Drought Impact on Phenology and Green Biomass Production of Alpine Mountain Forest—Case Study of South Tyrol 2001–2012 Inspected with MODIS Time Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Meteorological Conditions
2.3. MODIS Time Series
2.3.1. Phenology and Green Biomass Production Measures
2.4. Ancillary Datasets
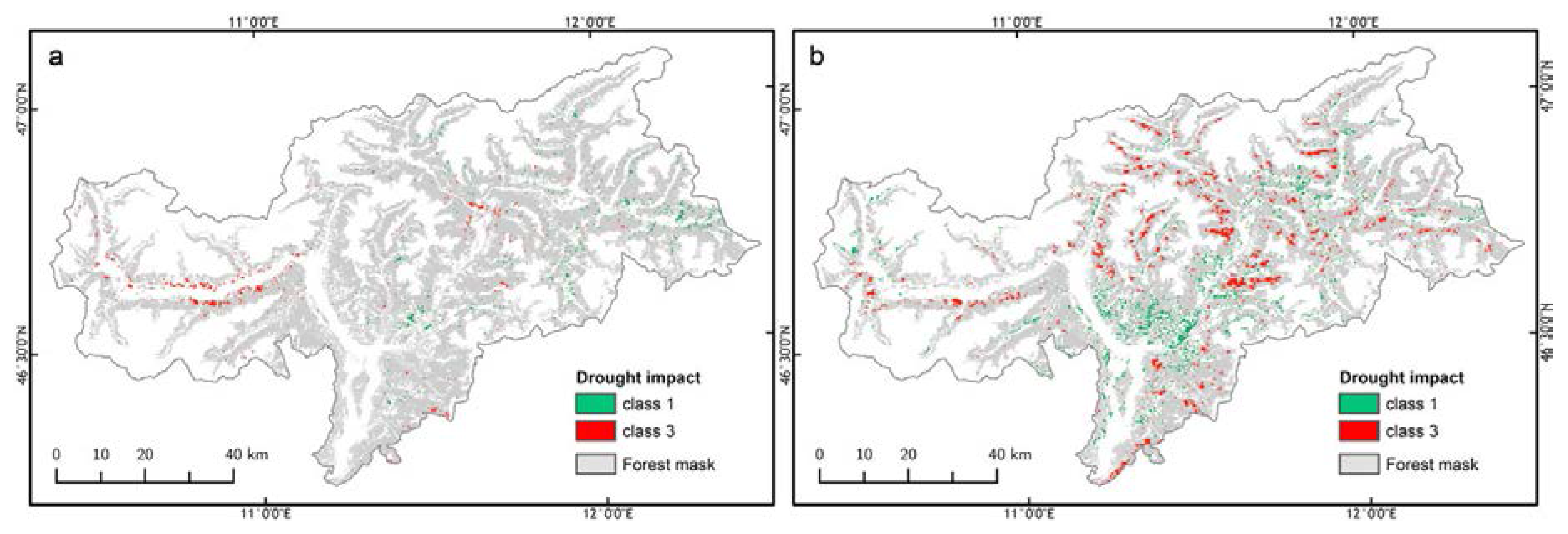
2.5. Identification of Drought Impact in Alpine Mountain Forest
2.6. Drought Impact Analysis
- forest type (3 levels: coniferous, mixed, broadleaved);
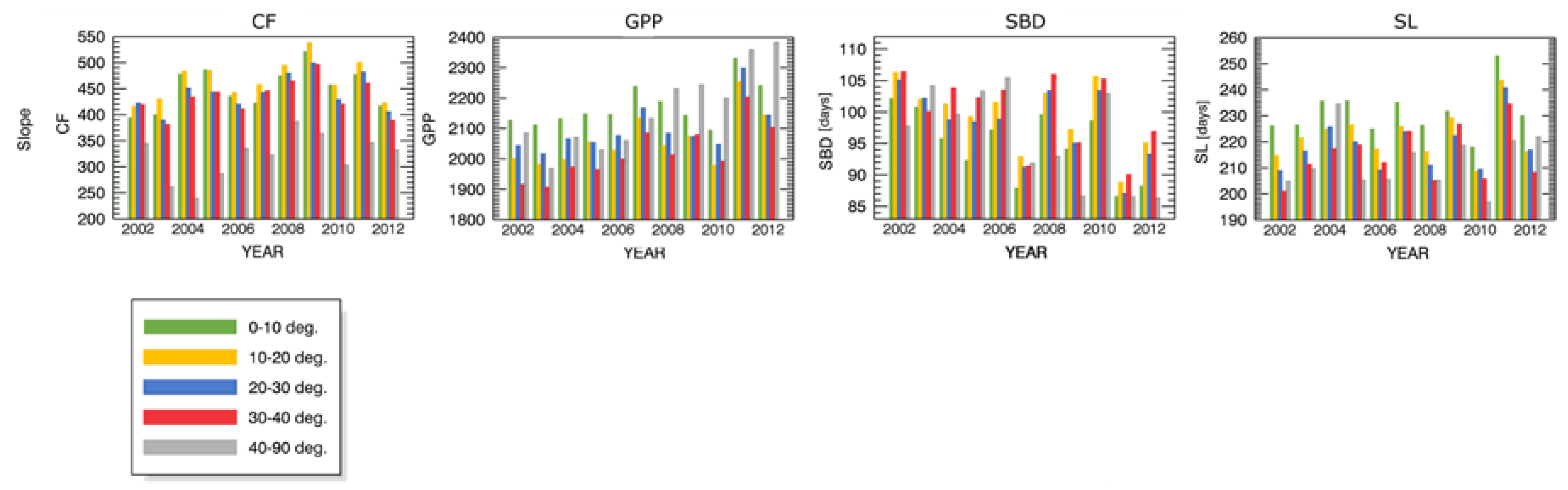
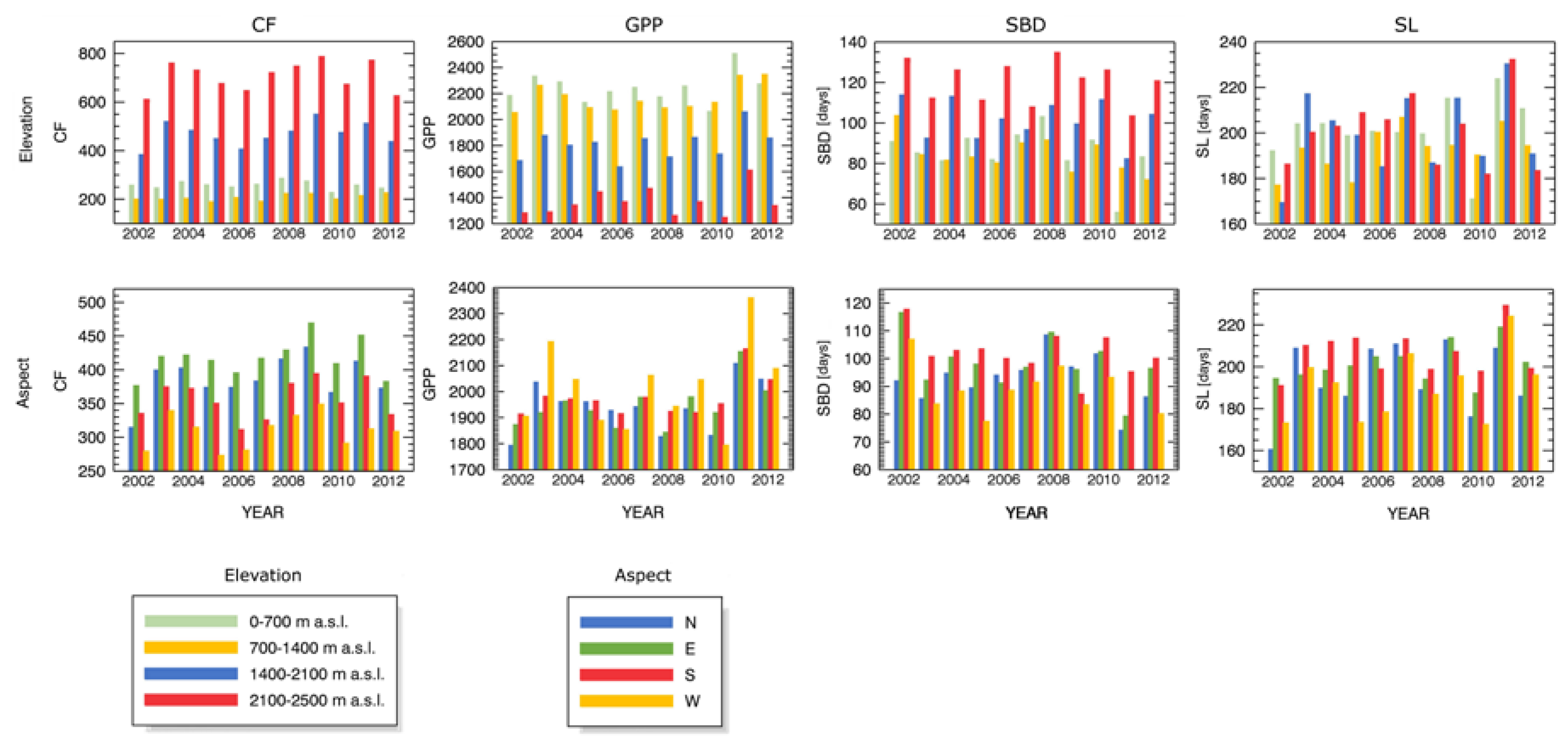
- elevation (4 levels: 0–700 m a.s.l., 700–1400 m a.s.l., 1400–2100 m a.s.l., 2100–2500 m a.s.l.; elevation stratification after [12]);
- exposition (4 levels: N, E, S, W);
- inclination (5 levels: 0°–10°, 10°–20°, 20°–30°, 30°–40°, 40°–90°).
3. Results
3.1. Drought Conditions
3.2. Forest Temporal Response to Drought Conditions
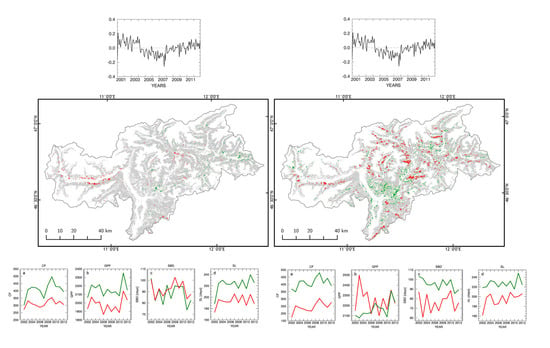
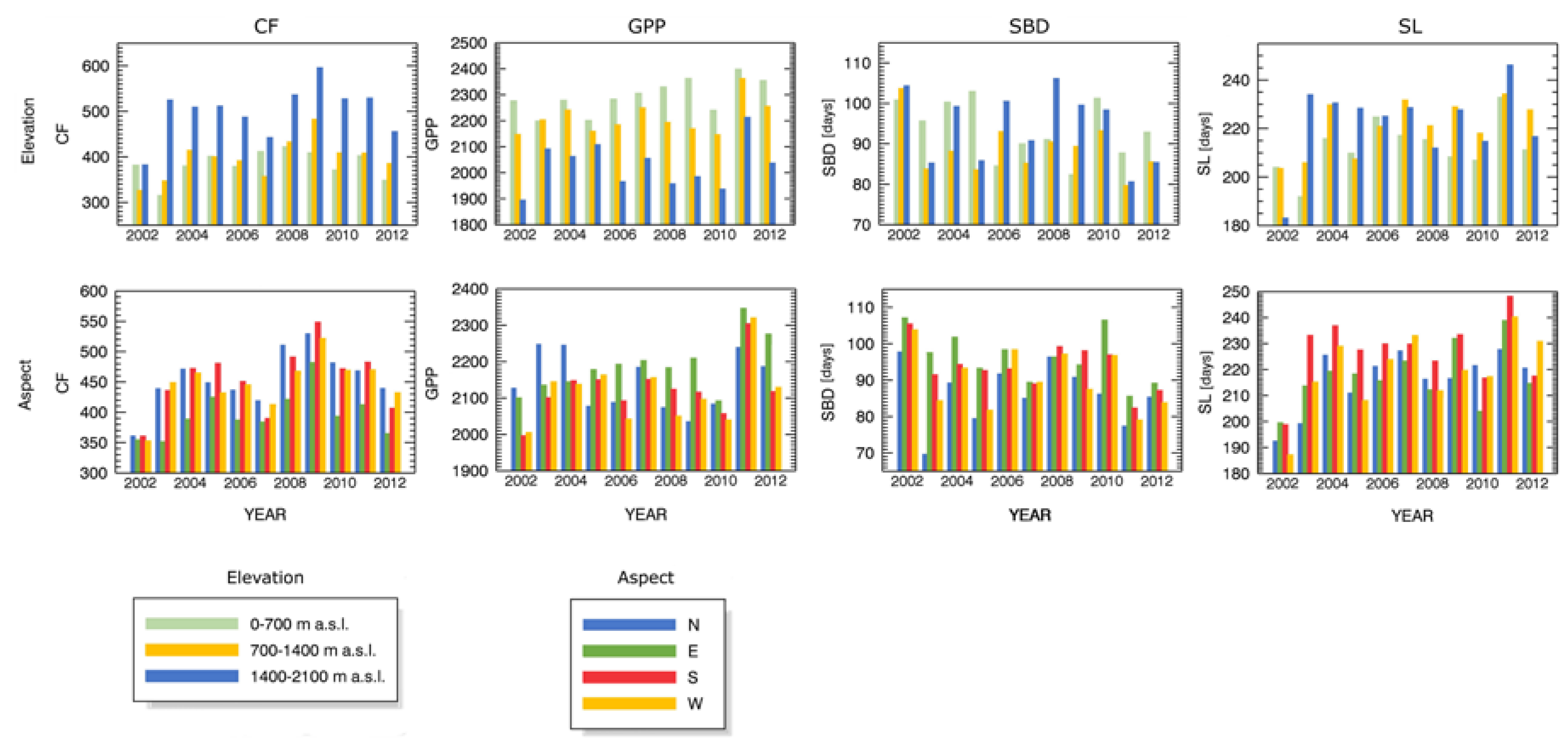
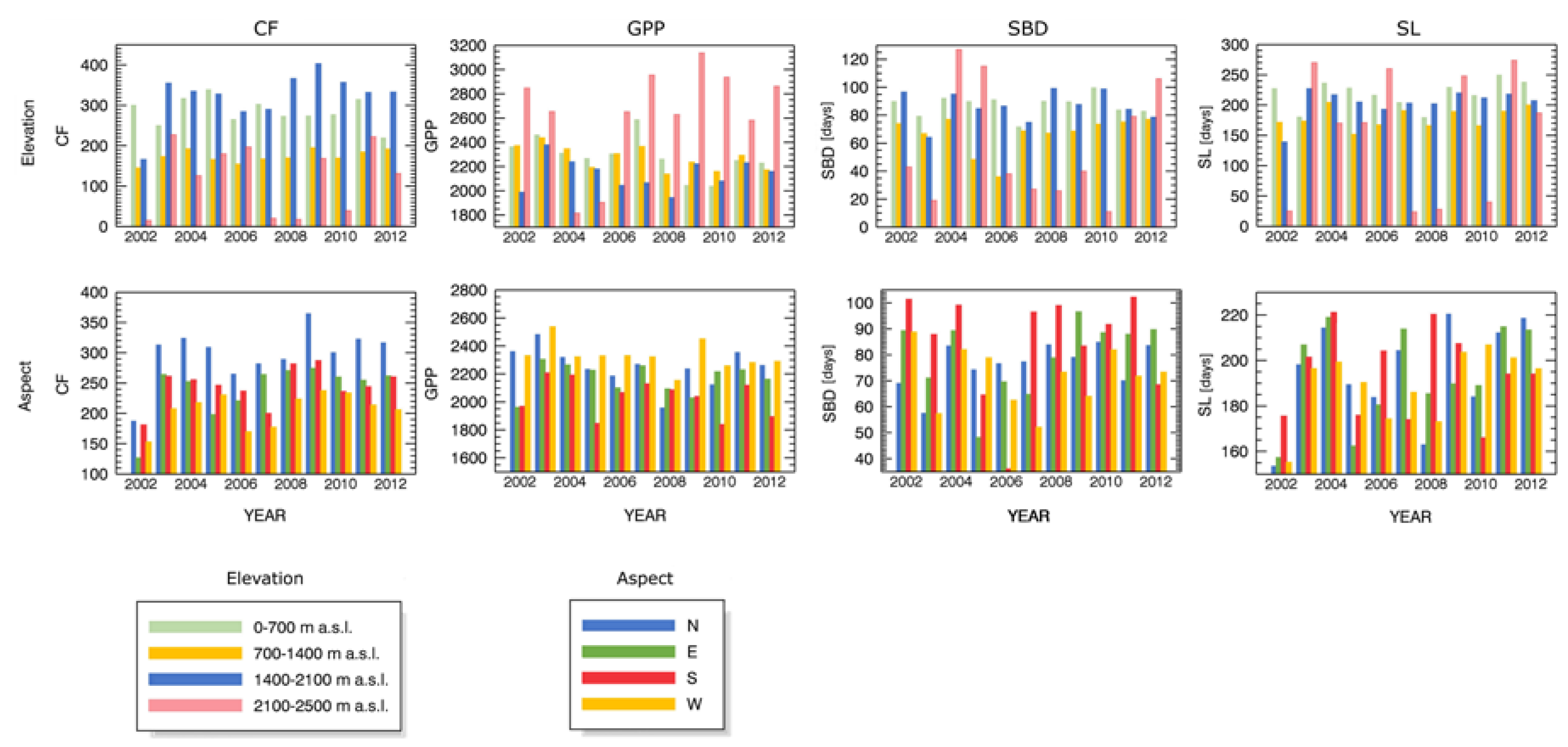
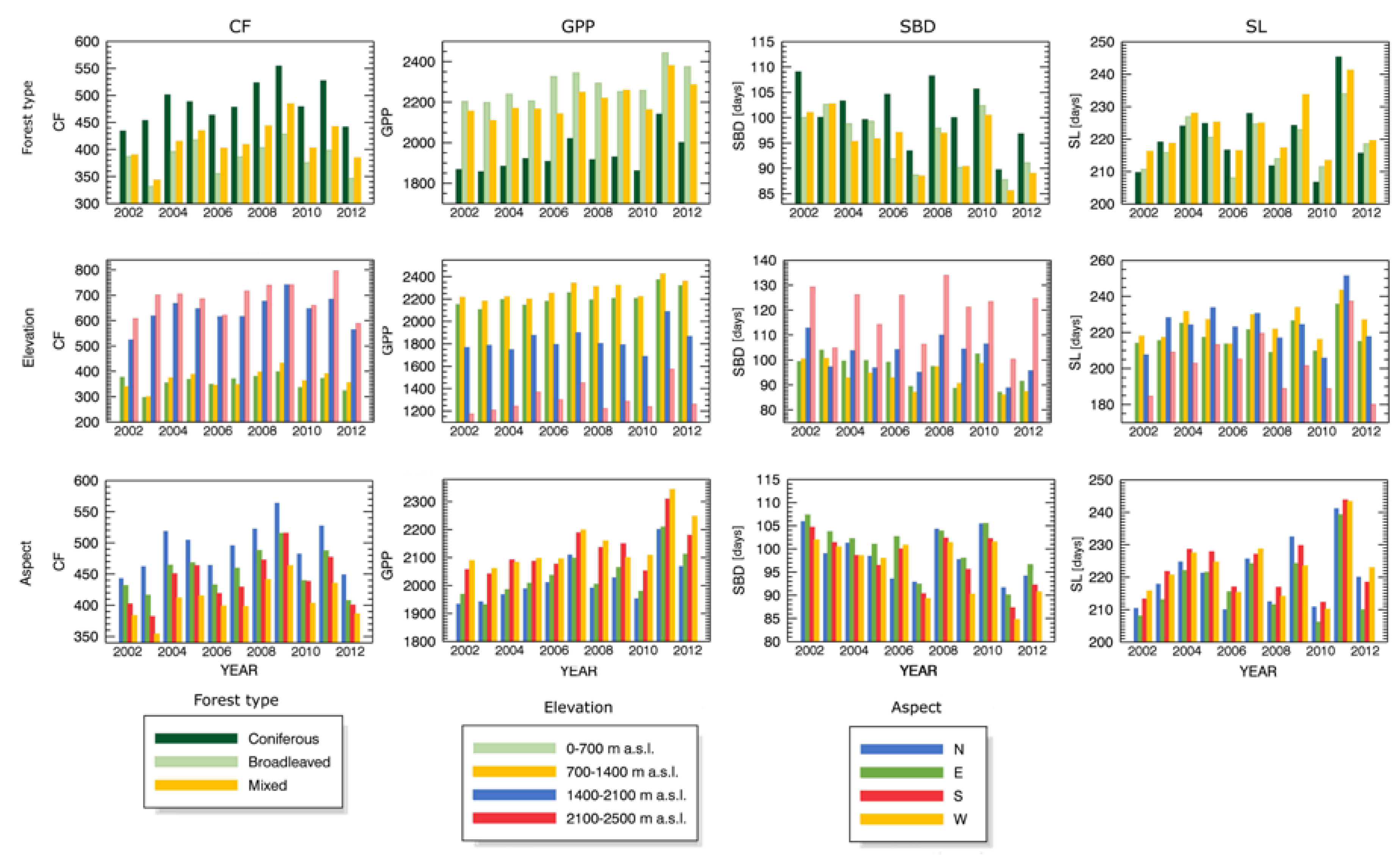
3.3. Forest Spatial Response to Meteorological Drought Conditions
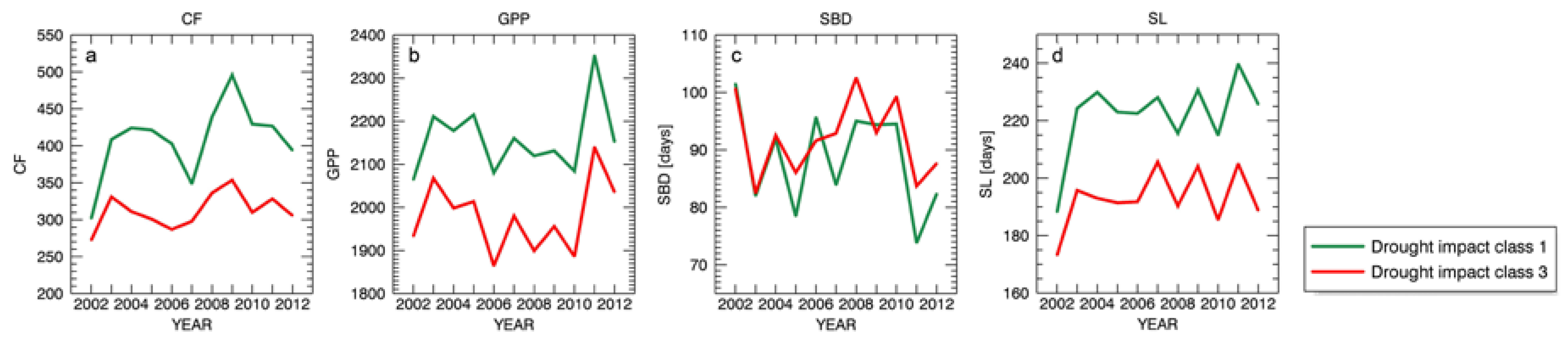
3.4. Drought Impact on Phenology and Green Biomass Production
3.4.1. Changes in Forest Canopy Water Content—4nNDII7 PC
3.4.2. Changes in Forest Canopy Photosynthetic Activity—3nNDVI PC
4. Discussion
4.1. Meteorological Drought Conditions and Forest Temporal Response
4.2. Alpine Forest Response to Identified Meteorological Drought Conditions
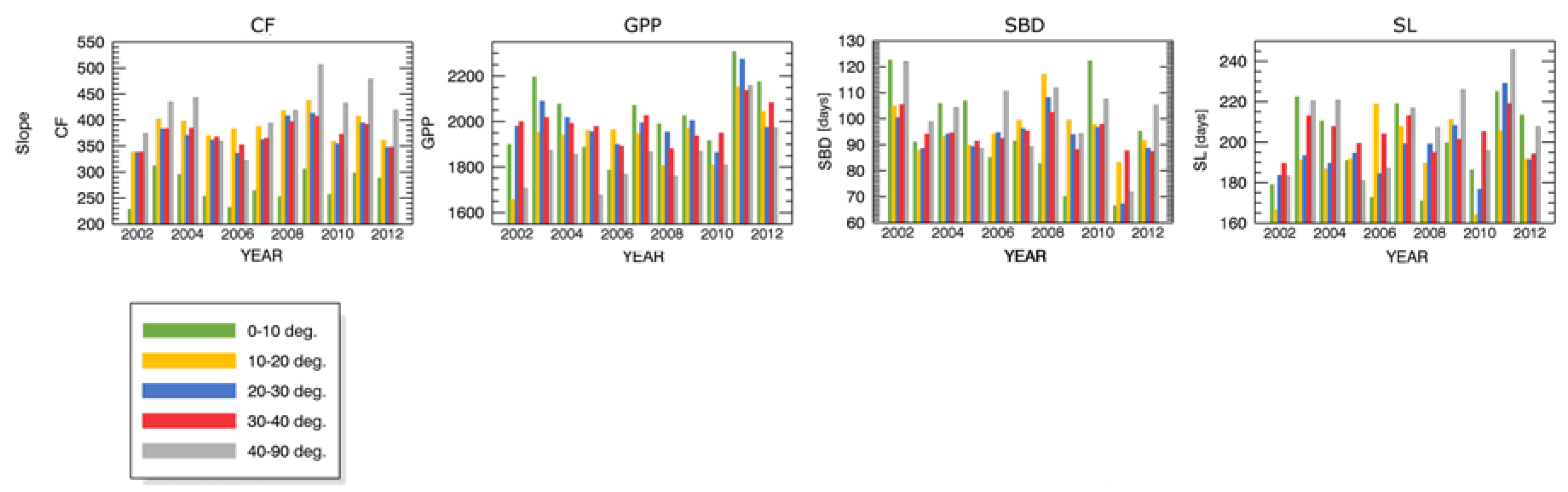
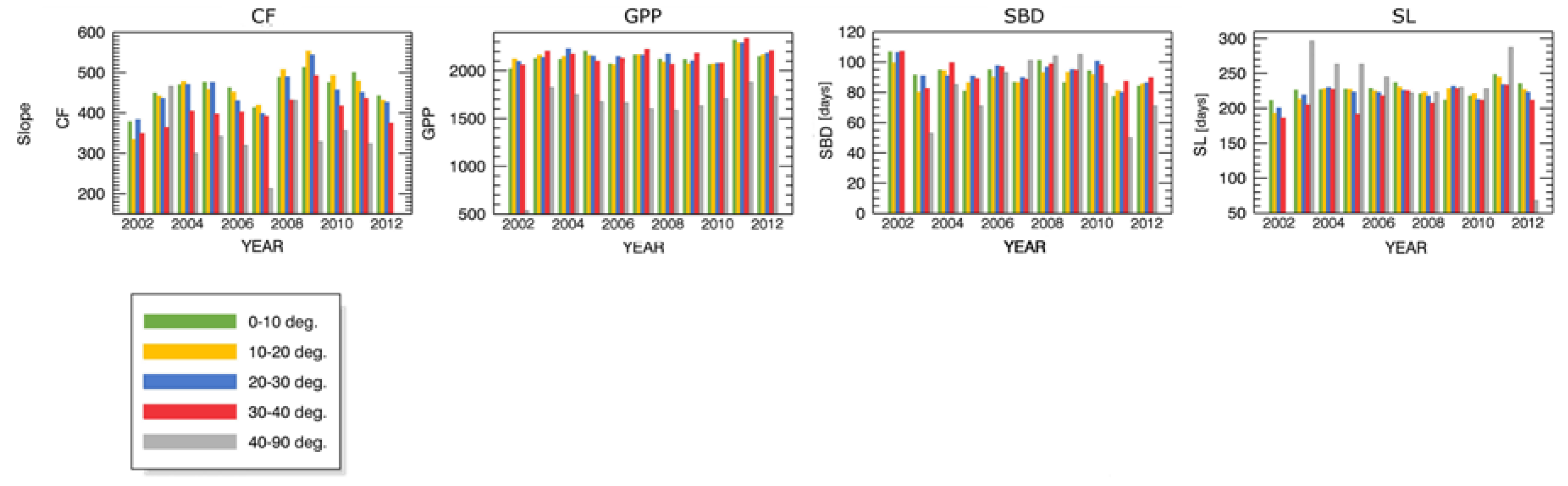
4.2.1. Changes in Foliage Water Content 2004–2007 (4nNDII7 PC)
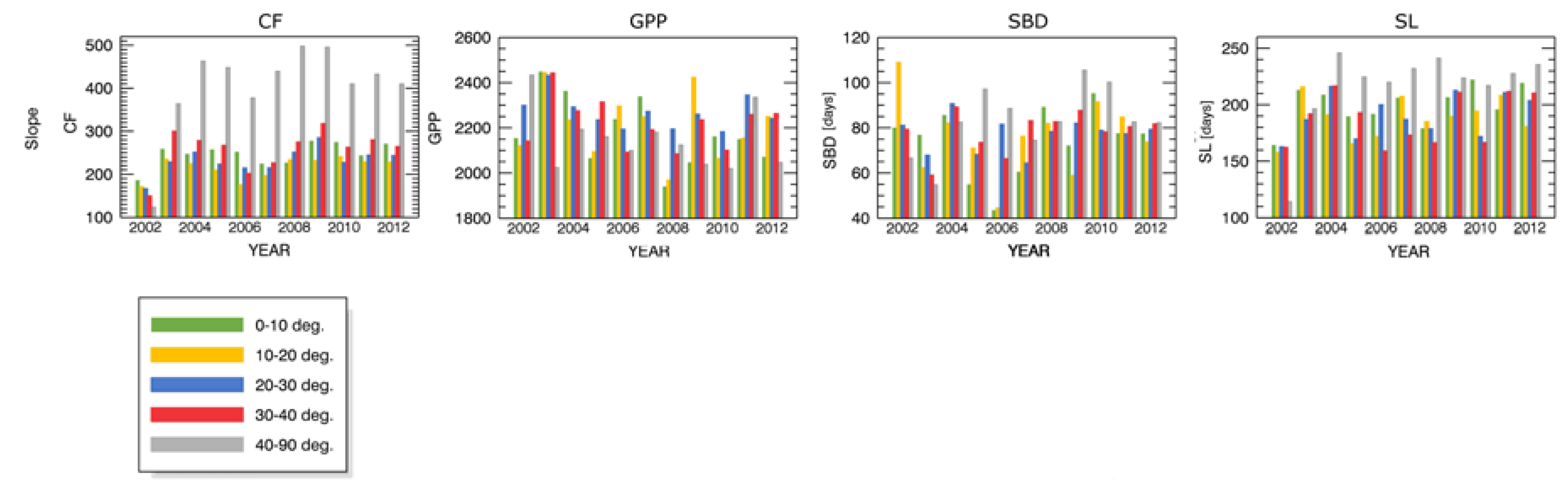
4.2.2. Changes in Foliage Photosynthetic Activity (3nNDVI)
4.3. Drought Impact under Different Biophysical Conditions
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Time | Time * Impact Class | Error | |||||
|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | |
| CF | 8.744 | 503.359 | 0.000 | 17.489 | 25.996 | 0.000 | 121,146.020 |
| GPP | 9.200 | 167.685 | 0.000 | 18.401 | 3.728 | 0.000 | 125,600.330 |
| SBD | 9.024 | 138.845 | 0.000 | 18.047 | 4.729 | 0.000 | 125,012.369 |
| SL | 9.351 | 125.195 | 0.000 | 18.702 | 3.434 | 0.000 | 129,545.465 |
| Drought Impact Class 1 | Drought Impact Class 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | Error df | df | F | p | Error df | |
| CF | 9.823 | 8.862 | 0.000 | 6100.107 | 10.0 | 4.048 | 0.000 | 6160.000 |
| GPP | 8.835 | 1.925 | 0.045 | 5486.281 | 10.0 | 2.390 | 0.008 | 6160.000 |
| SBD | 8.365 | 1.455 | 0.165 | 5194.880 | 10.0 | 0.980 | 0.458 | 6160.000 |
| SL | 9.616 | 2.413 | 0.008 | 5971.693 | 10.0 | 2.219 | 0.014 | 6160.000 |
Appendix A.1 Changes in Forest Canopy Water Content—4nNDII7 PC
Appendix B
| Time | Time * Impact Class | Error | |||||
|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | |
| CF | 8.892 | 724.295 | 0.000 | 17.784 | 63.582 | 0.000 | 214,367.891 |
| GPP | 9.279 | 241.944 | 0.000 | 18.558 | 48.716 | 0.000 | 223,701.831 |
| SBD | 9.021 | 189.351 | 0.000 | 18.042 | 22.824 | 0.000 | 217,481.749 |
| SL | 9.366 | 173.060 | 0.000 | 18.732 | 20.009 | 0.000 | 225,791.834 |
| Drought Impact Class 1 | Drought Impact Class 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | Error df | df | F | p | Error df | |
| CF | 8.486 | 38.342 | 0.000 | 9309.011 | 9.821 | 4.124 | 0.000 | 11,225.627 |
| GPP | 9.695 | 23.771 | 0.000 | 10635.826 | 9.891 | 0.604 | 0.810 | 11,305.458 |
| SBD | 8.335 | 15.525 | 0.000 | 9143.595 | 9.205 | 0.523 | 0.862 | 10,521.605 |
| SL | 9.252 | 14.318 | 0.000 | 10149.226 | 10.000 | 1.846 | 0.048 | 11,430.000 |
Appendix B.1 Changes in Forest Canopy Photosynthetic Activity—3nNDVI PC
References
- European Environment Agency. EEA Climate Change, Impacts and Vulnerability in Europe 2012; EEA: Copenhagen, Denmark, 2012. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Philipona, R.; Behrens, K.; Ruckstuhl, C. How declining aerosols and rising greenhouse gases forced rapid warming in Europe since 1980s. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Lloyd-Hughes, B.; Saunders, M.A. A drought climatology for Europe. Int. J. Climatol. 2002, 22, 1571–1592. [Google Scholar] [CrossRef]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar]
- Beniston, M. Exploring the behaviour of atmospheric temperatures under dry conditions in Europe: Evolution since the mid-20th century and projections for the end of the 21st century. Int. J. Climatol. 2013, 33, 457–462. [Google Scholar] [CrossRef]
- Auer, I.; Reinhard, B.; Jurkovic, A.; Lipa, W.; Orlik, A.; Potzmann, R.; Sch, W.; Ungersb, M.; Matulla, C.; Briffa, K.; et al. HISTALP—Historical instrumental climatological surface time series of the Greater Alpine Region. Int. J. Climatol. 2007, 27, 17–46. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Schmidli, J.; Schmutz, C.; Frei, C.; Wanner, H.; Schär, C. Mesoscale precipitation variability in the Alpine region during the 20th century. Int. J. Climatol. 2002, 22, 1049–1074. [Google Scholar] [CrossRef]
- Calanca, P. Climate change and drought occurrence in the Alpine region: How severe are becoming the extremes? Glob. Planet. Chang. 2007, 57, 151–160. [Google Scholar] [CrossRef]
- Theurillat, J.-P.; Guisan, A. Potential Impact of Climate Change on Vegetation in the European Alps: A Review. Clim. Chang. 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J. Veg. Sci. 2007, 18, 571–582. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Vittoz, P.; Rulence, B.; Largey, T.; Freléchoux, F. Effects of Climate and Land-Use Change on the Establishment and Growth of Cembran Pine (Pinus cembra L.) over the Altitudinal Treeline Ecotone in the Central Swiss Alps. Arct. Antarct. Alp. Res. 2008, 40, 225–232. [Google Scholar] [CrossRef]
- Minerbi, S.; Cescatti, A.; Cherubini, P. Scots Pine dieback in the Isarco Valley due to severe drought in the summer of 2003. For. Obs. 2006, 2, 89–143. [Google Scholar]
- Zimmermann, N.E.; Jandl, R.; Hanewinkel, M.; Kunstler, G.; Kölling, C.; Gasparini, P.; Breznikar, A.; Meier, E.S.; Normand, S.; Ulmer, U.; et al. Potential Future Ranges of Tree Species in the Alps. In Management Strategies to Adapt Alpine Space Forests to Climate Change Risks; Cerbu, G.A., Hanewinkel, M., Gerosa, G., Jandl, R., Eds.; InTech: Rijeka, Croatia, 2013; pp. 37–48. [Google Scholar]
- Courbaud, B.; Kunstler, G.; Morin, X. What is the future of the ecosystem services of the Alpine forest against a backdrop of climate change? J. Alp. Res. 2010, 98-4. [Google Scholar] [CrossRef]
- Kapos, V.; Iremonger, S. Achieving Global and Regional Perspectives on Forest Biodiversity and Conservation. In Proceedings of the Conference on Assessment of Biodiversity for Improved Forest Planning, Monte Verità, Switzerland, 7–11 October 1996; Bachmann, P., Köhl, M., Päivinen, R., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 3–13. [Google Scholar]
- Körner, C. Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Schoene, D.H.F.; Bernier, P.Y. Adapting forestry and forests to climate change: A challenge to change the paradigm. For. Policy Econ. 2012, 24, 12–19. [Google Scholar] [CrossRef]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an Inciting Mortality Factor in Scots Pine Stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Dobbertin, M.; Mayer, P.; Wohlgemuth, T.; Feldmeyer-Christe, E.; Graf, U.; Zimmermann, N.E.; Rigling, A. The Decline of Pinus sylvestris L. Forests in the Swiss Rhone Valley—A Result of Drought Stress? Phyton (B. Aires) 2005, 45, 153–156. [Google Scholar]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Arpaci, A.; Malowerschnig, B.; Sass, O.; Vacik, H. Using multi variate data mining techniques for estimating fire susceptibility of Tyrolean forests. Appl. Geogr. 2014, 53, 258–270. [Google Scholar] [CrossRef]
- Etzold, S.; Waldner, P.; Thimonier, A.; Schmitt, M.; Dobbertin, M. Tree growth in Swiss forests between 1995 and 2010 in relation to climate and stand conditions: Recent disturbances matter. For. Ecol. Manag. 2014, 311, 41–55. [Google Scholar] [CrossRef]
- Pichler, P.; Oberhuber, W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manag. 2007, 242, 688–699. [Google Scholar] [CrossRef]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth response of five co-occurring conifers to drought across a wide climatic gradient in Central Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- He, B.; Cui, X.; Wang, H.; Chen, A. Drought: The most important physical stress of terrestrial ecosystems. Acta Ecol. Sin. 2014, 34, 179–183. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, C.; Zhu, Q.; Chen, H.; Yu, G.; Li, W.; Zhou, X.; Wang, W.; Zhang, W. Regional drought-induced reduction in the biomass carbon sink of Canada’s boreal forests. Proc. Natl. Acad. Sci. USA 2012, 109, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Rigling, A.; Waldner, P.O.; Forster, T.; Bräker, O.U.; Pouttu, A. Ecological interpretation of tree-ring width and intraannual density fluctuations in Pinus sylvestris on dry sites in the central Alps and Siberia. Can. J. For. Res. 2001, 31, 18–31. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Motta, R.; Carrer, M. Summer climate variability over the last 250 years differently affected tree species radial growth in a mesic Fagus–Abies–Picea old-growth forest. For. Ecol. Manag. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Eilmann, B.; Weber, P.; Rigling, A.; Eckstein, D. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Coppola, A.; Leonelli, G.; Salvatore, M.C.; Pelfini, M.; Baroni, C. Weakening climatic signal since mid-20th century in European larch tree-ring chronologies at different altitudes from the Adamello-Presanella Massif (Italian Alps). Quat. Res. 2012, 77, 344–354. [Google Scholar] [CrossRef]
- Reichstein, M.; Ciais, P.; Papale, D.; Valentini, R.; Running, S.; Viovy, N.; Cramer, W.; Granier, A.; Ogée, J.; Allard, V.; et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modelling analysis. Glob. Chang. Biol. 2007, 13, 634–651. [Google Scholar] [CrossRef]
- Fagan, M.; DeFries, R. Measurement and Monitoring of the World’s Forests: A Review and Summary of Remote Sensing Technical Capability, 2009–2015. Resources for the Future, Washington DC. Available online: http://www.rff.org/Publications/Pages/PublicationDetails.aspx?PublicationID=20971 (accessed on 12 January 2018).
- Dobbertin, M.; Brang, P. Crown defoliation improves tree mortality models. For. Ecol. Manag. 2001, 141, 271–284. [Google Scholar] [CrossRef]
- De Beurs, K.; Townsend, P. Estimating the effect of gypsy moth defoliation using MODIS. Remote Sens. Environ. 2008, 112, 3983–3990. [Google Scholar] [CrossRef]
- Spruce, J.P.; Sader, S.; Ryan, R.E.; Smoot, J.; Kuper, P.; Ross, K.; Prados, D.; Russell, J.; Gasser, G.; McKellip, R. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens. Environ. 2011, 115, 427–437. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photograpic Infrared Linear combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Rahimzadeh Bajgiran, P.; Shimizu, Y.; Hosoi, F.; Omasa, K. MODIS vegetation and water indices for drought assessment in semi-arid ecosystems of Iran. J. Agric. Meteorol. 2009, 65, 349–355. [Google Scholar] [CrossRef]
- Van Wagtendonk, J.W.; Root, R.R.; Key, C.H. Comparison of AVIRIS and Landsat ETM+ detection capabilities for burn severity. Remote Sens. Environ. 2004, 92, 397–408. [Google Scholar] [CrossRef]
- Hayes, D.J.; Cohen, W.B.; Sader, S.A.; Irwin, D.E. Estimating proportional change in forest cover as a continuous variable from multi-year MODIS data. Remote Sens. Environ. 2008, 112, 735–749. [Google Scholar] [CrossRef]
- Caccamo, G.; Chisholm, L.A.; Bradstock, R.A.; Puotinen, M.L. Assessing the sensitivity of MODIS to monitor drought in high biomass ecosystems. Remote Sens. Environ. 2011, 115, 2626–2639. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Oskorbin, P.A.; Petrov, I.A.; Ranson, K.J. Siberian pine decline and mortality in southern siberian mountains. For. Ecol. Manag. 2013, 310, 312–320. [Google Scholar] [CrossRef]
- Ivits, E.; Cherlet, M.; Mehl, W.; Sommer, S. Ecosystem functional units characterized by satellite observed phenology and productivity gradients: A case study for Europe. Ecol. Indic. 2013, 27, 17–28. [Google Scholar] [CrossRef]
- Reed, B.C.; Brown, J.F.; VanderZee, D.; Loveland, T.R.; Merchant, J.W.; Ohlen, D.O. Measuring phenological variability from satellite imagery. J. Veg. Sci. 1994, 5, 703–714. [Google Scholar] [CrossRef]
- Jönsson, P.; Eklundh, L. TIMESAT—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Report to the Standing Forestry Committee Ad Hoc Working Group III on “Climate Change and Forestry”, November 2010. European Commission: Brussels. Available online: https://ec.europa.eu/agriculture/sites/agriculture/files/fore/publi/wg3-112010_en.pdf (accessed on 12 January 2018).
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Wells, N.; Steve, G.; Hayes, M.J. A Self-Calibrating Palmer Drought Severity Index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- Preisendorfer, R.W. Principal Component Analysis in Meteorology and Oceanography; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- South Tyrol in Figures; Report No. 8; Benvenuto, O.; Gobbi, G. (Eds.) Provincial Statistics Institute of Autonomous Province of South Tyrol: Bolzano, Italy, 2012. [Google Scholar]
- Provincia Autonomica di Bolzano. Tipologie Forestali dell’Alto Adige Volume 1; Provincia Autonomica di Bolzano—Alto Adige: Bolzano, Italy, 2010; Volume 1. [Google Scholar]
- Büntgen, U.; Trouet, V.; Frank, D.; Leuschner, H.H.; Friedrichs, D.; Luterbacher, J.; Esper, J. Tree-ring indicators of German summer drought over the last millennium. Quat. Sci. Rev. 2010, 29, 1005–1016. [Google Scholar] [CrossRef]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schröder, C.; Wilmking, M. Drought matters—Declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Gillner, S.; Vogt, J.; Roloff, A. Climatic response and impacts of drought on oaks at urban and forest sites. Urban For. Urban Green. 2013, 12, 597–605. [Google Scholar] [CrossRef]
- Wells, N. PDSI; National Agricultural Decision Support System, University of Nebraska-Lincoln: Lincoln, NE, USA, 2003. [Google Scholar]
- Lewińska, K.; Ivits, E.; Schardt, M.; Zebisch, M. Alpine Forest Drought Monitoring in South Tyrol: PCA Based Synergy between scPDSI Data and MODIS Derived NDVI and NDII7 Time Series. Remote Sens. 2016, 8, 639. [Google Scholar] [CrossRef]
- Colditz, R.R.; Conrad, C.; Wehrmann, T.; Schmidt, M.; Dech, S. TiSeG: A Flexible Software Tool for Time-Series Generation of MODIS Data Utilizing the Quality Assessment Science Data Set. IEEE Trans. Geosci. Remote Sens. 2008, 46, 3296–3308. [Google Scholar] [CrossRef]
- Udelhoven, T. TimeStats: A Software Tool for the Retrieval of Temporal Patterns from Global Satellite Archives. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2011, 4, 310–317. [Google Scholar] [CrossRef]
- Wang, D.; Morton, D.; Masek, J.; Wu, A.; Nagol, J.; Xiong, X.; Levy, R.; Vermote, E.; Wolfe, R. Impact of sensor degradation on the MODIS NDVI time series. Remote Sens. Environ. 2012, 119, 55–61. [Google Scholar] [CrossRef]
- Ivits, E.; Cherlet, M.; Horion, S.; Fensholt, R. Global biogeographical pattern of ecosystem functional types derived from earth observation data. Remote Sens. 2013, 5, 3305–3330. [Google Scholar] [CrossRef]
- geoland2 Technical Note on HR Forest Layer Product Specification, Issue 1.4. Publication of the FP7 geoland2 project 2012.
- European Environment Agency (EEA). Corine Land Cover 2006; European Environment Agency: Kobenhavn, Denmark, 2010; Available online: https://www.eea.europa.eu/data-and-maps/data/clc-2006-vector-4 (accessed on 12 January 2018).
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop 10.1 Beta 1, 2011.
- Venegas, S.A. Statistical Methods for Signal Detection in Climate; Danish Center for Earth System Science (DCESS): Copenhagen, Denmark, 2001. [Google Scholar]
- Ivits, E.; Horion, S.; Fensholt, R.; Cherlet, M. Drought footprint on European ecosystems between 1999 and 2010 assessed by remotely sensed vegetation phenology and productivity. Glob. Chang. Biol. 2014, 20, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Vacchiano, G.; Garbarion, M.; Borgogno Mondino, E.; Renzo, M. Evidences of drought stress as a predisposing factor to Scots pine decline in Valle d’Aosta. Eur. J. For. Res. 2012, 131, 989–1000. [Google Scholar] [CrossRef]
- Vanoni, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Drought and frost contribute to abrupt growth decreases before tree mortality in nine temperate tree species. For. Ecol. Manag. 2016, 382, 51–63. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Jolly, W.M.; Dobbertin, M.; Zimmermann, N.E.; Reichstein, M. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Gutsch, M.; Lasch-Born, P.; Suckow, F.; Reyer, C.P.O. Evaluating the productivity of four main tree species in Germany under climate change with static reduced models. Ann. For. Sci. 2016, 73, 401–410. [Google Scholar] [CrossRef]
- Primicia, I.; Camarero, J.J.; Janda, P.; Čada, V.; Morrissey, R.C.; Trotsiuk, V.; Bače, R.; Teodosiu, M.; Svoboda, M. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Scherrer, D.; Bader, M.K.-F.; Körner, C. Drought-sensitivity ranking of deciduous tree species based on thermal imaging of forest canopies. Agric. For. Meteorol. 2011, 151, 1632–1640. [Google Scholar] [CrossRef]
- Feichtinger, L.M.; Eilmann, B.; Buchmann, N.; Rigling, A. Growth adjustments of conifers to drought and to century-long irrigation. For. Ecol. Manag. 2014, 334, 96–105. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2014, 111, 91–113. [Google Scholar] [CrossRef]
- Gruber, A.; Strobl, S.; Veit, B.; Oberhuber, W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010, 30, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Hartl-Meier, C.; Dittmar, C.; Zang, C.; Rothe, A. Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 2014, 28, 819–829. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Gebetsroither, E.; Züger, J.; Loibl, W. Drought in Alpine Areas under Changing Climate Conditions. In Management Strategies to Adapt Alpine Space Forests to Climate Change Risks; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Gebetsroither, E.; Züger, J.; Schmatz, D.; Psomas, A. Future Climate of the European Alps. In Management Strategies to Adapt Alpine Space Forests to Climate Change Risks; Zimmermann, N.E., Ed.; InTech: Rijeka, Croatia, 2013; pp. 27–36. [Google Scholar]
- Studer, S.; Appenzeller, C.; Defila, C. Inter-Annual Variability and Decadal Trends in Alpine Spring Phenology: A Multivariate Analysis Approach. Clim. Chang. 2005, 73, 395–414. [Google Scholar] [CrossRef]
- Studer, S.; Stöckli, R.; Appenzeller, C.; Vidale, P.L. A comparative study of satellite and ground-based phenology. Int. J. Biometeorol. 2007, 51, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Swidrak, I.; Schuster, R.; Oberhuber, W. Comparing growth phenology of co-occurring deciduous and evergreen conifers exposed to drought. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 609–617. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gobiet, A.; Kotlarski, S.; Beniston, M.; Heinrich, G.; Rajczak, J.; Stoffel, M. 21st century climate change in the European Alps—A review. Sci. Total Environ. 2014, 493, 1138–1151. [Google Scholar] [CrossRef] [PubMed]


| nNDVI | nNDII7 | |||||||
|---|---|---|---|---|---|---|---|---|
| 1nNDVI | 2nNDVI | 3nNDVI | 4nNDVI | 1nNDII7 | 2nNDII7 | 3nNDII7 | 4nNDII7 | |
| 1scPDSI | −0.573 | 0.713 * | −0.310 * | −0.374 | −0.717 * | −0.374 | −0.189 | −0.608 * |
| 2scPDSI | −0.321 | 0.608 * | −0.360 * | −0.349 | −0.199 * | −0.278 | −0.260 | −0.502 * |
| 3scPDSI | −0.155 | −0.337 ** | −0.632 * | −0.160 | −0.559 * | −0.257 | −0.131 | −0.023 * |
| 4scPDSI | −0.489 | −0.013 ** | −0.257 * | −0.261 | −0.010 * | −0.243 | −0.288 | −0.583 * |
| 4nNDII7 | 3nNDVI | |||
|---|---|---|---|---|
| Drought Impact | Drought Impact | |||
| [%] | Class 1 | Class 3 | Class 1 | Class 3 |
| Coniferous | 91.04 | 95.38 | 76.10 | 96.85 |
| Broadleaved | 1.88 * | 0.58 * | 3.65 * | 0.33 * |
| Mixed | 7.08 * | 4.05 * | 20.25 | 2.82 * |
| 0–700 m a.s.l. | 1.88 * | 3.90 * | 13.11 | 0.50 * |
| 700–1400 m a.s.l. | 33.38 | 54.77 | 63.24 | 31.29 |
| 1400–2100 m a.s.l. | 64.74 | 33.96 | 20.66 | 68.13 |
| 2100–2500m a.s.l. | - | 7.37 | 2.99 * | 0.50 * |
| N | 25.29 | 27.17 | 4.65 | 62.07 |
| E | 17.63 | 18.79 | 30.71 | 11.78 |
| S | 27.75 | 34.54 | 49.21 | 5.15 |
| W | 29.34 | 19.51 | 15.44 | 21.00 |
| 0–10 deg. | 18.50 | 3.76 | 14.27 | 3.90 |
| 10–20 deg. | 34.54 | 20.38 | 36.43 | 20.08 |
| 20–30 deg. | 36.56 | 51.01 | 40.91 | 56.85 |
| 30–40 deg | 10.26 | 23.70 | 7.97 | 18.67 |
| 40–90 deg. | 0.14 * | 1.16 * | 0.41 * | 0.50 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewińska, K.E.; Ivits, E.; Schardt, M.; Zebisch, M. Drought Impact on Phenology and Green Biomass Production of Alpine Mountain Forest—Case Study of South Tyrol 2001–2012 Inspected with MODIS Time Series. Forests 2018, 9, 91. https://doi.org/10.3390/f9020091
Lewińska KE, Ivits E, Schardt M, Zebisch M. Drought Impact on Phenology and Green Biomass Production of Alpine Mountain Forest—Case Study of South Tyrol 2001–2012 Inspected with MODIS Time Series. Forests. 2018; 9(2):91. https://doi.org/10.3390/f9020091
Chicago/Turabian StyleLewińska, Katarzyna Ewa, Eva Ivits, Mathias Schardt, and Marc Zebisch. 2018. "Drought Impact on Phenology and Green Biomass Production of Alpine Mountain Forest—Case Study of South Tyrol 2001–2012 Inspected with MODIS Time Series" Forests 9, no. 2: 91. https://doi.org/10.3390/f9020091