Root Infection of Canker Pathogens, Fusarium circinatum and Diplodia sapinea, in Asymptomatic Trees in Pinus radiata and Pinus pinaster Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Sampling
2.2. Isolation in Roots and Morphological Identification
2.3. Molecular Identification
2.4. Mating Type Determination
2.5. Haplotypes
2.6. Pathogenicity Tests
2.7. Isolation from Field-Collected Soil
3. Results
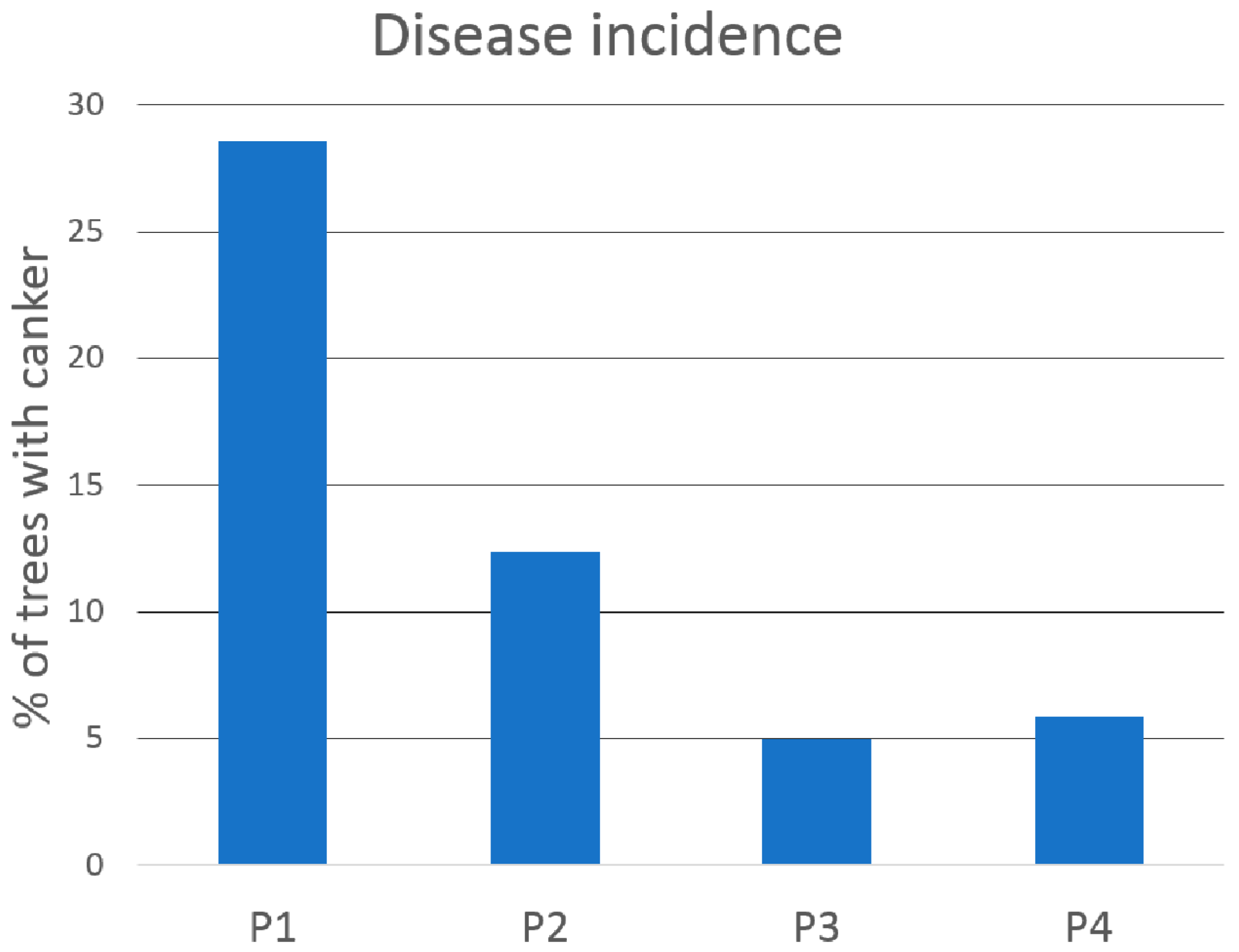
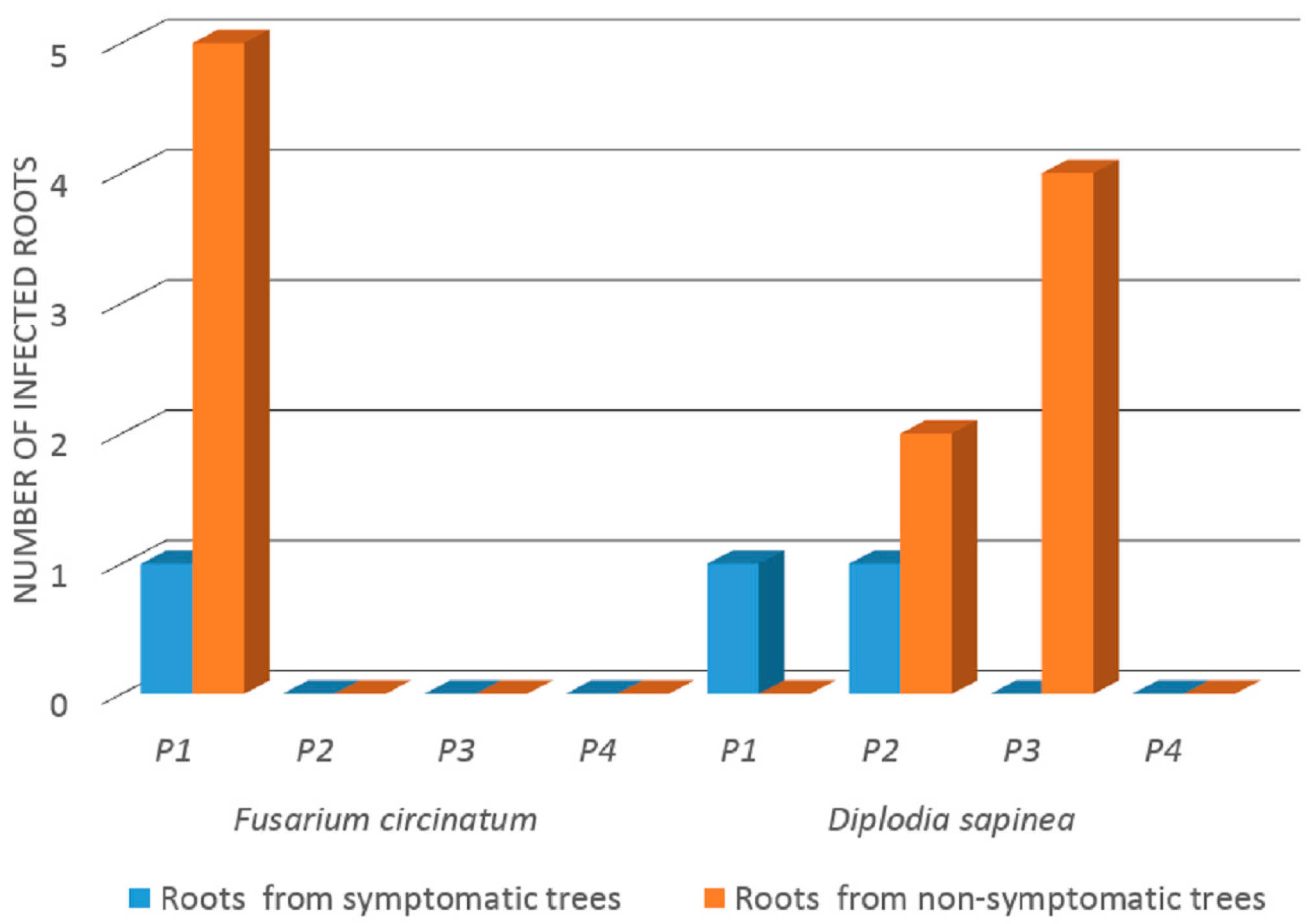
3.1. Incidence of F. circinatum and D. sapinea in Roots
3.2. Isolate Characterization: Genetic Variability and Pathogenicity
3.2.1. Mating Types
3.2.2. Haplotypes
3.2.3. Pathogenicity Test
3.3. Detection in Field-Collected Soil
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriacea: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Burgess, T.I.; Wingfield, M.J.; Wingfield, B.D. Global distribution of Diplodia pinea genotypes revealed using simple sequence repeat (SSR) markers. Australas. Plant Pathol. 2004, 33, 513–519. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- Bihon, W.; Wingfield, M.J.; Slippers, B.; Duong, T.A.; Wingfield, B.D. MAT gene idiomorphs suggest a heterothallic sexual cycle in a predominantly asexual and important pine pathogen. Fungal Genet. Biol. 2014, 62, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Swart, W.J.; Wingfield, M.J. Biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Dis. 1991, 75, 761–766. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Kruger, E.L.; Stanosz, G.R. Sphaeropsis sapinea and water stress in a red pine plantation in Central Wisconsin. Phytopathology 1997, 87, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Inman, A.R.; Kirkpatrick, S.C.; Gordon, T.R.; Pathology, P.; Shaw, D.V. Limiting effects of low temperature on growth and spore germination in Gibberella circinata, the cause of pitch canker in pine species. Plant Dis. 2008, 92. [Google Scholar] [CrossRef]
- Landeras, E.; García, P.; Fernández, Y.; Braña, M. Outbreak of pitch canker caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First report of pitch canker on pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of pitch canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (Southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. EPPO Reporting Service; No. 05; EPPO: Paris, France, 1 May 2006. [Google Scholar]
- Bihon, W.; Slippers, B.; Burgess, T.; Wingfield, M.J.; Wingfield, B.D. Diverse sources of infection and cryptic recombination revealed in South African Diplodia pinea populations. Fungal Biol. 2012, 116, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.J.; Ah Chee, A.; Sale, P.O.; Taylor, J.T.; Hadar, E.; Hadar, Y.; Farrell, R.L. Variation among New Zealand isolates of Sphaeropsis sapinea. For. Pathol. 2002, 32, 109–121. [Google Scholar] [CrossRef]
- Smith, H.; Wingfield, M.J.; de Wet, J.; Coutinho, T. A Genotypic diversity of Sphaeropsis sapinea from South Africa and Northern Sumatra. Plant Dis. 2000, 84, 139–142. [Google Scholar] [CrossRef]
- Nadal, M.; Moret, A.; Ferrer, R. Léxico de las Enfermedades de las Plantas Producidas por Hongos; Phytoma: Valencia, España, 2005. [Google Scholar]
- García-serna, I.; Iturritxa, E. Diversidas de aislados de Diplodia Pinea procedentes de Chile, Nueva Zelanda y las comunidades de País Vasco y Navarra. Cuad. Soc. Esp. Cienc. For. 2008, 26, 161–164. [Google Scholar]
- Zwolinski, J.B.; Swart, W.J.; Wingfield, M.J. Economic impact of a post-hail outbreak of dieback induced by Sphaeropsis sapinea. For. Pathol. 1990, 20, 405–411. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Trobaugh, J.; Guthmiller, M.A.; Sanosz, J.C. Sphaeropsis shoot blight and altered nutrition in red pine plantations treated with paper mill waste sludge. For. Ecol. Manag. 2004, 34, 245–253. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Robin, C.; Reynaud, G.; Déqué, M.; Badeau, V.; Piou, D.; Husson, C.; Marçais, B. Simulating the effects of a climate-change scenario on the geographical range and activity of forest-pathogenic fungi. Can. J. Plant Pathol. 2007, 29, 101–120. [Google Scholar] [CrossRef]
- Keen, A.; Smits, T.F.C. Application of a mathematical function for a temperature optimum curve to establish differences in growth between isolates of a fungus. Neth. J. Plant Pathol. 1989, 95, 37–49. [Google Scholar] [CrossRef]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f.sp. pini, with Monterey pine seeds and seedlings in California. Plant Pathol. 1998, 47, 649–656. [Google Scholar] [CrossRef]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Barrows-Broaddus, J. Colonization of clones and seed of loblolly pine following inoculation with Fusarium subglutinans. Plant Dis. 1990, 74, 1002–1005. [Google Scholar] [CrossRef]
- Evira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests 2015, 6, 3353–3368. [Google Scholar] [CrossRef]
- Anderson, R.L. New method for assessing contamination of slash and loblolly pine seeds by Fusarium moniliforme var. subglutinans. Plant Dis. 1986, 70, 452–453. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First report of Fusarium subglutinans f.sp. pini on pine seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Wingfield, M.; Knox-Davies, P. Association of Diplodia pinea with a root disease of pines in South Africa. Plant Dis. 1980, 64, 221–223. [Google Scholar] [CrossRef]
- Whitehill, J.G.; Lehman, J.S.; Bonello, P. Ips pini (Curculionidae: Scolytinae) is a vector of the fungal pathogen, Sphaeropsis sapinea (Coelomycetes), to Austrian pines, Pinus nigra (Pinaceae). Environ. Entomol. 2007, 36, 114–120. [Google Scholar] [CrossRef]
- Sinclair, W.A.; Johnson, W.T.; Lyon, H.H. Diseases of Trees and Shrubs; Cornell University Press: Ithaca, NY, USA, 1987; ISBN 0801415179. [Google Scholar]
- Stanosz, G.R.; Swart, W.J.; Smith, D.R. RAPD marker and isozyme characterization of Sphaeropsis sapinea from diverse coniferous hosts and locations. Mycol. Res. 1999, 103, 1193–1202. [Google Scholar] [CrossRef]
- Palmer, M.A.; Nicholls, T.H. Shoot blight and collar rot of Pinus resinosa caused by Sphaeropsis sapinea in forest tree nurseries. Plant Dis. 1985, 69, 739–740. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Leisso, R. Diplodia shoot blight and asymptomatic persistence of Diplodia pinea on or in stems of jack pine nursery seedlings. For. Pathol. 2007, 37, 145–154. [Google Scholar] [CrossRef]
- Flowers, J.L.; Nuckles, E.; Hartman, J.R.; Vaillancourt, L.J. Latent infection of Austrian and Scots pine tissues by Sphaeropsis sapinea. Plant Dis. 2001, 85, 1107–1112. [Google Scholar] [CrossRef]
- Flowers, J.; Hartman, J.; Vaillancourt, L. Detection of latent Sphaeropsis sapinea infections in austrian pine tissues using nested-polymerase chain reaction. Phytopathology 2003, 93, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Wingfied, M.J.; Coutinho, T.A. The role of latent Sphaeropsis sapinea infections in post-hail associated die-back of Pinus patula. For. Ecol. Manag. 2002, 164, 177–184. [Google Scholar] [CrossRef]
- Vujanovic, V.; St-Arnaud, M.; Neumann, P.J. Susceptibility of cones and seeds to fungal infection in a pine (Pinus spp.) collection. For. Pathol. 2000, 30, 305–320. [Google Scholar] [CrossRef]
- Bihon, B.W.; Slippers, B.; Burgess, T.; Wingfield, M.J.; Wingfield, B.D. Sources of Diplodia pinea endophytic infections in Pinus patula and P. radiata seedlings in South Africa. For. Pathol. 2011, 41, 370–375. [Google Scholar] [CrossRef]
- Fabre, B.; Piou, D.; Desprez-Loustau, M.L.; Marçais, B. Can the emergence of pine Diplodia shoot blight in France be explained by changes in pathogen pressure linked to climate change? Glob. Chang. Biol. 2011, 17, 3218–3227. [Google Scholar] [CrossRef]
- Swett, C.L.; Kirkpatrick, S.C.; Gordon, T. Evidence for a hemibiotrophic association of the pitch canker pathogen, Fusarium circinatum, with Pinus radiata. Plant Dis. 2016, 1–27. [Google Scholar] [CrossRef]
- Martín-Rodrigues, N.; Sanchez-Zabala, J.; Salcedo, I.; Majada, J.; González-Murua, C.; Duñabeitia, M.K. New insights into radiata pine seedling root infection by Fusarium circinatum. Plant Pathol. 2015, 64, 1336–1348. [Google Scholar] [CrossRef]
- Swett, C.L.; Gordon, T.R. Exposure to a pine pathogen enhances growth and disease resistance in Pinus radiata seedlings. For. Pathol. 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Stanosz, G.; Blodgett, J.; Smith, D.; Kruger, E. Water stress and Sphaeropsis sapinea as a latent pathogen of red pine seedlings. New Phytol. 2001, 149, 531–538. [Google Scholar] [CrossRef]
- Blaschke, M.; Cech, T.L. Absterbende Weißkiefern—eine langfristige Folge des Trockenjahres 2003? (Declining scots pines: A consequence of the drought in 2003?). Forstsch. Aktuell 2007, 40, 32–34. [Google Scholar]
- Bihon, W.; Burgess, T.; Slippers, B.; Wingfield, M.J.; Wingfield, B.D. Distribution of Diplodia pinea and its genotypic diversity within asymptomatic Pinus patula trees. Australas. Plant Pathol. 2011, 40, 540–548. [Google Scholar] [CrossRef]
- García Serna, I. Diplodia pinea (Desm.) Kickx y Fusarium circinatum Nirenberg & O’Donnell, Principales Hongos de Chancro de las Masas Forestales de Pinus radiata D. Don del País Vasco. Ph.D. Thesis, Universidad del País Vasco, Leioa, Spain, 2011. [Google Scholar]
- Serrano, Y.; Iturritxa, E.; Elvira-Recuenco, M.; Raposo, R. Survival of Fusarium circinatum in soil and Pinus radiata needle and branch segments. Plant Pathol. 2016, 66, 934–940. [Google Scholar] [CrossRef]
- Aegerter, B.J.; Gordon, T.R. Rates of pitch canker induced seedling mortality among Pinus radiata families varying in levels of genetic resistance to Gibberella circinata (anamorph Fusarium circinatum). For. Ecol. Manag. 2006, 235, 14–17. [Google Scholar] [CrossRef]
- Nirenberg, H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Can. J. Bot. 1981, 59, 1599–1609. [Google Scholar] [CrossRef]
- Slippers, B.; Crous, P.W.; Denman, S.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 2004, 96, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Phillips, A.J.L. The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef]
- Dissanayake, A.; Phillips, A.; Li, X.; KD, H. Botryosphaeriaceae : Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Maddau, L.; Franceschini, A.; Phillips, A.J.L. Botryosphaeriaceae species associated with lentisk dieback in Italy and description of Diplodia insularis sp. nov. Mycosphere 2016, 7, 962–977. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.W.T.; Dai, D.Q.; Dai, Y.C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Hernawati Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Castro-Medina, F.; Mohali, S.R.; Gubler, W.D. Botryosphaeriaceae species associated with cankers and dieback symptoms of Acacia mangium and Pinus caribaea var. hondurensis in Venezuela. Plant Dis. 2016, 100, 2455–2464. [Google Scholar] [CrossRef]
- Smith, D.R.; Stanosz, G.R. A species-specific PCR assay for detection of Diplodia pinea and D. scrobiculata in dead red and Jack pines with collar rot symptoms. Plant Dis. 2006, 90, 307–313. [Google Scholar] [CrossRef]
- Schweigkofler, W.; O’Donnell, K.; Garbelotto, M. Detection and quantification of airborne conidia of Fusarium circinatum, the causal agent of pine pitch canker, from two California sites by using a real-time PCR approach combined with a simple spore trapping method. Appl. Environ. Microbiol. 2004, 70, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.M.; Covert, S.F. Molecular mating type assay for Fusarium circinatum. Appl. Environ. Microbiol. 2000, 66, 5506–5508. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, M.; Armengol, J.; Grünwald, N.J. Evidence for multiple introductions and clonality in spanish populations of Fusarium circinatum. Phytopathology 2013, 103, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Iturritxa, E.; Ganley, R.J.; Wright, J.; Heppe, E.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. A genetically homogenous population of Fusarium circinatum causes pitch canker of Pinus radiata in the Basque Country, Spain. Fungal Biol. 2011, 115, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alia, R.; Raposo, R. Adaptive potential of maritime pine (Pinus pinaster) populations to the emerging pitch canker pathogen, Fusarium circinatum. PLoS ONE 2014, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Serra-Varela, M.J.; Alía, R.; Pórtoles, J.; Gonzalo, J.; Soliño, M.; Grivet, D.; Raposo, R. Incorporating exposure to pitch canker disease to support management decisions of Pinus pinaster Ait. in the face of Climate change. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Escribano, L.; Iturritxa, E.; Elvira-Recuenco, M.; Berbegal, M.; Campos, J.A.; Renobales, G.; García, I.; Raposo, R. Herbaceous plants in the understory of a pitch canker-affected Pinus radiata plantation are endophytically infected with Fusarium circinatum. Fungal Ecol. 2018, 32, 65–71. [Google Scholar] [CrossRef]
- Smith, H.; Wingfield, M.J.; Crous, P.W.; Coutinho, T. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S. Afr. J. Bot. 1996, 62, 86–88. [Google Scholar] [CrossRef]
- Santini, A.; Pepori, A.; Ghelardini, L.; Capretti, P. Persistence of some pine pathogens in coarse woody debris and cones in a Pinus pinea forest. For. Ecol. Manag. 2008, 256, 502–506. [Google Scholar] [CrossRef]
- Oblinger, B.W.; Smith, D.R.; Stanosz, G.R. Red pine harvest debris as a potential source of inoculum of Diplodia shoot blight pathogens. For. Ecol. Manag. 2011, 262, 663–670. [Google Scholar] [CrossRef]
- Romón, P.; Troya, M.; Fernández De Gamarra, M.E.; Eguzkitza, A.; Iturrondobeitia, J.C.; Goldarazena, A. Fungal communities associated with pitch canker disease of Pinus radiata caused by Fusarium circinatum in northern Spain: Association with insects and pathogen-saprophyte antagonistic interactions. Can. J. Plant Pathol. 2008, 30, 241–253. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Fernández, M.; Diez, J.J. Epidemiology and management of Pine Pitch Canker disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Swett, C.L.; Gordon, T.R. First report of grass species (Poaceae) as naturally occurring hosts of the pine pathogen Gibberella circinata. Plant Dis. 2012, 96, 908. [Google Scholar] [CrossRef]
- Swett, C.L.; Porter, B.; Fourie, G.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. Association of the pitch canker pathogen Fusarium circinatum with grass hosts in commercial pine production areas of South Africa. South. For. 2014, 76, 161–166. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; Mckenzie, E.; Hyde, K. Are some endophytes of Musa acuminata latent pathogens? Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Junker, C.; Draeger, S.; Schulz, B. A fine line—Endophytes or pathogens in Arabidopsis thaliana. Fungal Ecol. 2012, 5, 657–662. [Google Scholar] [CrossRef]
- Flowers, J.L.; Hartman, J.R.; Vaillancourt, L.J. Histology of Diplodia pinea in diseased and latently infected Pinus nigra shoots. For. Pathol. 2006, 36, 447–459. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Albers, J.S. Surveys for asymptomatic persistence of Sphaeropsis sapinea on or in stems of red pine seedlings from seven Great Lakes region nurseries. For. Pathol. 2005, 35, 233–244. [Google Scholar] [CrossRef]
| Species | IC | Location | Tree Code | NC | DUB | DLB | Haplotype SSR bp Peaks 1 | MAT 2 |
|---|---|---|---|---|---|---|---|---|
| Diplodia sapinea | 176 | Araba | 224 | 0 | 0 | 0 | 409-500-267-312-173-112-157-162-71-108 | 1-1-1 |
| Diplodia sapinea | 178 | Araba | 224 | 0 | 0 | 0 | 409-500-267-312-173-112-157-162-71-108 | 1-1-1 |
| Diplodia sapinea | 175 | Araba | 231 | 0 | 0 | 0 | 409-500-263-312-173-112-157-162-71-108 | 1-1-1 |
| Diplodia sapinea | 181 | Araba | 237 | 0 | 1 | 0 | 409-500-267-312-173-112-157-162-71-108 | 1-1-1 |
| Diplodia sapinea | 183 | Araba | 237 | 0 | 1 | 0 | 409-500-263-312-173-112-157-171-69-108 | 1-2-1 |
| Diplodia sapinea | 177 | Araba | 248 | 0 | 0 | 1 | 409-500-263-312-173-112-157-162-69-108 | 1-1-1 |
| Diplodia sapinea | 182 | Bizkaia | 335 | 2 | 0 | 1 | 409-500-267-312-173-112-157-162-71-108 | 1-2-1 |
| Diplodia sapinea | 209 | Gipuzkoa-R | 63 | 1 | 0 | 1 | 409-500-263-312-173-112-157-171-71-108 | 1-1-1 |
| Diplodia sapinea | 212 | Gipuzkoa-R | 231 | 0 | 0 | 0 | 409-500-263-314-173-112-157-162-71-108 | 1-2-1 |
| Diploida sapinea | 213 | Gipuzkoa-R | 231 | 0 | 0 | 0 | 409-500-267-312-173-112-157-162-71-108 | 1-1-1 |
| Diplodia sapinea | 211 | Gipuzkoa-R | 336 | 0 | 0 | 0 | 409-500-267-312-173-112-157-162-71-108 | 1-1-1 |
| Fusarium circinatum | 104 | Bizkaia | 286 | 0 | 0 | 0 | 178-153-251-190-173-124-204-244 | 2 |
| Fusarium circinatum | 105 | Bizkaia | 484 | 0 | 0 | 0 | 178-153-251-190-173-124-204-244 | 2 |
| Fusarium circinatum | 106 | Bizkaia | 486 | 0 | 0 | 0 | 178-153-251-190-173-124-204-244 | 2 |
| Fusarium circinatum | 107 | Bizkaia | 570 | 0 | 0 | 0 | 178-153-251-190-173-124-204-244 | 2 |
| Fusarium circinatum | 108 | Bizkaia | 459 | 1 | 0 | 1 | 178-153-251-190-173-124-204-244 | 2 |
| Fusarium circinatum | 109 | Bizkaia | 4 | 0 | 1 | 0 | 178-153-251-190-173-124-204-244 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Escribano, L.; Iturritxa, E.; Aragonés, A.; Mesanza, N.; Berbegal, M.; Raposo, R.; Elvira-Recuenco, M. Root Infection of Canker Pathogens, Fusarium circinatum and Diplodia sapinea, in Asymptomatic Trees in Pinus radiata and Pinus pinaster Plantations. Forests 2018, 9, 128. https://doi.org/10.3390/f9030128
Hernandez-Escribano L, Iturritxa E, Aragonés A, Mesanza N, Berbegal M, Raposo R, Elvira-Recuenco M. Root Infection of Canker Pathogens, Fusarium circinatum and Diplodia sapinea, in Asymptomatic Trees in Pinus radiata and Pinus pinaster Plantations. Forests. 2018; 9(3):128. https://doi.org/10.3390/f9030128
Chicago/Turabian StyleHernandez-Escribano, Laura, Eugenia Iturritxa, Ana Aragonés, Nebai Mesanza, Mónica Berbegal, Rosa Raposo, and Margarita Elvira-Recuenco. 2018. "Root Infection of Canker Pathogens, Fusarium circinatum and Diplodia sapinea, in Asymptomatic Trees in Pinus radiata and Pinus pinaster Plantations" Forests 9, no. 3: 128. https://doi.org/10.3390/f9030128





