Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range
Abstract
:1. Introduction
- How do oaks perform in regard to their general growth, both within and beyond their natural range, over a long-term basis?
- Has the oak growth revealed a change in growth dynamic during the last decades?
- Are there site-specific differences in the growth reaction of oaks to drought events, particularly in terms of resistance and resilience?
2. Materials and Methods
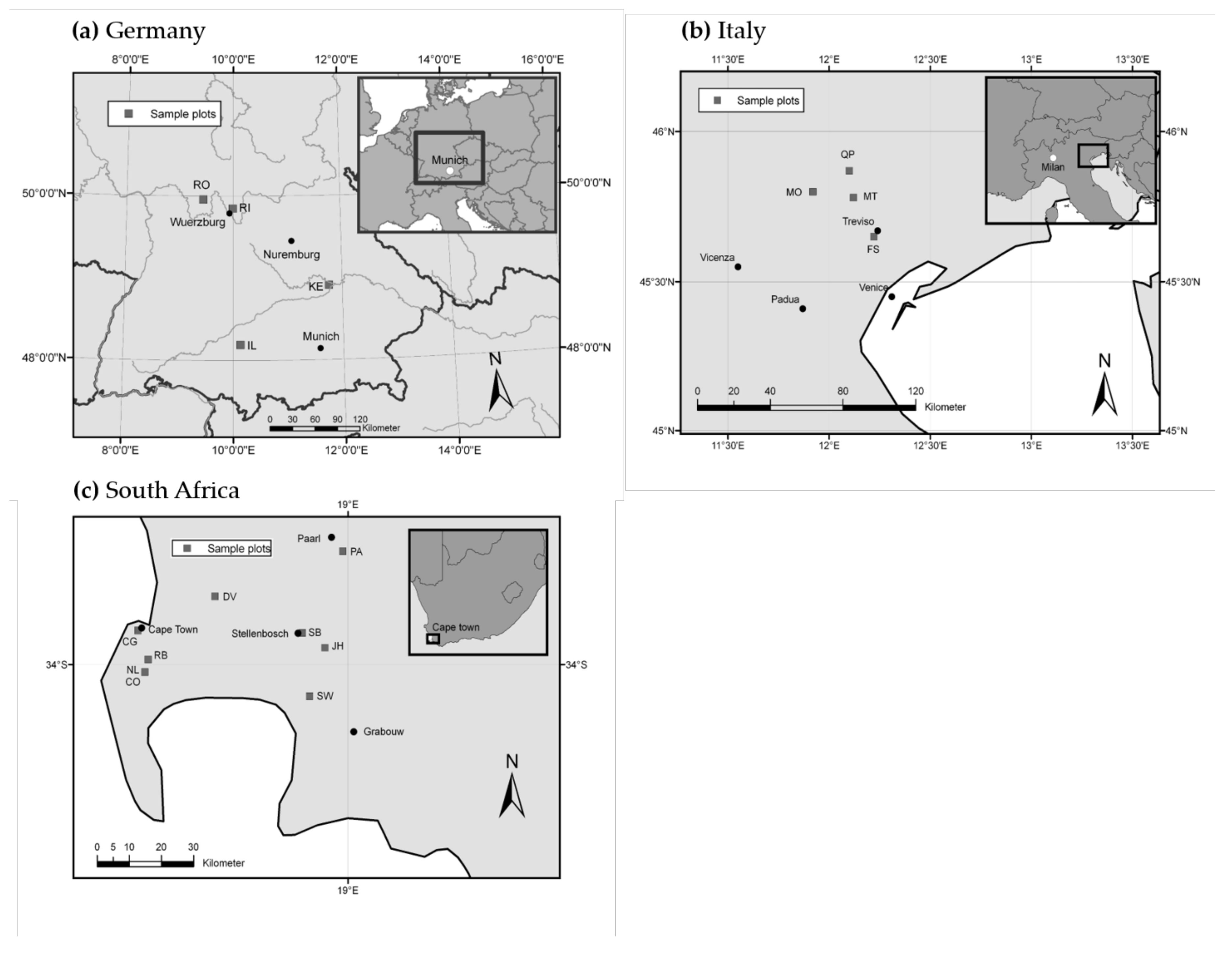
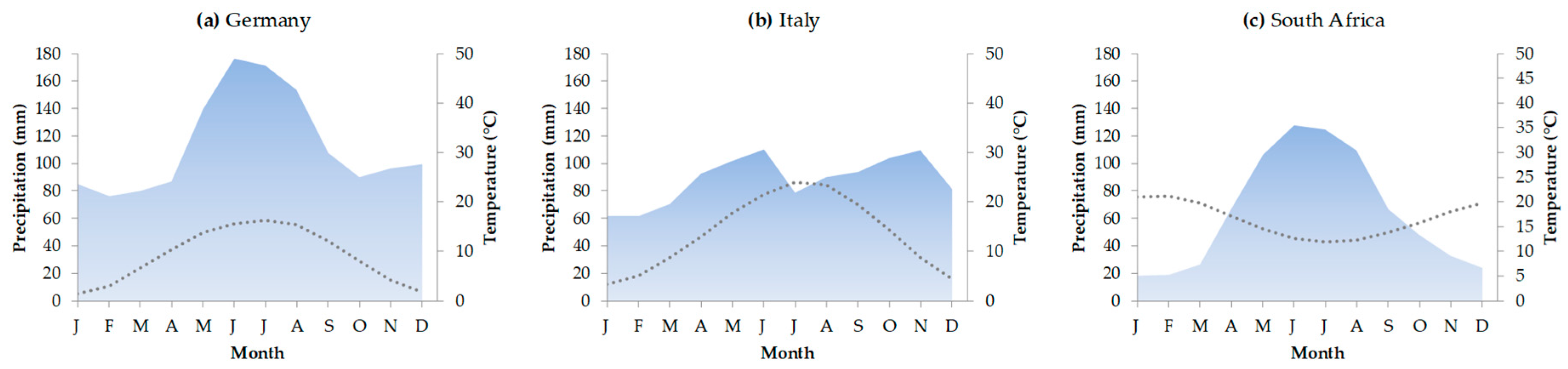
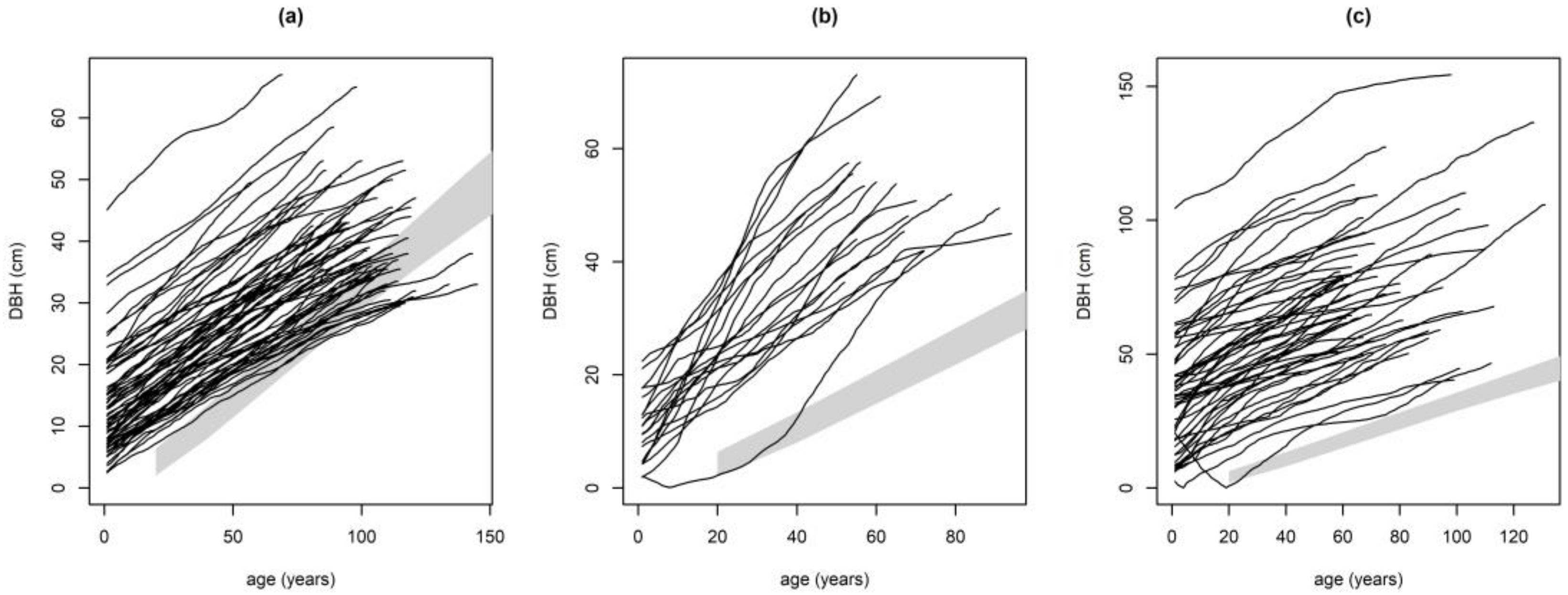
2.1. Study Areas and Climatic Conditions
2.2. Tree-Ring Data
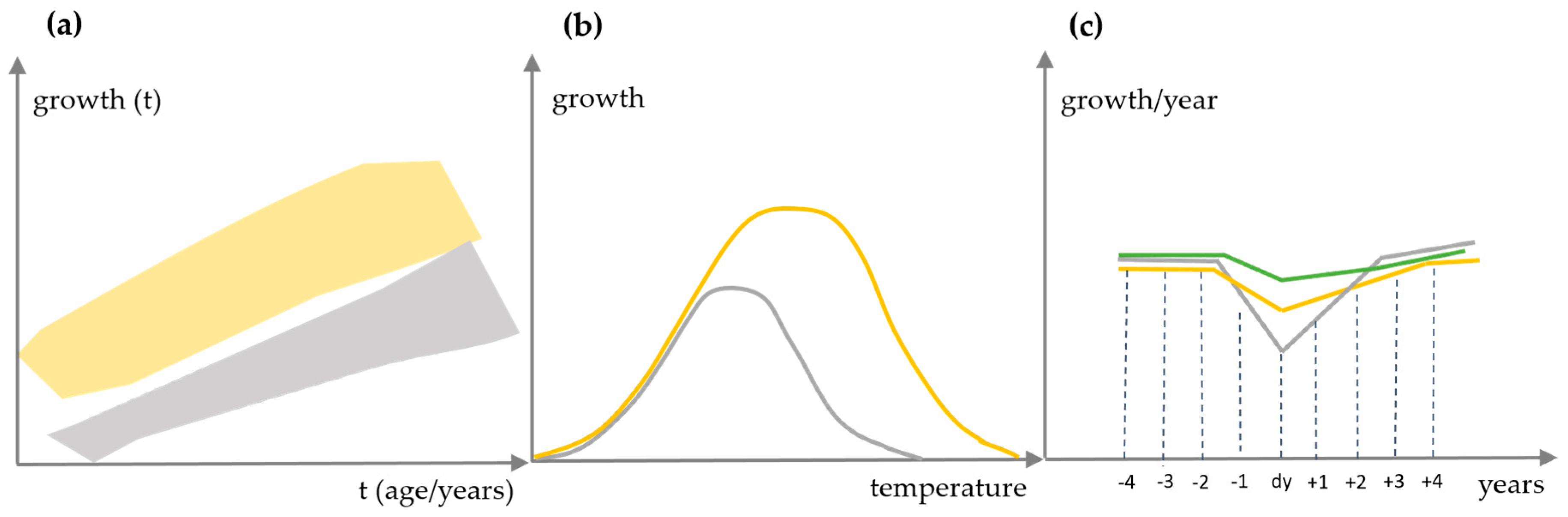
2.3. Evaluation of Long-Term Growth Trends
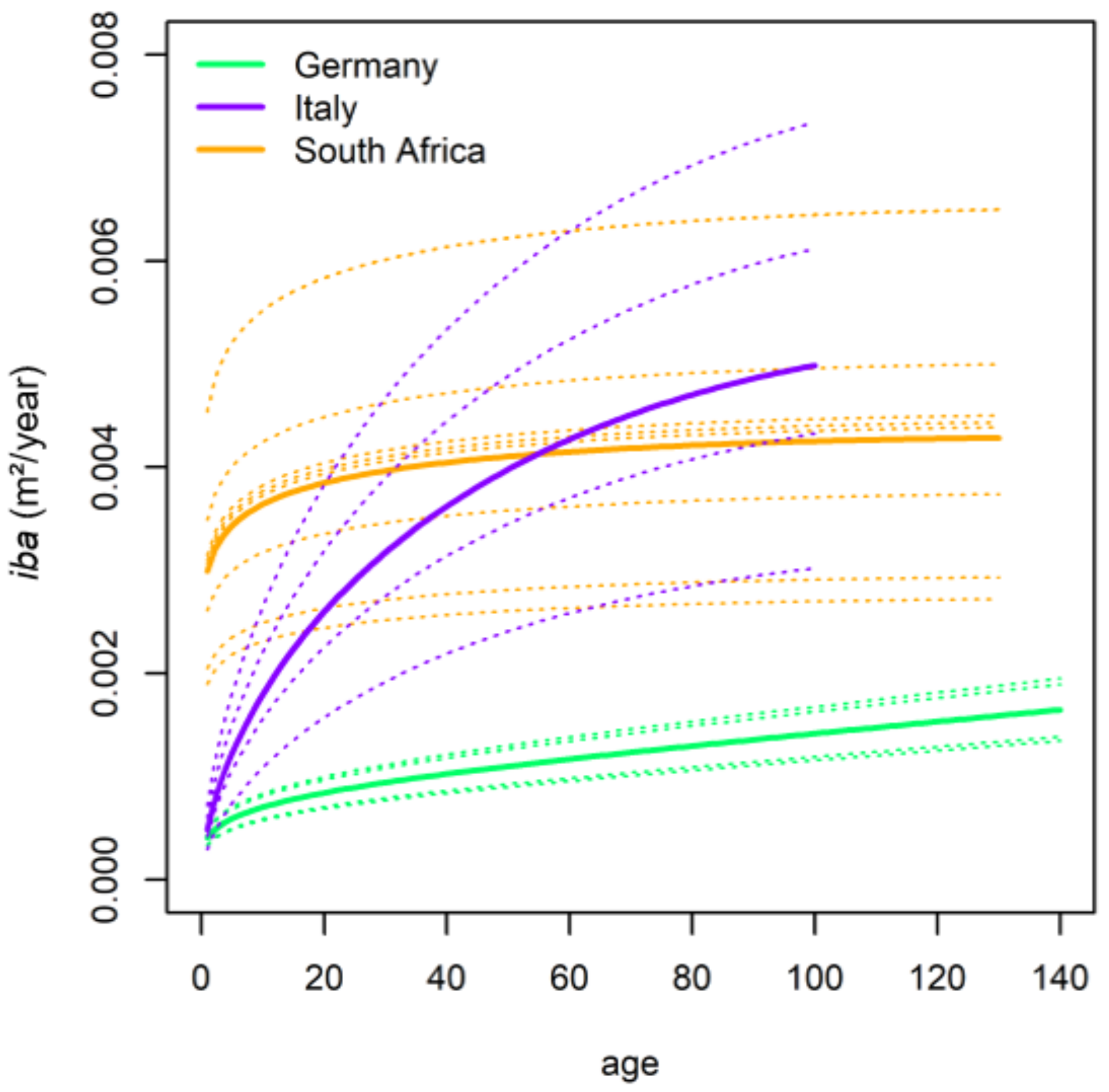
2.3.1. Performance of Growth Level within and beyond the Natural Range
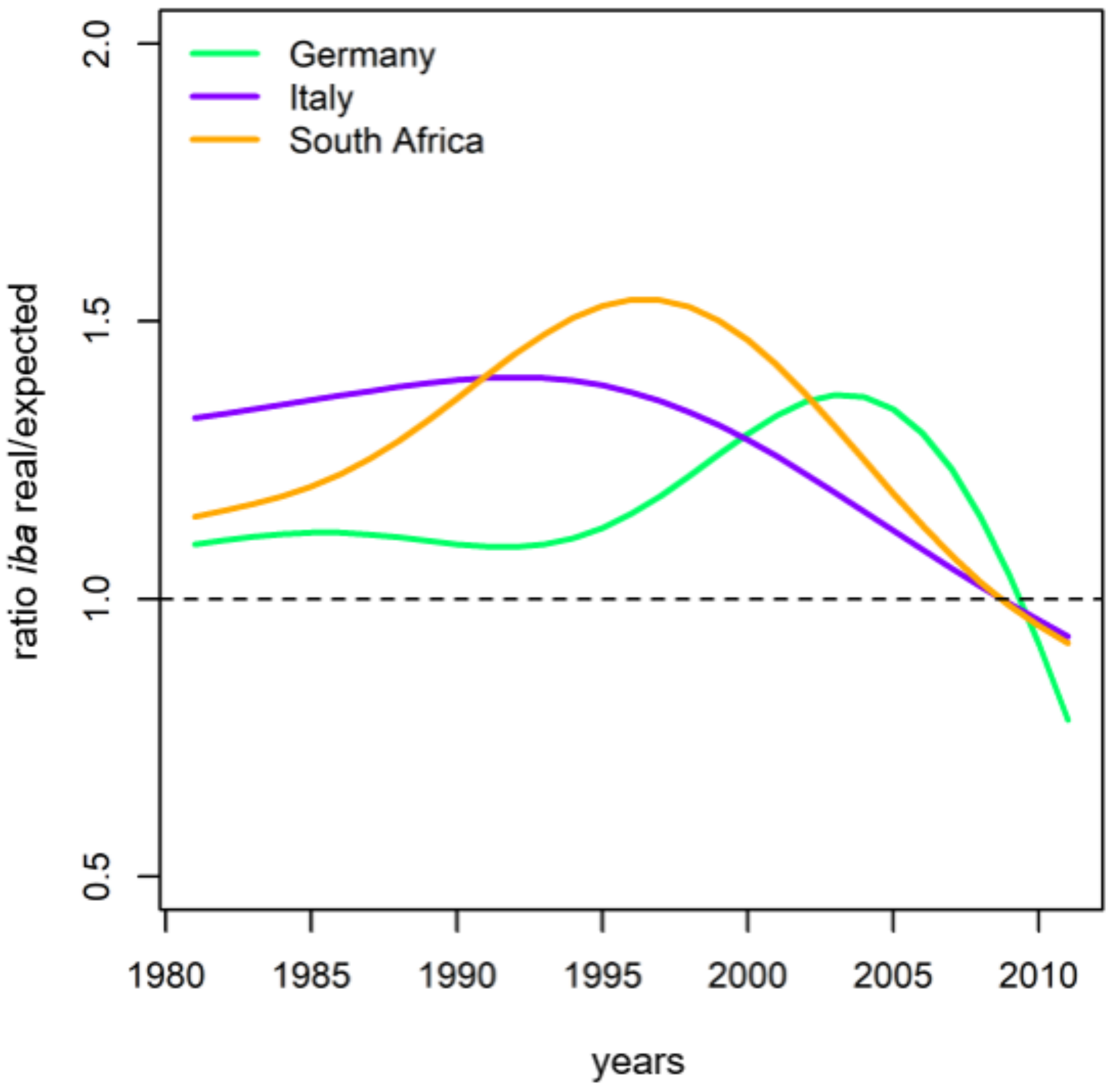
2.3.2. Long-Term Growth Behaviour and Recent Growth Trends
2.3.3. Change of Size-Growth-Relation During the Last Decades
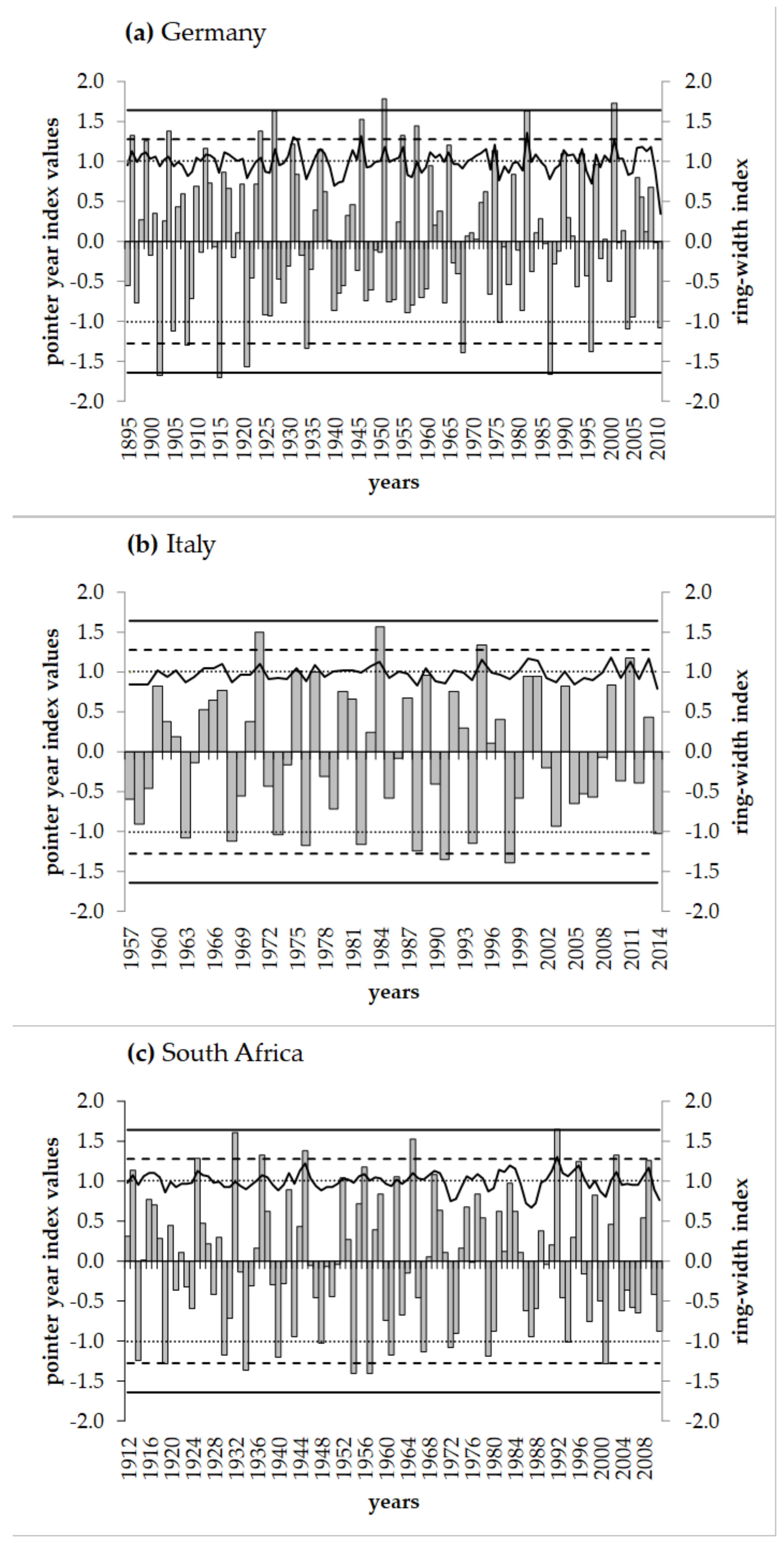
2.4. Reactions of Oak Growth on Drought Events
3. Results
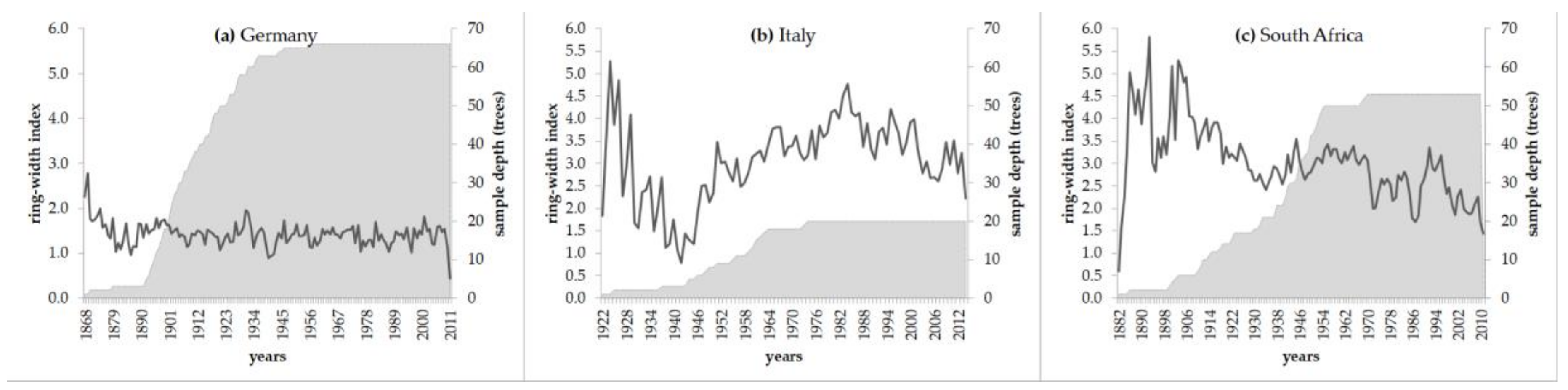
3.1. Tree-Ring Data and Their Basic Statistics
3.2. Evaluation of Long-Term Growth Trends
Performance of Growth Level within and beyond the Natural Range
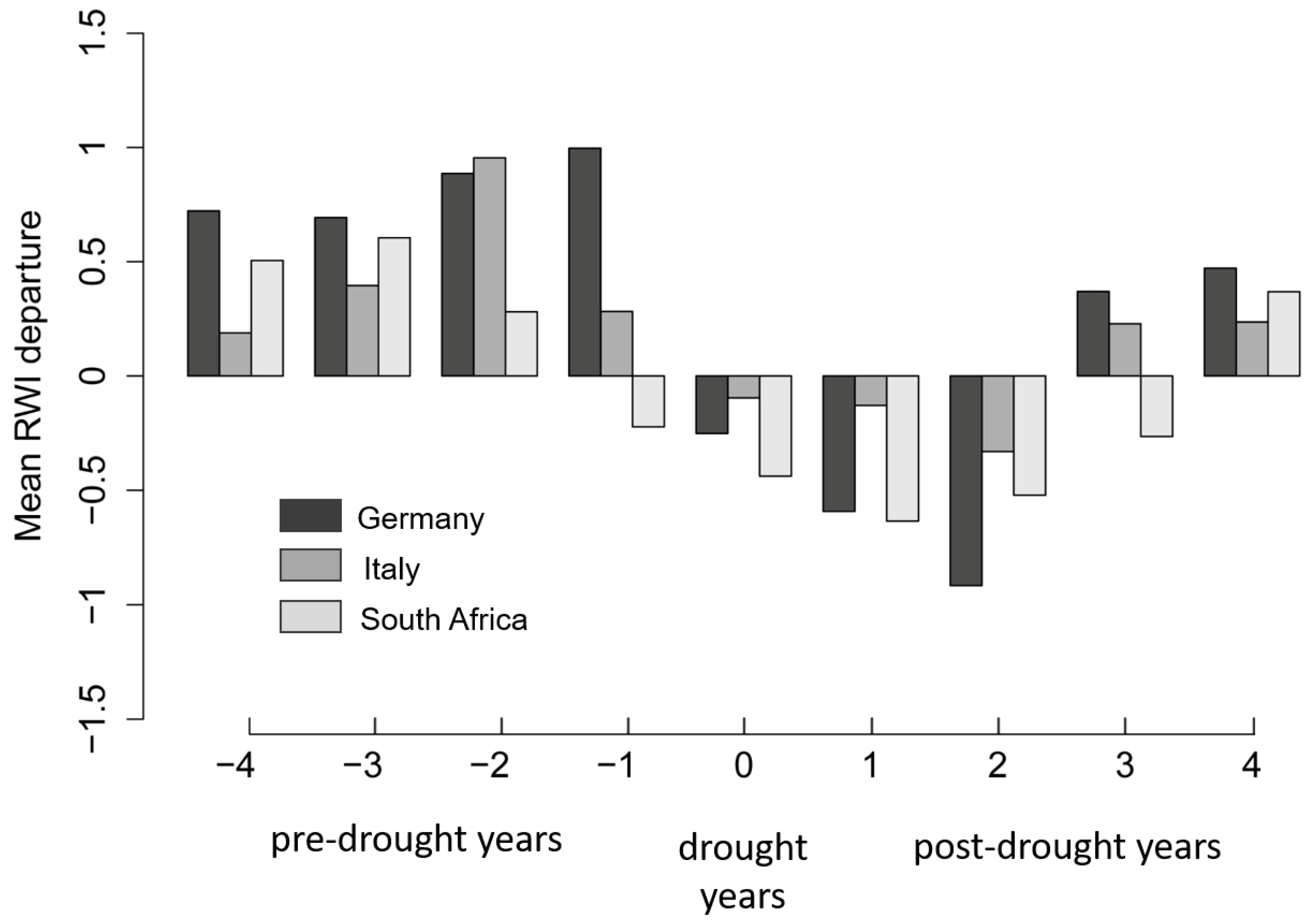
3.3. Reactions of Oak Growth on Drought Events
4. Discussion
4.1. General Growth Level and Growth Trends
4.2. Reactions of Oak Growth on Drought Events/Periods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Fixed effects | Parameter | Estimate | Std. Error | p | |
|---|---|---|---|---|---|
| −6.8817 | 0.5538 | 0.0000 | |||
| 0.1465 | 0.0286 | 0.0000 | |||
| 0.0087 | 0.0074 | 0.0000 | |||
| Random effects | Parameter | Std. Dev. | |||
| 0.4535 | |||||
| 0.3690 | |||||
| 0.9399 | |||||
| 0.0415 | |||||
| 0.0127 | |||||
| 0.3912 | |||||
| Estimated country-Specific Values | |||||
| Parameter | Germany | Italy | South Africa | ||
| −0.7810 | −0.2355 | 1.0166 | |||
| 0.0187 | 0.0220 | −0.0408 | |||
| −0.0050 | 0.0143 | −0.0093 | |||
| Germany | |||||
| Fixed effects | Parameter | Estimate | Std. Error | p | |
| −5.6240 | 0.1466 | 0.0000 | |||
| 0.4170 | 0.0408 | 0.0000 | |||
| −0.3183 | 0.0664 | 0.0000 | |||
| −0.1127 | 0.0288 | 0.0000 | |||
| Random effects | Level | Parameter | Std. Dev. | ||
| Plot | 1.1782 | ||||
| Plot | 9.1077 | ||||
| Tree in plot | 1.0198 | ||||
| Tree in plot | 0.3064 | ||||
| Residual | 0.3405 | ||||
| Italy | |||||
| Fixed effects | Parameter | Estimate | Std. Error | p | |
| −3.8436 | 0.2453 | 0.0000 | |||
| 0.6911 | 0.0369 | 0.0000 | |||
| −1.1793 | 0.1384 | 0.0000 | |||
| −0.4106 | 0.0495 | 0.0000 | |||
| Random effects | Level | Parameter | Std. Dev. | ||
| Plot | 0.4091 | ||||
| Plot | 5.4971 | ||||
| Tree in plot | 0.2442 | ||||
| Tree in plot | 0.3220 | ||||
| Residual | 0.4468 | ||||
| South Africa | |||||
| Fixed effects | Parameter | Estimate | Std. Error | p | |
| −5.0762 | 0.0710 | 0.0000 | |||
| 0.3033 | 0.0219 | 0.0000 | |||
| −0.3774 | 0.0330 | 0.0000 | |||
| −0.2556 | 0.0241 | 0.0000 | |||
| Random effects | Level | Parameter | Std. Dev. | ||
| Plot | 0.3023 | ||||
| Plot | 3.5391 | ||||
| Tree in plot | 0.3023 | ||||
| Tree in plot | 0.0217 | ||||
| Residual | 0.5122 | ||||
| Fixed effects | Parameter | Estimate | Std. Error | p | |
|---|---|---|---|---|---|
| 1.1494 | 0.1914 | 0.0000 | |||
| 0.1114 | 0.2961 | 0.7070 | |||
| 0.1309 | 0.2507 | 0.6020 | |||
| Non-parametric smoother | p | ||||
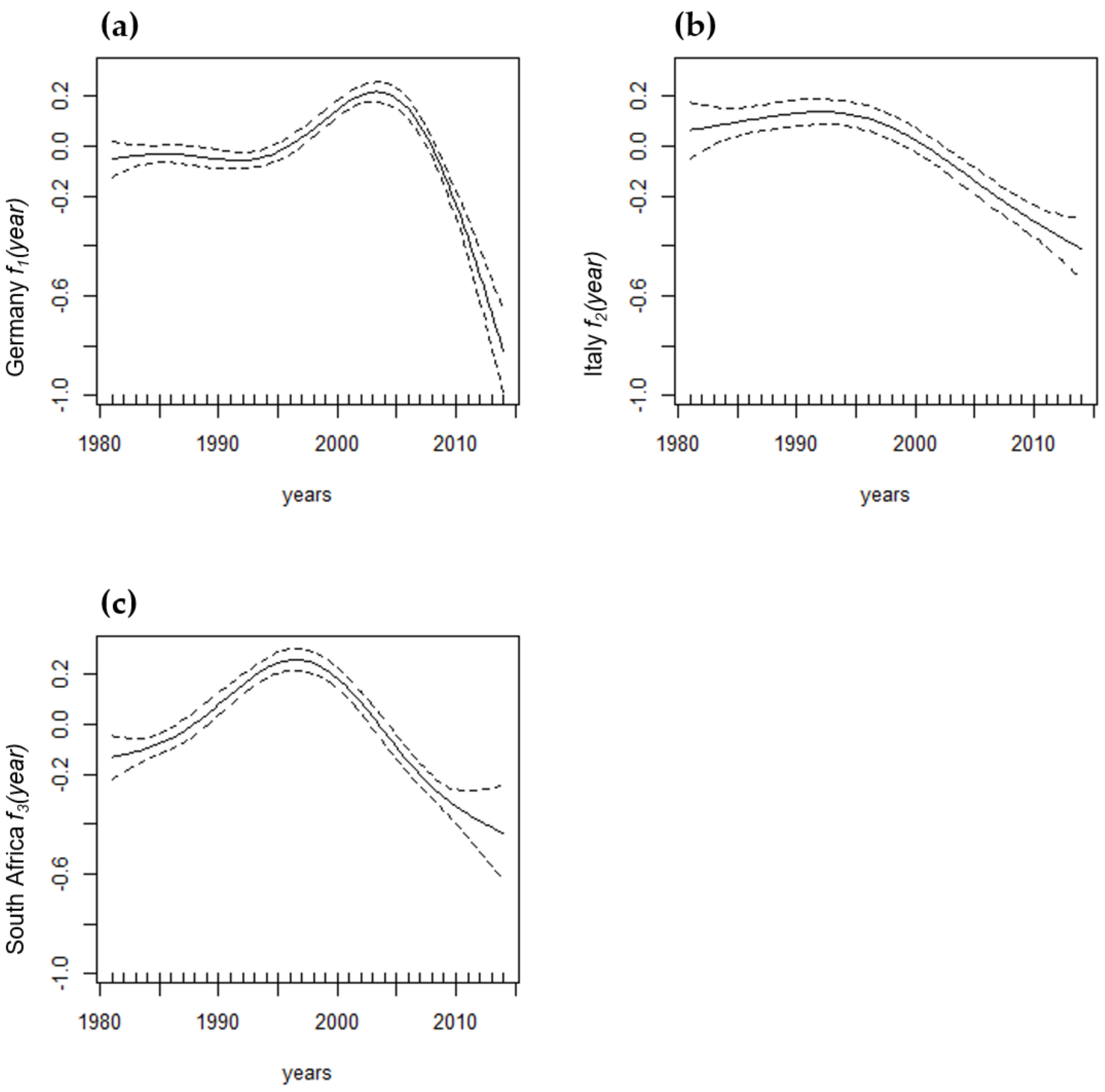
| f1(year) see Figure A1 | 0.0000 | ||||
| f2(year) see Figure A1 | 0.0000 | ||||
| f3(year) see Figure A1 | 0.0000 | ||||
| Random effects | Parameter | Std. Dev. | |||
| 0.3508 | |||||
| 0.6122 | |||||
| 0.4764 | |||||
| Site | Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −4 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | |
| Pre-Drought Years | Drought Years | Post-Drought Years | |||||||
| Germany | |||||||||
| IL | 0.55629803 | −0.03131591 | 0.91157843 | 0.48650042 | 0.37747352 | −0.40987714 | −0.34604707 | 0.3198875 | 0.35851158 |
| KE | 0.23774573 | 0.40799534 | 0.07888110 | 0.69931780 | −0.36785892 | −0.15835306 | −0.81287509 | 0.77624272 | 1.0969131 |
| RI | 0.47632093 | 0.69020222 | 0.75826215 | 1.0784091 | −0.66902923 | −0.89093999 | −0.76053921 | −0.03171566 | 0.00048237 |
| RO | 1.05106777 | 0.69237553 | 0.93242868 | 0.64102589 | −0.00791415 | −0.29594034 | −0.85371995 | 0.24127894 | −0.09626109 |
| Italy | |||||||||
| FS | 0.17592635 | 0.47263100 | 0.66785080 | −0.09426894 | 0.26321477 | 0.35170359 | −0.29685685 | 0.43268679 | 0.49510446 |
| MT | −0.09948952 | −0.43667626 | 0.37670988 | 0.35216058 | −0.07425614 | −0.06914289 | −0.17069694 | −0.43803122 | −0.41282043 |
| MO | 0.36724712 | 1.4248831 | 1.29156969 | −0.06582161 | −0.17323267 | −0.84175735 | −0.61972081 | 0.68742789 | −0.19081160 |
| QP | −0.1130041 | −0.3646164 | 0.9829210 | 0.5270011 | −1.0304076 | −0.4681115 | −0.3114193 | −0.1379355 | 0.5459765 |
| South Africa | |||||||||
| RB | −0.15392203 | −0.19508340 | 1.09611125 | −0.02774566 | −0.25770315 | −1.04564693 | 0.626315598 | −0.001743126 | −0.307411933 |
| SW | 0.09559793 | 0.81468107 | −0.01210531 | −0.09462245 | −0.21608670 | −0.36220396 | −0.58564310 | −0.41582023 | 0.42060171 |
| JH | 0.62226984 | 0.04366845 | 0.06405663 | −0.15354578 | −0.16597562 | −0.52549416 | −0.38744865 | 0.07184230 | 0.26197139 |
| PA | 0.67328311 | 0.56504161 | −0.42335109 | −0.48853342 | −1.0279399 | −0.92447381 | −0.00649988 | 0.70648546 | 0.03965515 |
| SB | 0.49508870 | 0.27462479 | 0.78459160 | −0.46170574 | 0.03104207 | −0.57616940 | −0.86129925 | −0.26494444 | −0.03688565 |
| DV | −0.11743943 | 0.96822879 | 0.00946096 | −0.08080230 | −1.0062275 | −0.20950409 | −0.27623300 | −0.31475344 | 0.03968543 |
| CG | 0.72068534 | 0.78664249 | 0.40765661 | −0.13206849 | −0.79011497 | −0.81164017 | −0.07628534 | −0.14777659 | 0.38371760 |
| NL | 0.3090942 | 11.867.157 | −0.5120751 | −0.4253017 | −0.3917846 | −0.5892783 | −0.4236440 | 0.1466858 | 0.5765544 |
| CO | 0.4886513 | 0.0296665 | 0.2186459 | 0.1438576 | −0.2926300 | −0.2974016 | −0.4423230 | −0.6742106 | 0.2710374 |
References
- Koprowski, M. Spatial distribution of introduced Norway spruce growth in lowland Poland: The influence of changing climate and extreme weather events. Quat. Int. 2013, 283, 139–146. [Google Scholar] [CrossRef]
- Goldblum, D. The geography of white oak’s (Quercus alba L.) response to climatic variables in North America and speculation on its sensitivity to climate change across its range. Dendrochronologia 2010, 28, 73–83. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Nahm, M.; Radoglou, K.; Rennenberg, H.; Halyvopoulos, G.; Matzarakis, A. Seasonal and interannual ecophysiological responses of beech (Fagus sylvatica) at its south-eastern distribution limit in Europe. For. Ecol. Manage. 2009, 257, 1157–1164. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12. [Google Scholar] [CrossRef]
- Büntgen, U.; Trouet, V.; Frank, D.; Leuschner, H.H.; Friedrichs, D.; Luterbacher, J.; Esper, J. Tree-ring indicators of German summer drought over the last millennium. Quat. Sci. Rev. 2010, 29, 1005–1016. [Google Scholar] [CrossRef]
- Körner, C. Ökologie. In Strasburger Lehrbuch für Botanik; Spektrum Akademischer Verlag: Heidelberg, Germany, 2002; pp. 886–1043. ISBN 9783827410108. (In German) [Google Scholar]
- Gundersen, P.; Boxman, A.W.; Lamersdorf, N.; Moldan, F.; Andersen, B.R. Experimental manipulation of forest ecosystems: Lessons from large roof experiments. For. Ecol. Manage. 1998, 101, 339–352. [Google Scholar] [CrossRef]
- Pretzsch, H.; Rötzer, T.; Matyssek, R.; Grams, T.E.E.; Häberle, K.H.; Pritsch, K.; Kerner, R.; Munch, J.C. Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: From reaction pattern to mechanism. Trees 2014, 28, 1305–1321. [Google Scholar] [CrossRef]
- Rothe, A.; Mellert, K.H. Effects of forest management on nitrate concentrations in seepage water of forests in southern Bavaria, Germany. Water Air Soil Pollut. 2004, 156, 337–355. [Google Scholar] [CrossRef]
- Nixon, K.C. Global and neotropical distribution and diversity of oak (genus Quercus) and oak forests. In Ecology and Conservation of Neotropical Montane Oak Forests; Kappelle, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–13. ISBN 978-3-540-28909-8. [Google Scholar]
- Scotti-Saintagne, C.; Mariette, S.; Porth, I.; Goicoechea, P.G.; Barreneche, T.; Bodénès, C.; Burg, K.; Kremer, A. Genome scanning for interspecific differentiation between two closely related oak species [Quercus robur L. and Q. petraea (Matt.) Liebl.]. Genetics 2004, 168, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Mariette, S.; Cottrell, J.; Csaikl, U.M.; Goikoechea, P.; König, A.; Lowe, A.J.; van Dam, B.C.; Barreneche, T.; Bodénès, C.; Streiff, R.; et al. Comparison of levels of genetic diversity detected with AFLP and microsatellite markers within and among mixed Q. petraea (MATT.) LIEBL. and Q. robur L. stands. Silvae Genet. 2002, 51, 72–79. [Google Scholar]
- Roloff, A.; Bärtels, A. Flora der Gehölze, 4th ed.; Ulmer, Eugen: Stuttgart, Germany, 2014; ISBN 978-3-8001-8246-6. (In German) [Google Scholar]
- Kleinschmitt, J.R.G.; Roloff, A. Comparison of morphological and genetic traits of pedunculate oak (Q. robur L.) and sessile oak (Q. petraea (Matt.) Liebl.). Silvae Genet. 1995, 44, 256–269. [Google Scholar]
- Poynton, R.J. Tree Planting in Southern Africa; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2009.
- Leuschner, C. Mechanismen der Konkurrenzüberlegenheit der Rotbuche; Ber. Reinh-Tüxen-Ges.: Hannover, Germany, 1998. (In German) [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen: In ökologischer, dynamischer und historischer Sicht, 6th ed.; Ulmer, Eugen: Stuttgart, Germany, 2010; ISBN 978-3825281045. [Google Scholar]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant. Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Schütze, G.; Bielak, K. Changes of forest stand dynamics in Europe. Facts from long-term observational plots and their relevance for forest ecology and management. For. Ecol. Manage. 2014, 316, 65–77. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. Tree species mixing can increase maximum stand density. Can. J. For. Res. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Kelly, P.M.; Leuschner, H.H.; Briffa, K.R.; Harris, I.C. The climatic interpretation of pan-European signature years in oak ring-width series. Holocene 2002, 12, 689–694. [Google Scholar] [CrossRef]
- Mette, T.; Dolos, K.; Meinardus, C.; Bräuning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Van der Werf, G.W.; Sass-Klaassen, U.G.W.; Mohren, G.M.J. The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 2007, 25, 103–112. [Google Scholar] [CrossRef]
- Sohar, K.; Läänelaid, A.; Eckstein, D.; Helama, S.; Jaagus, J. Dendroclimatic signals of pedunculate oak (Quercus robur L.) in Estonia. Eur. J. For. Res. 2014, 133, 535–549. [Google Scholar] [CrossRef]
- Gillner, S.; Vogt, J.; Roloff, A. Climatic response and impacts of drought on oaks at urban and forest sites. Urban. For. Urban. Green. 2013, 12, 597–605. [Google Scholar] [CrossRef]
- Freising, V.C.K. Klimahüllen für 27 Waldbaumarten. Available online: https://www.lwf.bayern.de/mam/cms04/boden-klima/dateien/afz-klimahuellen-fuer-27-baumarten.pdf (accessed on 24 February 2018).
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of mixed versus pure stands of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- DWD. Deutscher Wetterdienst. Available online: http://www.dwd.de (accessed on 25 November 2017).
- Roloff, A.; Weisgerber, H.; Lang, U.M.; Stimm, B. Bäume Mitteleuropas: Von Aspe bis Zirbelkiefer. Mit den Porträts aller Bäume des Jahres von 1989 bis 2010, 1st ed.; Wiley-VCH: Weinheim, Germany, 2010; ISBN 978-3527328253. [Google Scholar]
- Rinn, F. Products and Services for Tree and Wood Analysis. Available online: http://www.rinntech.com/ (accessed on 26 February 2018).
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C.; Buras, A.; Cecile, J.; Mudelsee, M.; et al. Package dplR: Dendrochronology Program Library in R. Available online: https://cran.r-project.org/web/packages/dplR/index.html (accessed on 26 February 2018).
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Cook, E.R.; Briffa, K.R.; Meko, D.M.; Graybill, D.A.; Funkhouser, G. The “segment length curse” in long tree-ring chronology development for palaeoclimatic studies. Holocene 1995, 5, 229–237. [Google Scholar] [CrossRef]
- Esper, J.; Cook, E.R.; Schweingruber, F.H. Low-frequency signals in long tree-ring chronologies for reconstructing past temperature variability. Science 2002, 295, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Tree rings and climate; Academic Press: London, UK, 1976. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G.; Jansma, E.; Korpela, M.; Westfall, R.D.; Baldwin, J. Using simulations and data to evaluate mean sensitivity (ζ) as a useful statistic in dendrochronology. Dendrochronologia 2013, 31, 250–254. [Google Scholar] [CrossRef]
- Buras, A. A comment on the expressed population signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D.; Wigley, T.M.L.; Pilcher, J.R.; Baillie, M.G.L. Climate reconstruction from tree rings: Part 1, basic methodology and preliminary. J. Climatol. 1983, 3, 233–242. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. Inequality in paleorecords. Ecology 2008, 89, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Melvin, T.M.; Briffa, K.R. A “signal-free” approach to dendroclimatic standardisation. Dendrochronologia 2008, 26, 71–86. [Google Scholar] [CrossRef]
- Zang, C.; Rothe, A.; Weis, W.; Pretzsch, H. Zur baumarteneignung bei klimawandel: Ableitung der Trockenstress-Anfälligkeit wichtiger waldbaumarten aus jahrringbreiten. Allgemeine Forst-und Jagdzeitung 2011, 182, 98–112. (In German) [Google Scholar]
- Kahle, H.-P.; Karjalainen, T.; Schuck, A.; Ågren, G.; Kellomäki, S.; Mellert, K.; Prietzel, J.; Rehfuess, K.E.; Spiecker, H. Causes and Consequences of Forest Growth Trends in Europe; Kahle, H.-P., Ed.; Koninklijke Brill NV: Leiden, The Netherlands, 2008; ISBN 9789004167056. [Google Scholar]
- Kint, V.; Aertsen, W.; Campioli, M.; Vansteenkiste, D.; Delcloo, A.; Muys, B. Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Change 2012, 115, 343–363. [Google Scholar] [CrossRef]
- Wilmking, M.; D’Arrigo, R.; Jacoby, G.C.; Juday, G.P. Increased temperature sensitivity and divergent growth trends in circumpolar boreal forests. Geophys. Res. Lett. 2005, 32, 2–5. [Google Scholar] [CrossRef]
- Bošela, M.; Kulla, L.; Marušák, R. Detrending ability of several regression equations in tree-ring research: A case study based on tree-ring data of Norway spruce (Picea abies [L.]). J. For. Sci. 2011, 57, 491–499. [Google Scholar] [CrossRef]
- Assmann, E. The Principles of Forest Yield Study; Pergamon Press: Oxford, UK, 1970. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Wood, S.N.; Goude, Y.; Shaw, S. Generalized additive models for large data sets. J. R. Stat. Soc. Ser. C 2015, 64, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H. Progress in Botany; Lüttge, U.E., Beyschlag, W., Büdel, B., Francis, D., Eds.; Progress in Botany: Berlin, Germany; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-02168-8. [Google Scholar]
- Pretzsch, H.; Biber, P.; Uhl, E.; Hense, P. Coarse Root–Shoot allometry of Pinus radiata modified by site conditions in the Western Cape province of South Africa. J. For. Sci. 2012, 74, 237–246. [Google Scholar]
- Neuwirth, B.; Schweingruber, F.H.; Winiger, M. Spatial patterns of central European pointer years from 1901 to 1971. Dendrochronologia 2007, 24, 79–89. [Google Scholar] [CrossRef]
- Cropper, J.P. Tree-Ring skeleton plotting by computer. Tree-Ring Bull. 1979, 39, 47–60. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Potop, V.; Boroneanţ, C.; Možný, M.; Štěpánek, P.; Skalák, P. Observed spatiotemporal characteristics of drought on various time scales over the Czech Republic. Theor. Appl. Climatol. 2014, 115, 563–581. [Google Scholar] [CrossRef]
- Thornthwaite, D.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 39, 55–94. [Google Scholar] [CrossRef]
- Orwig, D.A.; Abrams, M.D. Variation in radial growth responses to drought among species, site, and canopy strata. Trees-Struct. Funct. 1997, 11, 474–484. [Google Scholar] [CrossRef]
- Lough, J.M.; Fritts, H.C. The Southern Oscillation and tree rings: 1600–1961. J. Clim. Appl. Meteorol. 1985, 24, 952–966. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; The University of Arizona Press: Tucson, ZA, USA, 2012. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I.; Angulo, M.; El Kenawy, A. A New global 0.5 gridded dataset (1901–2006) of a multiscalar drought index: Comparison with current drought index datasets based on the Palmer Drought Severity Index. J. Hydrometeorol. 2010, 11, 1033–1043. [Google Scholar] [CrossRef]
- Dolos, K.; Mette, T.; Wellstein, C. Silvicultural climatic turning point for European beech and sessile oak in Western Europe derived from national forest inventories. For. Ecol. Manage. 2016, 373, 128–137. [Google Scholar] [CrossRef]
- Barsoum, N.; Eaton, E.L.; Levanič, T.; Pargade, J.; Bonnart, X.; Morison, J.I.L. Climatic drivers of oak growth over the past one hundred years in mixed and monoculture stands in southern England and northern France. Eur. J. For. Res. 2014, 134, 33–51. [Google Scholar] [CrossRef]
- Leibing, C.; Signer, J.; van Zonneveld, M.; Jarvis, A.; Dvorak, W. Selection of provenances to adapt tropical pine forestry to climate change on the basis of climate analogs. Forests 2013, 4, 155–178. [Google Scholar] [CrossRef]
- Eilmann, B.; de Vries, S.M.G.; den Ouden, J.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For. Ecol. Manage. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2012, 3, 203–207. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Schütze, G.; Perkins, D.; Rötzer, T.; Caldentey, J.; Koike, T.; van Con, T.; et al. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, O. Eichenertragstafeln. In Ertragstafeln der Wichtigsten Baumarten; Schober, R., Ed.; JD Sauerländer’s Verlag: Frankfurt am Main, Germany, 1971; pp. 134–138. [Google Scholar]
- IPCC. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kauppi, P.E.; Posch, M.; Pirinen, P. Large impacts of climatic warming on growth of boreal forests since 1960. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Kato, T.; Guo, Z.; Yang, Y.; Hu, H.; Shen, H.; Zhao, X.; Kishimoto-Mo, A.W.; Tang, Y.; Houghton, R.A. Evidence for environmentally enhanced forest growth. Proc. Natl. Acad. Sci. 2014, 111, 9527–9532. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Leuzinger, S.; Zotz, G.; Asshoff, R.; Körner, C. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol. 2005, 25, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kniesel, B.M.; Günther, B.; Roloff, A.; von Arx, G. Defining ecologically relevant vessel parameters in Quercus robur L. for use in dendroecology: A pointer year and recovery time case study in Central Germany. Trees 2015, 29, 1041–1051. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Consequences of climate change on the tree of life in Europe. Nature 2011, 470, 531–534. [Google Scholar] [CrossRef] [PubMed]



| Study Site | Germany | ||||
| Value | |||||
| Average | Std. Dev. (Standard Deviation) | Min | Max | ||
| Overall timespan | 1868–2011 | ||||
| Common interval | 1956–2011 | ||||
| Mean growth rate (mm a−1) | 1.38 | 0.56 | 0.15 | 4.74 | |
| Expressed population signal (EPS) | 0.947 | ||||
| Mean sensitivity (MS) | 0.276 | ||||
| Mean inter-series correlation (Rbar) | 0.234 | ||||
| Diameter at breast height (cm) | 41.48 | 8.18 | 29 | 67 | |
| Tree height (m) | 30.13 | 2.91 | 24.1 | 37.5 | |
| Study Site | Italy | ||||
| Value | |||||
| Average | Std. Dev. | Min | Max | ||
| Overall timespan | 1922–2014 | ||||
| Common interval | 1974–2014 | ||||
| Mean growth rate (mm a−1) | 3.34 | 1.87 | 0.25 | 10.62 | |
| Expressed population signal (EPS) | 0.776 | ||||
| Mean sensitivity (MS) | 0.349 | ||||
| Mean inter-series correlation (Rbar) | 0.148 | ||||
| Diameter at breast height (cm) | 51.98 | 9.05 | 36.4 | 73.1 | |
| Tree height (m) | 28.66 | 5.28 | 18.2 | 39.7 | |
| Study Site | South Africa | ||||
| Value | |||||
| Average | Std. Dev. | Min | Max | ||
| Overall timespan | 1882–2011 | ||||
| Common interval | 1970–2010 | ||||
| Mean growth rate (mm a−1) | 2.74 | 1.68 | 0.29 | 12.48 | |
| Expressed population signal (EPS) | 0.927 | ||||
| Mean sensitivity (MS) | 0.216 | ||||
| Mean inter-series correlation (Rbar) | 0.193 | ||||
| Diameter at breast height (cm) | 76.8 | 25.18 | 36.3 | 154.4 | |
| Tree height (m) | 16.51 | 4.43 | 19.75 | 27.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range. Forests 2018, 9, 108. https://doi.org/10.3390/f9030108
Perkins D, Uhl E, Biber P, Du Toit B, Carraro V, Rötzer T, Pretzsch H. Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range. Forests. 2018; 9(3):108. https://doi.org/10.3390/f9030108
Chicago/Turabian StylePerkins, Diana, Enno Uhl, Peter Biber, Ben Du Toit, Vinicio Carraro, Thomas Rötzer, and Hans Pretzsch. 2018. "Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range" Forests 9, no. 3: 108. https://doi.org/10.3390/f9030108





