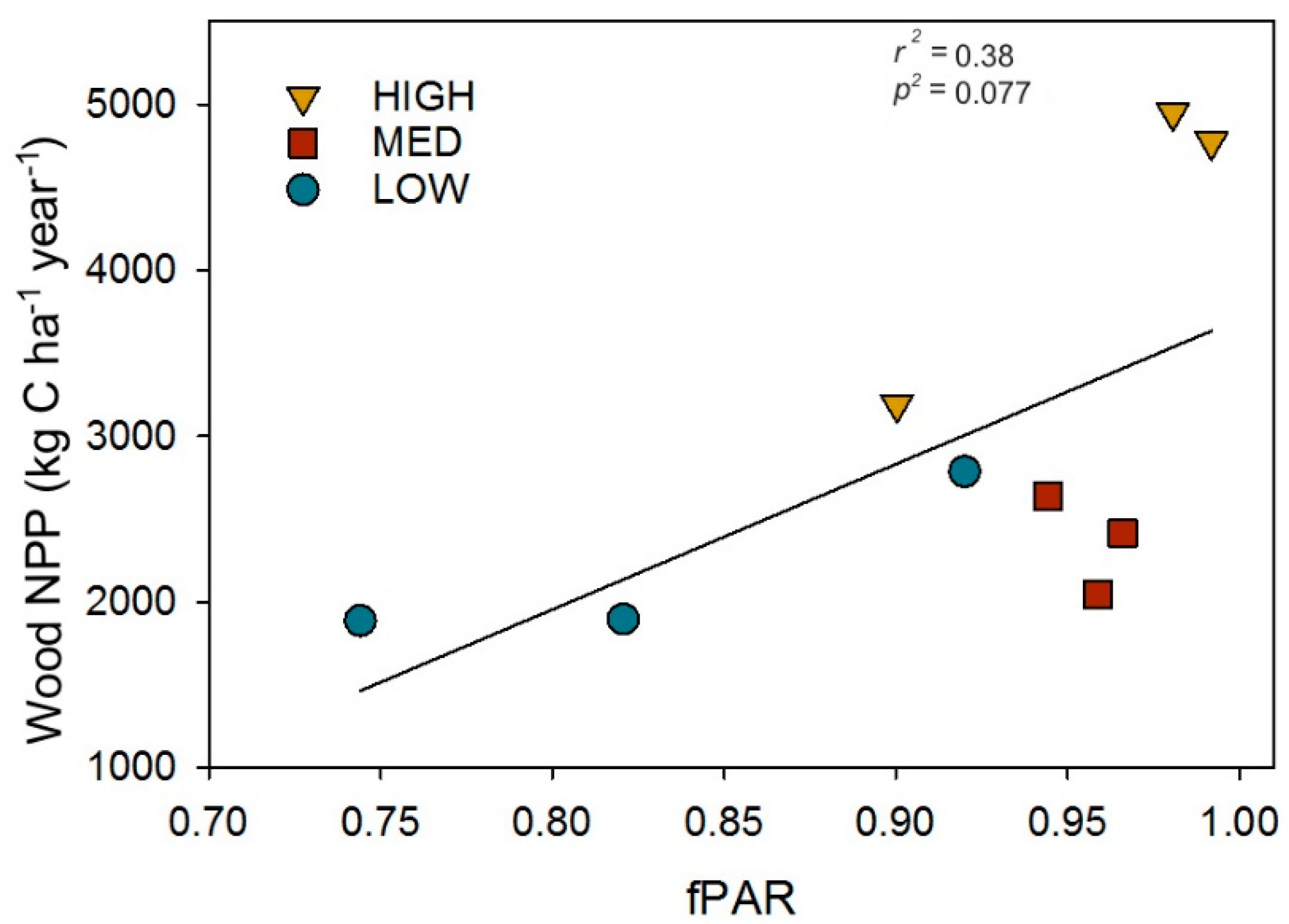
3.3. Post-Recovery Structure, Wood NPP, and Light Interception Interactions
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Term | Degrees of Freedom (DF) | Litter Trap LAI | NPP | ||||
|---|---|---|---|---|---|---|---|
| Sums of Squares | F-Value | p-Value | Sums of Squares | F-Value | p-Value | ||
| Stand | 2 | 0.17 | 3.40 | 0.0449 | 7646786 | 14.63 | 0.0004 |
| Time/Ecological Period | 3 | 0.51 | 6.86 | 0.0009 | 11348383 | 14.47 | 0.0001 |
| Stand × Time/Ecological Period | 6 | 0.31 | 2.07 | 0.0820 | 1369897 | 0.87 | 0.5381 |
| Disturbance Severity (covariate) | 1 | 0.02 | 0.79 | 0.3817 | 291892 | 1.12 | 0.3085 |
| Stem Density (Stems ha−1) | Wood Mass (Mg C ha−1) | ||||||
|---|---|---|---|---|---|---|---|
| Term | DF | Sums of Squares | F-Value | p-Value | Sums of Squares | F-Value | p-Value |
| Time | 1 | 4.78 | 15.21 | 0.0003 | 6.02 | 20.72 | <0.0001 |
| Stand | 2 | 11.93 | 18.98 | <0.0001 | 21.62 | 37.23 | <0.0001 |
| DBH Class | 4 | 90.67 | 72.11 | <0.0001 | 377.91 | 325.35 | <0.0001 |
| Time × Stand | 2 | 0.54 | 0.87 | 0.4265 | 0.82 | 01.40 | 0.2539 |
| Time × DBH Class | 4 | 4.24 | 3.37 | 0.0153 | 8.91 | 7.67 | <0.0001 |
| Stand × DBH Class | 8 | 6.19 | 2.46 | 0.0230 | 11.37 | 4.90 | 0.0001 |
| Time × Stand × DBH Class | 8 | 0.81 | 0.32 | 0.9540 | 0.78 | 0.33 | 0.9490 |
| Term | DF | H’ Combined | H’ Canopy | H’ Subcanopy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sums of Squares | F-Value | p-Value | Sums of Squares | F-Value | p-Value | Sums of Squares | F-Value | p-Value | ||
| Time | 1 | 0.11 | 2.26 | 0.1588 | 0.22 | 3.31 | 0.0939 | 0.01 | 0.16 | 0.6961 |
| Stand | 2 | 0.42 | 4.18 | 0.0419 | 0.26 | 1.94 | 0.1865 | 2.18 | 16.02 | 0.0004 |
| Time × Stand | 2 | 0.08 | 0.87 | 0.4446 | 0.08 | 0.60 | 0.5668 | 0.02 | 0.14 | 0.8699 |
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the World’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Goodale, C.L.; Apps, M.J.; Birdsey, R.A.; Field, C.B.; Heath, L.S.; Houghton, R.A.; Jenkins, J.C.; Kohlmaier, G.H.; Kurz, W.A.; Liu, S.; et al. Forest carbon sinks in the Northern Hemisphere. Ecol. Appl. 2002. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Collatz, G.J.; Masek, J.; Goward, S.N. Carbon consequences of forest disturbance and recovery across the conterminous United States. Glob. Biogeochem. Cycles 2012, 26, GB1005. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Harrold, K.H.; George, K.; Curtis, P.S. The legacy of harvest and fire on ecosystem carbon storage in a north temperate forest. Glob. Chang. Biol. 2007, 13, 1935–1949. [Google Scholar] [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; (Ted) Hogg, E.H.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Kashian, D.M.; Romme, W.H.; Tinker, D.B.; Turner, M.G.; Ryan, M.G. Carbon storage on landscapes with stand-replacing fires. Biol. Sci. 2006, 56, 598–606. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. Bio. Science 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Cohen, W.B.; Yang, Z.; Stehman, S.V.; Schroeder, T.A.; Bell, D.M.; Masek, J.G.; Huang, C.; Meigs, G.W. Forest disturbance across the conterminous United States from 1985–2012: The emerging dominance of forest decline. For. Ecol. Manag. 2016, 360, 242–252. [Google Scholar] [CrossRef]
- Goetz, S.J.; Bond-Lamberty, B.; Law, B.E.; Hicke, J.A.; Huang, C.; Houghton, R.A.; McNulty, S.; O’Halloran, T.; Harmon, M.; Meddens, A.J.H.; et al. Observations and assessment of forest carbon dynamics following disturbance in North America. J. Geophys. Res. 2012, 117, G02022. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Fisk, J.P.; Holm, J.A.; Bailey, V.; Bohrer, G.; Gough, C.M. Moderate forest disturbance as a stringent test for gap and big-leaf models. Biogeosciences 2015, 12, 513–526. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, S.; Stoy, P.C. Preface: Impacts of extreme climate events and disturbances on carbon dynamics. Biogeosciences 2016, 13, 3665–3675. [Google Scholar] [CrossRef]
- Skovsgaard, J.P. Analysing effects of thinning on stand volume growth in relation to site conditions: A case study for even-aged Sitka spruce (Picea sitchensis (Bong.) Carr.). Forestry 2009, 82, 87–104. [Google Scholar] [CrossRef]
- Reich, P.B. Key canopy traits drive forest productivity. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.B.; Wythers, K.R.; Bradford, J.B.; Reich, P.B. Influence of disturbance on temperate forest productivity. Ecosystems 2013, 16, 95–110. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Curtis, P.S. Canopy structural changes following widespread mortality of canopy dominant trees. Forests 2013, 4, 537–552. [Google Scholar] [CrossRef]
- Reinikainen, M.; D’Amato, A.W.; Bradford, J.B.; Fraver, S. Influence of stocking, site quality, stand age, low-severity canopy disturbance, and forest composition on sub-boreal aspen mixedwood carbon stocks. Can. J. For. Res. 2013, 44, 230–242. [Google Scholar] [CrossRef]
- Pedro, M.S.; Rammer, W.; Seidl, R. Tree species diversity mitigates disturbance impacts on the forest carbon cycle. Oecologia 2015, 177, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tanabe, S.; Hiura, T. Exploring the relationships among canopy structure, stand productivity, and biodiversity of temperate forest ecosystems. For. Sci. 2004, 50, 342–355. [Google Scholar]
- Parker, G.G.; Harding, D.J.; Berger, M.L. A portable LIDAR system for rapid determination of forest canopy structure. J. Appl. Ecol. 2004, 41, 755–767. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.M.; Curtis, P.S.; Hardiman, B.S.; Scheuermann, C.M.; Bond-Lamberty, B. Disturbance, complexity, and succession of net ecosystem production in North America’s temperate deciduous forests. Ecosphere 2016, 7. [Google Scholar] [CrossRef]
- Nave, L.E.; Gough, C.M.; Maurer, K.D.; Bohrer, G.; Hardiman, B.S.; Le Moine, J.; Munoz, A.B.; Nadelhoffer, K.J.; Sparks, J.P.; Strahm, B.D.; et al. Disturbance and the resilience of coupled carbon and nitrogen cycling in a north temperate forest. J. Geophys. Res. 2011, 116, G04016. [Google Scholar] [CrossRef]
- Gough, C.M.; Hardiman, B.S.; Nave, L.E.; Bohrer, G.; Maurer, K.D.; Vogel, C.S.; Nadelhoffer, K.J.; Curtis, P.S. Sustained carbon uptake and storage following moderate disturbance in a Great Lakes forest. Ecol. Appl. 2013, 23, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Haëntjens, E.J.; Curtis, P.S.; Fahey, R.T.; Vogel, C.S.; Gough, C.M. Net primary production of a temperate deciduous forest exhibits a threshold response to increasing disturbance severity. Ecology 2015, 96, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Fahey, R.; Stuart-Haëntjens, E.; Gough, C.; De La Cruz, A.; Stockton, E.; Vogel, C.; Curtis, P. Evaluating forest subcanopy response to moderate severity disturbance and contribution to ecosystem-level productivity and resilience. For. Ecol. Manag. 2016, 376, 135–147. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Hardiman, B.; Curtis, P.S. Wood net primary production resilience in an unmanaged forest transitioning from early to middle succession. For. Ecol. Manag. 2010, 260, 36–41. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Schmid, H.P.; Su, H.-B.; Curtis, P.S. Multi-year convergence of biometric and meteorological estimates of forest carbon storage. Agric. For. Meteorol. 2008, 148, 158–170. [Google Scholar] [CrossRef]
- Davies, G.M.; Gray, A. Don’t let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecol. Evol. 2015, 5, 5295–5304. [Google Scholar] [CrossRef] [Green Version]
- Hardiman, B.S.; Gough, C.M.; Halperin, A.; Hofmeister, K.L.; Nave, L.E.; Bohrer, G.; Curtis, P.S. Maintaining high rates of carbon storage in old forests: A mechanism linking canopy structure to forest function. For. Ecol. Manag. 2013, 298, 111–119. [Google Scholar] [CrossRef]
- Fahey, R.T.; Fotis, A.T.; Woods, K.D. Quantifying canopy complexity and effects on productivity and resilience in late-successional hemlock–hardwood forests. Ecol. Appl. 2015, 25, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Sheuermann, C.S.; Nave, L.E.; Fahey, T.R.; Nadelhoffer, K.J.; Gough, C.M. The coupling of ecosystem biological and physical structure with primary production is mixed in upper Great Lakes forests. Oecologia 2018. submitted. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gorelick, R. Combining richness and abundance into a single diversity index using matrix analogues of Shannon’s and Simpson’s indices. Ecography 2006, 29, 525–530. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; ISBN 978-94-015-7360-3. [Google Scholar]
- Liang, J.; Buongiorno, J.; Monserud, R.A.; Kruger, E.L.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Romme, W.H.; Knight, D.; Yavitt, J. Mountain pine beetle outbreaks in the Rocky Mountains: Regulators of primary productivity? Am. Nat. 1986, 127. [Google Scholar] [CrossRef]
- Brown, M.; Black, T.A.; Nesic, Z.; Foord, V.N.; Spittlehouse, D.L.; Fredeen, A.L.; Grant, N.J.; Burton, P.J.; Trofymow, J.A. Impact of mountain pine beetle on the net ecosystem production of lodgepole pine stands in British Columbia. Agric. For. Meteorol. 2010, 150, 254–264. [Google Scholar] [CrossRef]
- Pfeifer, E.M.; Hicke, J.A.; Meddens, A.J.H. Observations and modeling of aboveground tree carbon stocks and fluxes following a bark beetle outbreak in the western United States. Glob. Chang. Biol. 2011, 17, 339–350. [Google Scholar] [CrossRef]
- Campbell, J.; Alberti, G.; Martin, J.; Law, B.E. Carbon dynamics of a ponderosa pine plantation following a thinning treatment in the northern Sierra Nevada. For. Ecol. Manag. 2009, 257, 453–463. [Google Scholar] [CrossRef]
- Edburg, S.L.; Hicke, J.A.; Lawrence, D.M.; Thornton, P.E. Simulating coupled carbon and nitrogen dynamics following mountain pine beetle outbreaks in the western United States. J. Geophys. Res. 2011, 116, G04033. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.E. Catastrophic disturbance and the steady state in northern hardwood forests: A new look at the role of disturbance in the development of forest ecosystems suggests important implications for land-use policies. Am. Sci. 1979, 67, 660–669. [Google Scholar]
- Woods, K.D. Multi-decade biomass dynamics in an old-growth hemlock-northern hardwood forest, Michigan, USA. PeerJ 2014, 2, e598. [Google Scholar] [CrossRef] [PubMed]
- Halpin, C.R.; Lorimer, C.G. Long-term trends in biomass and tree demography in northern hardwoods: An integrated field and simulation study. Ecol. Monogr. 2016, 86, 78–93. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M. Biodiversity and ecosystem functioning: A mechanistic model. Proc. Natl. Acad. Sci. USA 1998, 95, 5632–5636. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Reichle, D.E. Dynamic Properties of Forest Ecosystems; Cambridge University Press: Cambridge, UK, 1981; ISBN 978-0-521-22508-3. [Google Scholar]
- Zhang, Y.; Chen, H.Y.H.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- Kelty, M.J. The role of species mixtures in plantation forestry. For. Ecol. Manag. 2006, 233, 195–204. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Fotis, A.; Murphy, S.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; John, W.; Queenborough, S.; Comita, L. Aboveground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2017. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Adler, P.B.; Hillerislambers, J.; Levine, J.M. A niche for neutrality. Ecol. Lett. 2007, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Fotis, A.T.; Curtis, P.S. Effects of structural complexity on within-canopy light environments and leaf traits in a northern mixed deciduous forest. Tree Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.M. The disturbing history of intermediate disturbance. Oikos 1999, 84, 145–147. [Google Scholar] [CrossRef]
- Sakai, T.; Akiyama, T. Quantifying the spatio-temporal variability of net primary production of the understory species, Sasa senanensis, using multipoint measuring techniques. Agric. For. Meteorol. 2005, 134, 60–69. [Google Scholar] [CrossRef]
- Cole, W.G.; Lorimer, C.G. Probabilities of small-gap capture by sugar maple saplings based on height and crown growth data from felled trees. Can. J. For. Resour. 2005, 35, 643–655. [Google Scholar] [CrossRef]
- Hart, J. Gap-Scale Disturbances in Central Hardwood Forests with Implications for Management. In Natural Disturbances and Historic Range of Variation Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C., Collins, B., Eds.; Springer: Cham, Switzerland, 2015; Volume 32, pp. 33–47. ISBN 978-3-319-21527-3. [Google Scholar]
- Portsmuth, A.; Niinemets, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
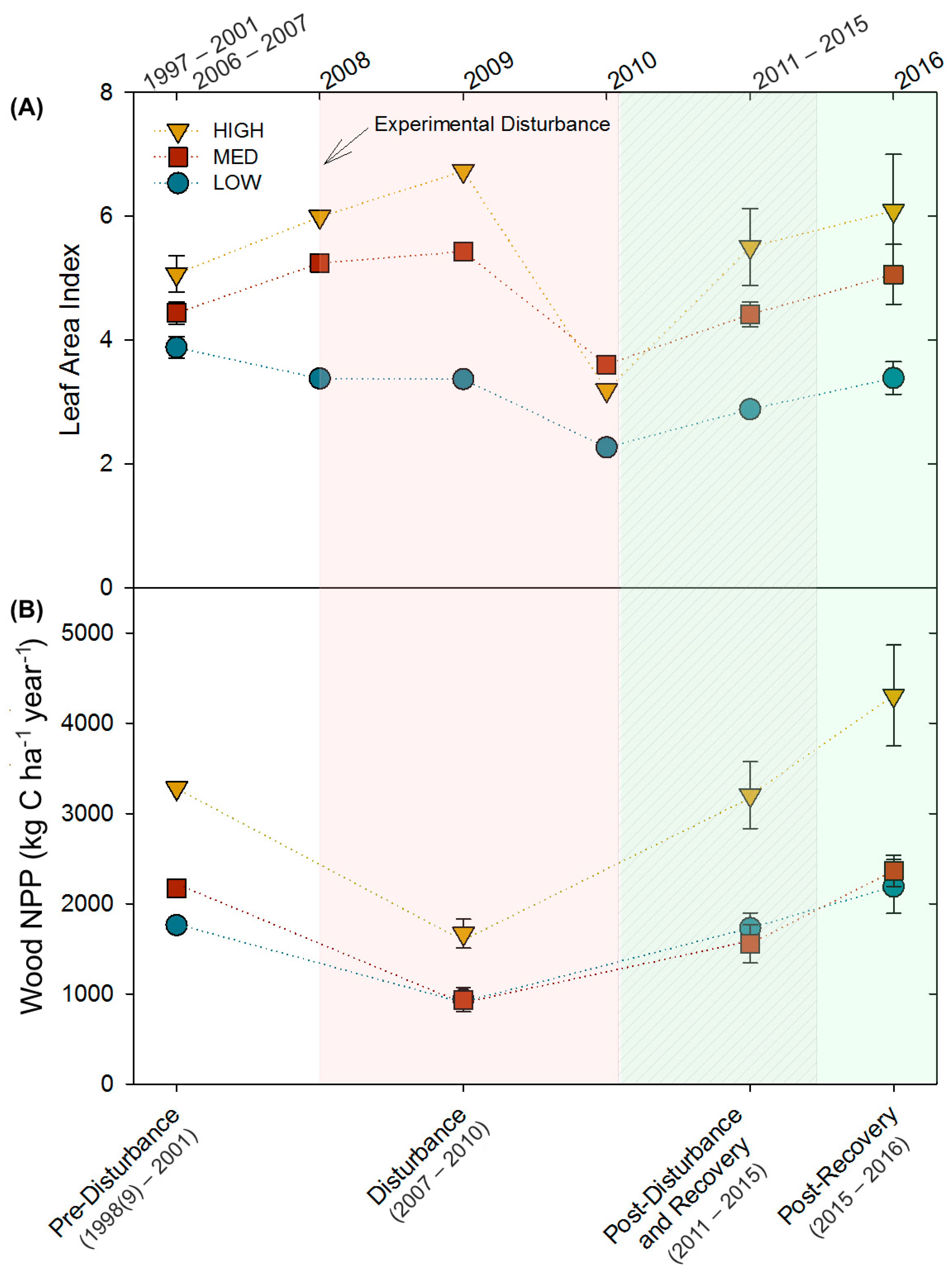
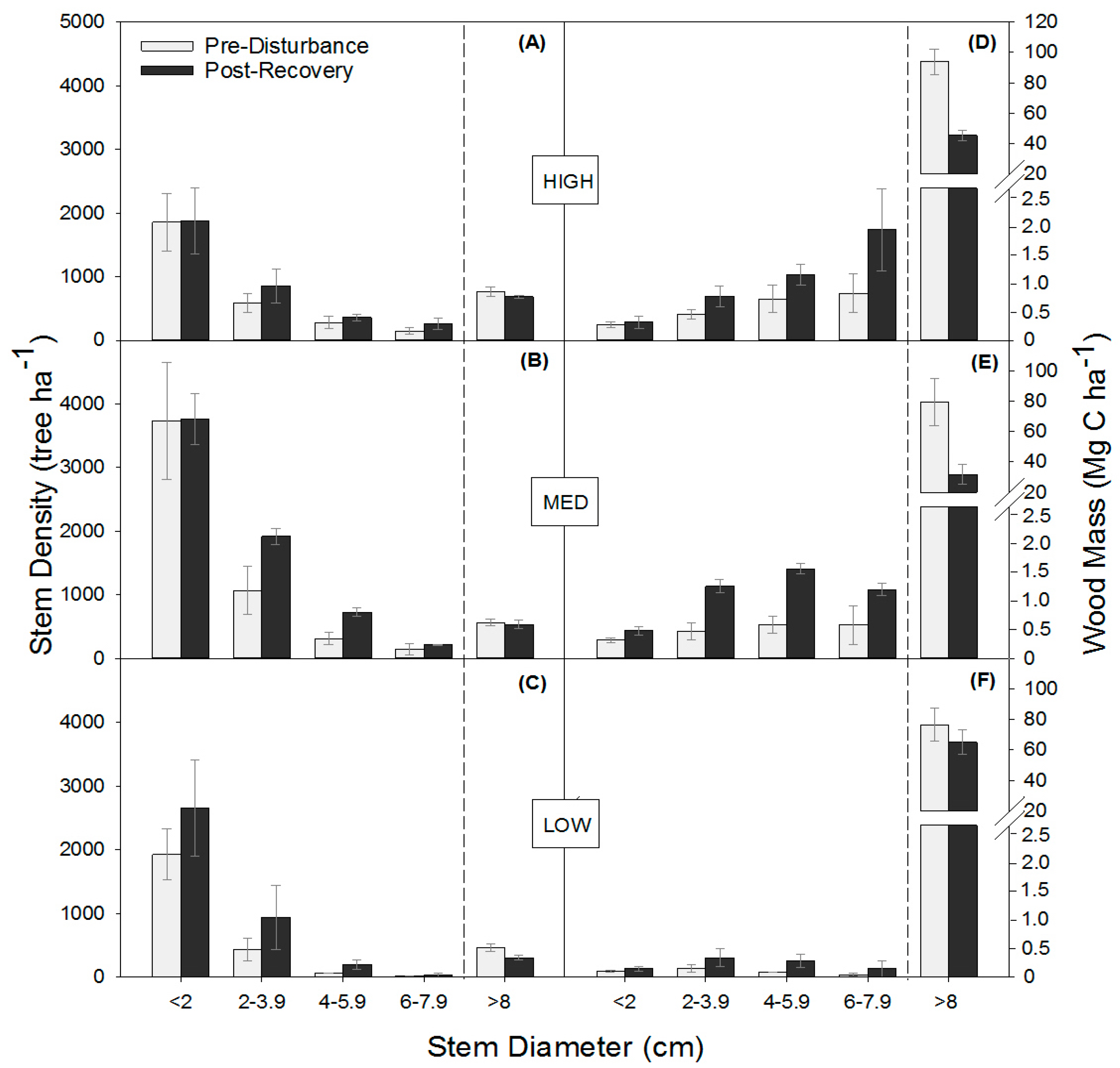
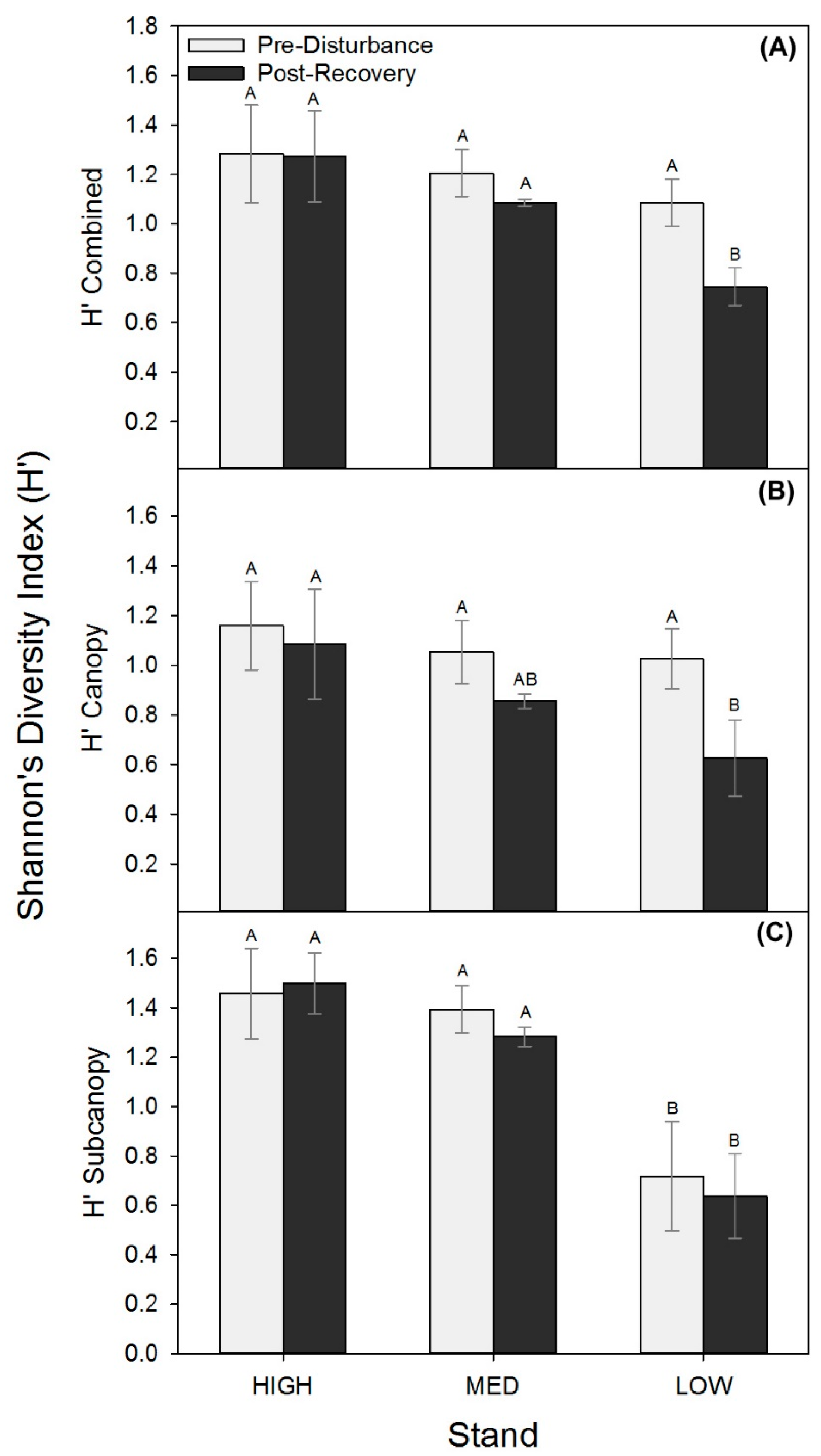
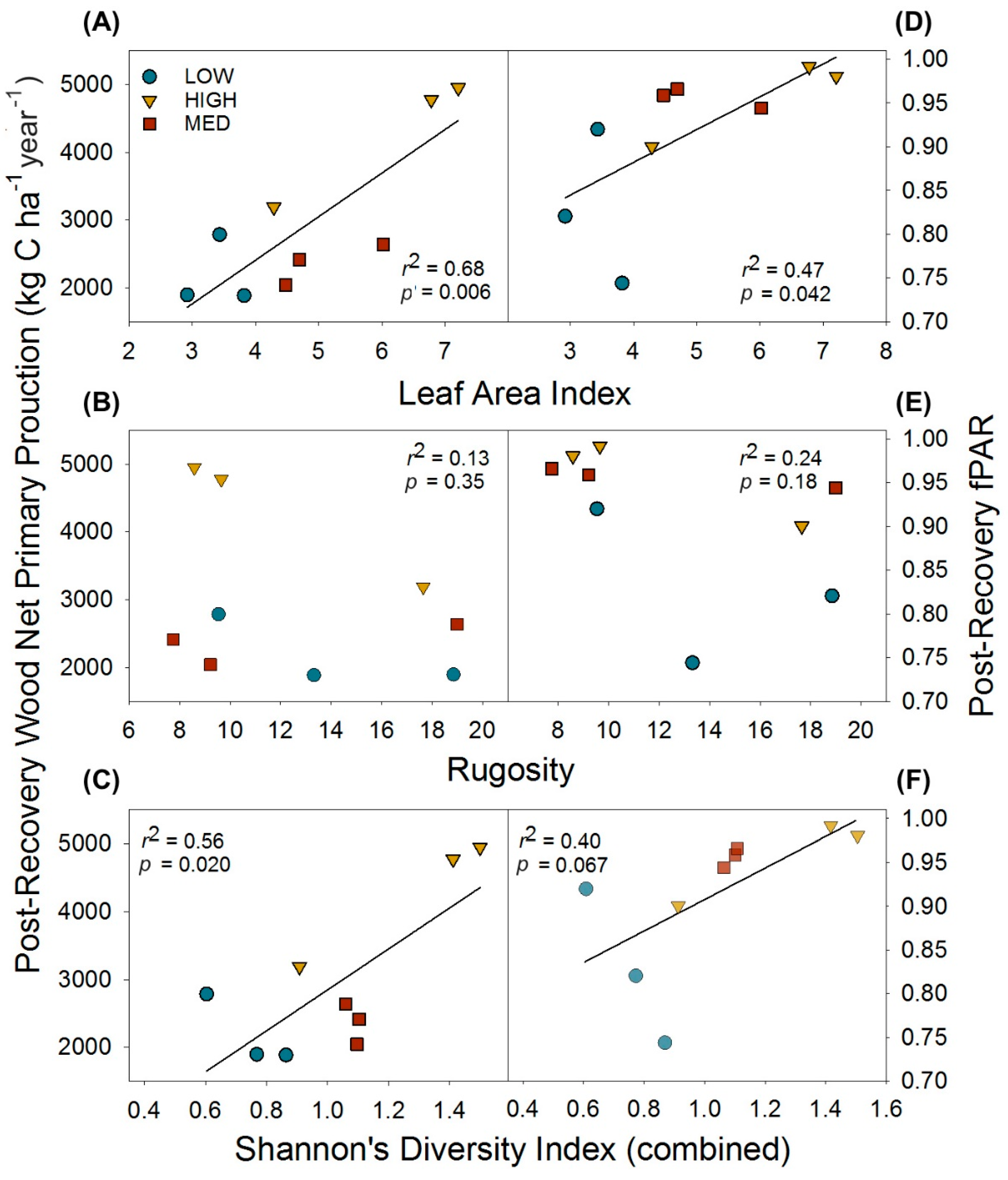
| Stand | Wood NPP (Mg C ha−1 year−1) | Canopy Wood Mass | Canopy Basal Area | LAI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total (Mg C ha−1) | Aspen and Birch (%) | Total (m2 ha−1) | Aspen and Birch /Mortality (%) | Total | Aspen and Birch (%) | Sapling Stem Density (stems ha−1) | Dominant Canopy Species (% BA contribution) | ||
| HIGH | 3.22 | 94.0 ± 8.3 | 74.1 ± 8.1 | 31.1 ± 2.5 | 64.6 ± 8.9 | 5.86 | 36.0 | 425 ± 151 | 1. Populus tremuloides (50%) 2. Acer rubrum (24%) 3. Populus grandidentata (14%) 4. Acer saccharum (7%) 5. Prunus serotina (1%) |
| MED | 2.14 | 79.3 ± 15.4 | 75.4 ± 2.7 | 27.4 ± 4.1 | 68.4 ± 3.5 | 4.58 | 38.5 | 454 ± 174 | 1. Populus grandidentata (56%) 2. Acer rubrum (26%) 3. Betula papyrifera (11%) 4. Quercus rubra (4%) 5. Fagus grandifolia (2%) |
| LOW | 1.76 | 76.6 ± 10.6 | 33.2 ± 9.0 | 25.1 ± 3.8 | 36.1 ± 9.7 | 3.37 | 29.5 | 71 ± 4 | 1. Quercus rubra (48%) 2. Populus grandidentata (32%) 3. Acer rubrum (13%) 4. Betula papyrifera (6%) 5. Pinus strobus (1%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagara, B.T.; Fahey, R.T.; Vogel, C.S.; Fotis, A.T.; Curtis, P.S.; Gough, C.M. Moderate Disturbance Has Similar Effects on Production Regardless of Site Quality and Composition. Forests 2018, 9, 70. https://doi.org/10.3390/f9020070
Sagara BT, Fahey RT, Vogel CS, Fotis AT, Curtis PS, Gough CM. Moderate Disturbance Has Similar Effects on Production Regardless of Site Quality and Composition. Forests. 2018; 9(2):70. https://doi.org/10.3390/f9020070
Chicago/Turabian StyleSagara, Benjamin T., Robert T. Fahey, Christoph S. Vogel, Alexander T. Fotis, Peter S. Curtis, and Christopher M. Gough. 2018. "Moderate Disturbance Has Similar Effects on Production Regardless of Site Quality and Composition" Forests 9, no. 2: 70. https://doi.org/10.3390/f9020070





