Hyperspectral Analysis of Pine Wilt Disease to Determine an Optimal Detection Index
Abstract
:1. Introduction
2. Data and Methods
2.1. Study Area
2.2. Target Tress and PWN Injection
2.3. Measurement of Leaf Reflectance
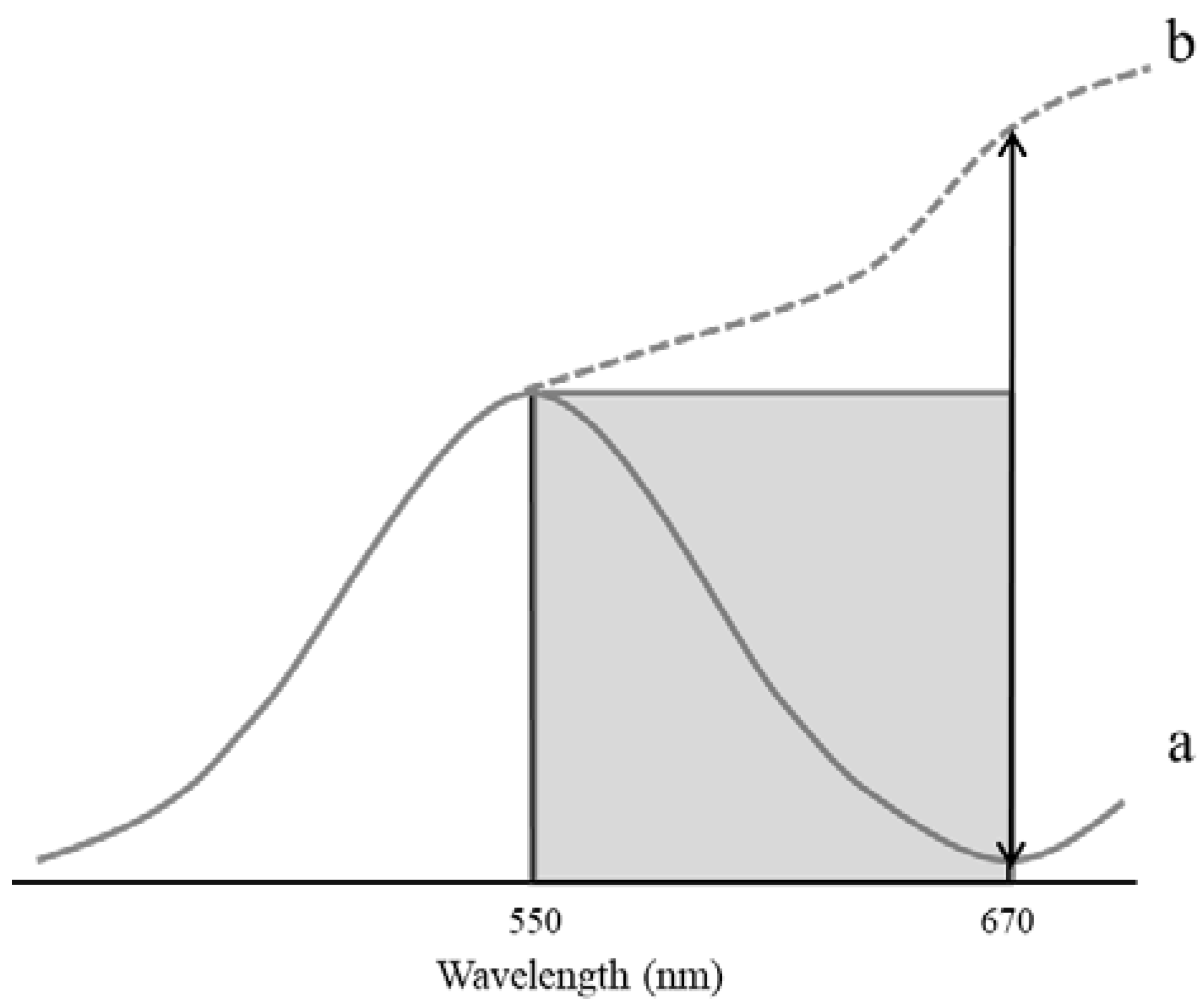
2.4. Vegetation Indices Calculation
2.5. Statistical Analysis
3. Results and Discussion
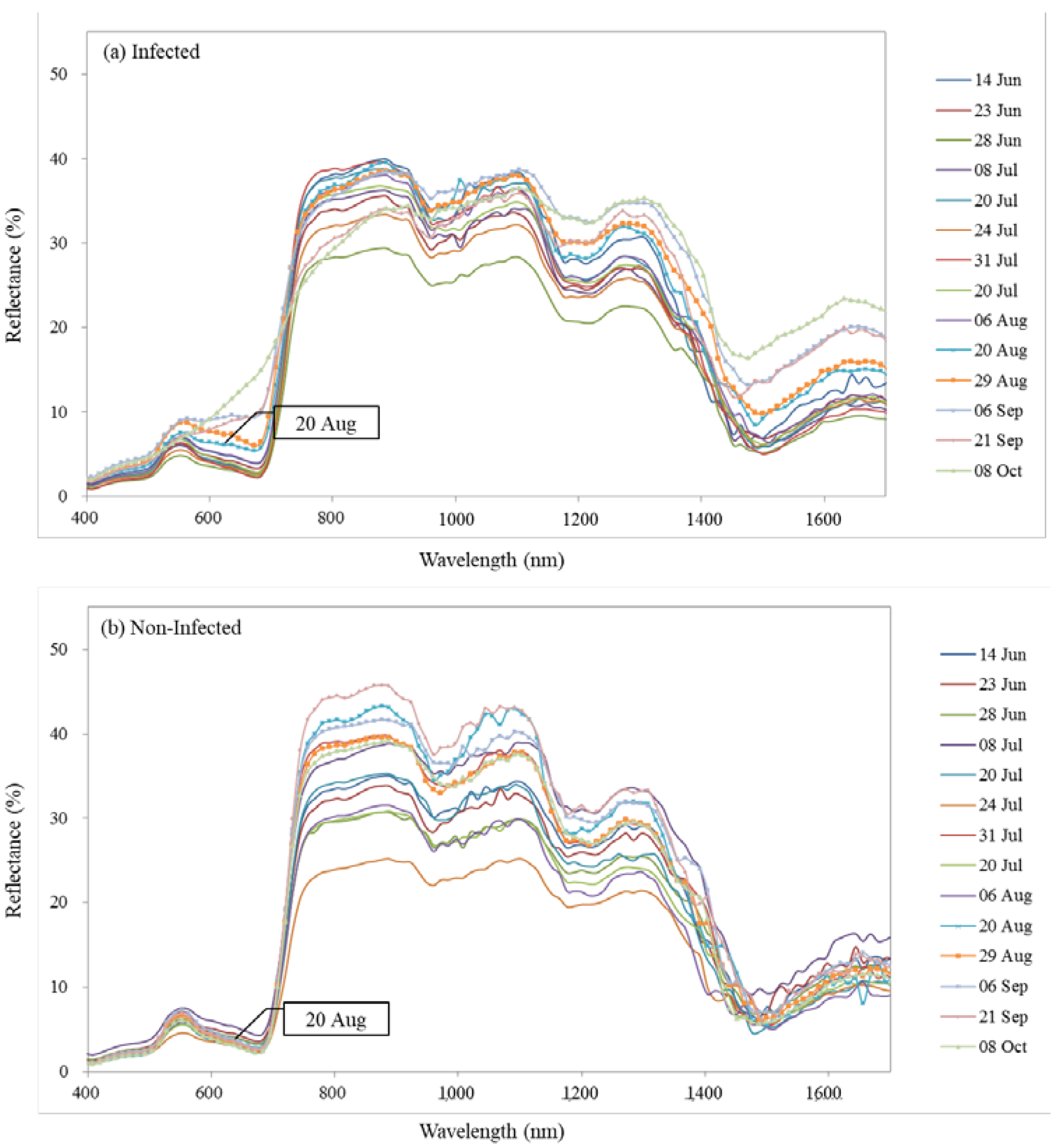
3.1. Changes in Leaf Reflectance Spectra
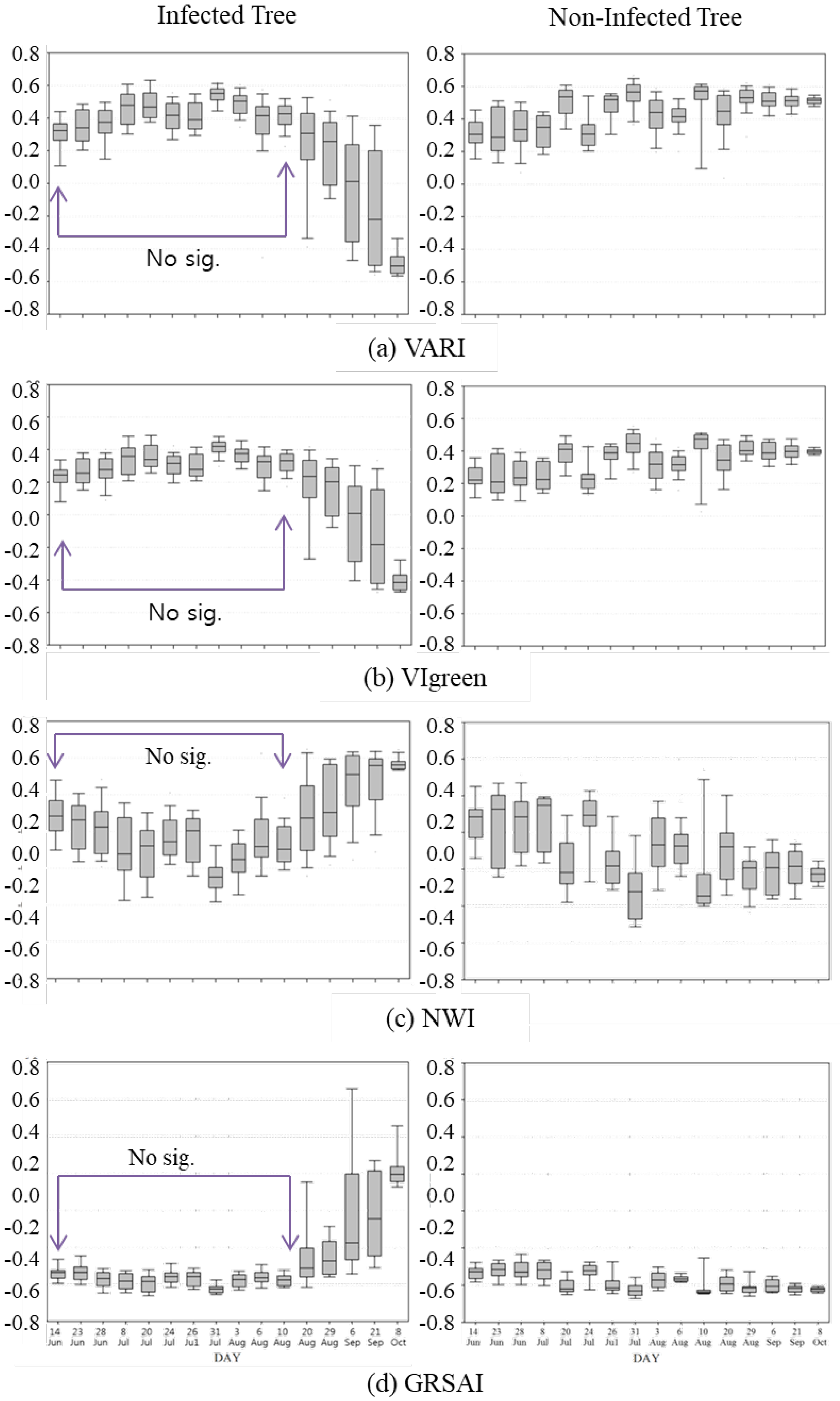
3.2. Vegetation Indices
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological change associated with migration of Bursaphelenchus xylophilus in seeding tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef]
- Hyun, M.W.; Kim, J.H.; Suh, D.Y.; Lee, S.K.; Kim, S.H. Fungi isolated from pine wood nematode, its vector Japanese pine sawyer, and the nematode-infected Japanese Black Pine Wood in Korea. Korean Soc. Mycol. 2007, 35, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M. National Eradication Programme for the Pinewood Nematode. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P., Eds.; Springer: Kato Bunmeisha, Japan, 2008; pp. 5–14. [Google Scholar]
- Sousa, D.; Naves, P.; Vieira, M. Prevention of pine wilt disease induced by Bursaphelenchus xylophilus and Monochanmus galloprovincialis by trunk injection of emamectin benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Kim, J.B.; Jo, M.H.; Oh, J.S.; Lee, K.J.; Park, S.J.; Um, H.H. Temporal and spatial correlation analysis of Bursaphelenchus xylophilus damage area. In Proceedings of the Korean Society of Agricultural and Forest Meteorology 2001 Spring Conference, Seoul, Korea, 1 June 2001; pp. 49–52. [Google Scholar]
- Shin, S.C. Pine Wilt Disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Kato Bunmeisha, Japan, 2008; pp. 26–32. [Google Scholar]
- Son, M.H.; Lee, W.K.; Lee, S.H.; Cho, H.K.; Lee, J.H. Natural spread pattern of damaged area by pine wilt disease using geostatistical analysis. J. Korean For. Soc. 2006, 95, 240–249. [Google Scholar]
- Kim, Y.S.; Jung, S.E.; Lee, W.K.; Kim, J.B.; Kwon, T.H. Analyzing vegetation index change of damaged trees by pine wilt disease using portable near infrared camera. J. Korean For. Soc. 2008, 97, 561–564. [Google Scholar]
- Kim, M.I.; Lee, W.K.; Kwon, T.H.; Kwak, D.A.; Kim, Y.S.; Lee, S.H. Early detecting damaged trees by pine wilt disease using DI (Detection Index) from portable near infrared camera. J. Korean For. Soc. 2011, 100, 374–381. [Google Scholar]
- Kim, E.N.; Kim, D.Y. An investigation of pine wilt damage by using ground remote sensing technique. Korean Assoc. Reg. Geogr. 2008, 14, 84–92. [Google Scholar]
- Kim, J.B.; Jo, M.H.; Kim, I.H.; Kim, Y.K. A study on the extraction of damaged area by pine wood nematode using high resolution IKONOS satellite images and GPS. J. Korean For. Soc. 2003, 92, 362–366. [Google Scholar]
- Jo, M.H.; Kim, J.B.; Oh, J.S.; Lee, K.J. Extraction method of damaged area by pinetree pest (Bursaphelenchus xylophilus) using high resolution IKONOS image. Korean Assoc. Geogr. Inf. Stud. 2001, 4, 72–78. [Google Scholar]
- Lee, H.Y.; Koo, C.D.; Sung, J.H.; Shin, J.H.; Yoo, J.H. Changes in water potential of pine seedlings inoculated with Bursaphelenchus xylophilus. J. Korean For. Soc. 2010, 99, 337–343. [Google Scholar]
- Jones, H.G.; Vaughan, R.A. Remote Sensing of Vegetation; Oxford University Press Inc.: New York, NY, USA, 2010; pp. 169–171. [Google Scholar]
- Kelly, N.M. Monitoring Sudden Oak Death in California Using High-Resolution Imagery; General Technical Report, PSW-GTR-184: 799-810; USADA-Forest Service: Washington, DC, USA, 2002.
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, VT, USA, 2005; p. 922. [Google Scholar]
- Fukuda, K.; Hogetsu, T.; Suzuki, K. Photosynthesis and water status of pine-wood nematode-infected pine seedlings. J. Jpn. For. Soc. 1992, 74, 1–8. [Google Scholar]
- Yoon, J.H.; Woo, K.S.; Moon, Y.S.; Koo, Y.B.; Lee, D.H. Change of water content and disease development on Pinus thunbergii seedlings inoculated with Bursaphelenchus xylophilus. J. Korean For. Soc. 2008, 97, 570–575. [Google Scholar]
- Uto, K.; Kosugi, Y. Evaluation of oak wilt index based on genetic programming. In Proceedings of the First Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS ’09), Grenoble, France, 26–28 August 2009; pp. 1–4. [Google Scholar]
- Uto, K.; Massaki, T.; Kosugi, Y.; Saito, E.; Ogata, T. Band selection for Japanese oak wilt extraction in autumnal tints of forest based on NWI. In Proceedings of the 2011 3rd Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Lisbon, Portugal, 6–9 June 2011; pp. 1–4. [Google Scholar]
- Jordan, C.F. Leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosysnthesis and transpiration. Int. J. Remote Sens. 1985, 6, 1335–1372. [Google Scholar] [CrossRef]
- Rock, B.N.; Vogelmann, J.E.; Williams, D.L.; Vogelmann, A.F.; Hoshizaki, T. Remote Detection of Forest Damage. BioScience 1986, 36, 439–445. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Rock, B.N.; Nobel, P.S. Measurement of leaf relative water content by infrared reflectance. Remote Sens. Environ. 1987, 22, 429–435. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water content by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems form high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, S.M. Plant Nematology, an Agricultural Training Aid; Nema Aid Publications: Sacramento, CA, USA, 1980. [Google Scholar]
- Field Spectroscopy Facility. Field Guide for the GER3700. 2005. Available online: http://fsf.nerc.ac.uk/resources/guides/pdf_guides/3700_guide_v3_roroc.pdf (accessed on 20 February 2013 ).
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third ERTS Symposium, Washington, DC, USA, 10–14 December 1973; NASA SP-351. pp. 309–317. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Lioret, P.; Muñoz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Gao, B. NDWI: A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and carotenoids from leaf to canopy scale: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Lee, J.Y.; Warner, T.A. Image classification with a region based approach in high spatial resolution imagery. In Proceedings of the International Society for Photogrammetry and Remote Sensing, Istanbul, Turkey, 12–23 July 2004; pp. 1141–1146. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E., III. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B and the carotenoid in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilization de la haute résolution spectrale pour suivre l’état des couverts végétaux. In Proceedings of the 4th International Colloquium on Spectral Signatures of Objects in Remote Sensing, Aussois, France, 18–22 January 1988; ESA SP-287. pp. 279–287. [Google Scholar]
- Uto, K.; Kosugi, Y.; Ogata, T. Hyperspectral analysis of Japanese oak wilt to determine normalized wilt index. In Proceedings of the Geoscience and Remote Sensing Symposium (IGARSS 2008), Boston, MA, USA, 6–11 July 2008; Volume 2, pp. 295–298. [Google Scholar]
- Jensen, J.R. Remote Sensing of the Environmental: An Earth Resource Perspective; Prentice Hall: Upper Saddle River, NJ, USA, 2002; p. 335. [Google Scholar]
- Carter, G.A. Primary and secondary effects of the water content on the spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Kiyohara, T.; Suzuki, K. Nematode population growth and disease development in the pine wilting disease. Eur. J. For. Pathol. 1978, 8, 285–292. [Google Scholar] [CrossRef]
- Hara, N.; Takeuch, Y.; Futai, K. Cytological changes in ray parenchyma cells of seedlings of three pine species infected with the pine wilt disease. Jpn. J. Nematol. 2006, 36, 23–32. [Google Scholar] [CrossRef]
- Keyworth, S.; Jarman, M.; Medcalf, K. Assessing the Extent and Severity of Erosion on the Upland Organic Soils of Scotland Using Earth Observation: A GIFTSS Implementation Test: Final Report. 2009. Available online: http://www.scotland.gov.uk/Resource/Doc/290944/0089386.pdf (accessed on 28 February 2014).
- Roberts, D.A.; Roth, K.L.; Perroy, R.L. Hyperspectral vegetation indices, In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 309–328. [Google Scholar]


| Vegetation Index | Equation | Reference |
|---|---|---|
| Normalized difference vegetation index (NDVI) | Rouse et al. [33] | |
| Green normalized difference vegetation index (GNDVI) | Gitelson et al. [40] | |
| Normalized difference water index (NDWI) | Gao [36] | |
| Vegetation index green (VIgreen) | Giltelson et al. [34] | |
| Plant senescing reflectance index (PSRI) | Merzlyak et al. [41] | |
| Simple ratio pigment index (SRPI) | Peñuelas et al. [35] | |
| Vegetation atmospherically resistant index (VARI) | Gitelson et al. [34] | |
| Pigment-specific normalized difference (PSND) | Chappelle et al. [42] | |
| Red edge position (REP) | Guyot & Baret [43] | |
| Normalized wilt index (NWI) | NDGI = (Rred − Rgreen)/(Rred + Rgreen) | Uto et al. [44] |
| Indices | 14 June | 23 June | 28 June | 8 July | 20 July | 24 July | 26 July | 31 July | 6 August | 10 August | 20 August | 29 August | 6 September | 21 September | 8 October |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | 1.437 | 0.865 | 0.525 | 4.314 | 0.950 | 3.050 | 0.166 | 0.490 | 1.714 | 1.296 | 3.144 | 6.619 | 9.228 | 10.269 | 27.932 |
| GNDVI | 1.208 | 1.126 | 1.593 | 3.711 | 0.226 | 1.944 | 2.807 | 2.379 | 0.443 | 1.971 | 2.838 | 5.005 | 9.717 | 14.113 | 7.088 |
| REP | 2.115 | 6.789 | 3.724 | 4.299 | 2.178 | 2.837 | 7.523 | 4.945 | 0.228 | 2.689 | 0.718 | 4.554 | 4.786 | 2.704 | 3.877 |
| NDWI | 1.500 | 3.423 | 2.327 | 6.747 | 1.854 | 5.102 | 0.182 | 4.285 | 0.069 | 0.646 | 1.230 | 6.681 | 8.902 | 8.968 | 12.478 |
| PSRI | 1.031 | 2.529 | 0.482 | 2.612 | 0.900 | 4.696 | 1.023 | 2.956 | 1.509 | 0.497 | 2.810 | 4.845 | 5.489 | 7.083 | 15.040 |
| SRPI | 0.691 | 3.396 | 0.196 | 2.374 | 2.111 | 2.049 | 1.464 | 1.367 | 1.318 | 2.741 | 1.121 | 5.271 | 6.529 | 9.525 | 10.857 |
| VARI * | 0.976 | 1.011 | 0.335 | 4.416 | 1.154 | 3.804 | 2.548 | 0.979 | 1.408 | 2.715 | 3.846 | 7.900 | 8.912 | 10.290 | 28.122 |
| VIgreen * | 1.054 | 0.636 | 0.383 | 3.948 | 1.745 | 3.420 | 2.990 | 1.857 | 1.445 | 3.594 | 4.074 | 7.844 | 8.823 | 10.222 | 27.622 |
| PSND | 1.320 | 1.048 | 0.570 | 4.444 | 0.859 | 3.097 | 0.550 | 0.754 | 1.726 | 1.154 | 2.958 | 6.437 | 9.230 | 10.257 | 25.554 |
| NWI * | 1.155 | 0.040 | 0.640 | 3.562 | 1.911 | 2.901 | 2.844 | 2.730 | 1.274 | 4.198 | 4.052 | 8.654 | 13.192 | 14.003 | 50.321 |
| GRSAI * | 0.674 | 0.860 | 2.355 | 4.242 | 1.489 | 2.427 | 2.186 | 0.532 | 1.412 | 2.660 | 3.915 | 5.535 | 6.033 | 8.582 | 11.138 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-R.; Lee, W.-K.; Lim, C.-H.; Kim, M.; Kafatos, M.C.; Lee, S.-H.; Lee, S.-S. Hyperspectral Analysis of Pine Wilt Disease to Determine an Optimal Detection Index. Forests 2018, 9, 115. https://doi.org/10.3390/f9030115
Kim S-R, Lee W-K, Lim C-H, Kim M, Kafatos MC, Lee S-H, Lee S-S. Hyperspectral Analysis of Pine Wilt Disease to Determine an Optimal Detection Index. Forests. 2018; 9(3):115. https://doi.org/10.3390/f9030115
Chicago/Turabian StyleKim, So-Ra, Woo-Kyun Lee, Chul-Hee Lim, Moonil Kim, Menas C. Kafatos, Seung-Ho Lee, and Sung-Soon Lee. 2018. "Hyperspectral Analysis of Pine Wilt Disease to Determine an Optimal Detection Index" Forests 9, no. 3: 115. https://doi.org/10.3390/f9030115






