1. Introduction
An understanding of processes underlying recovery following non-stand-replacing disturbance is essential for accurately predicting future carbon (C) dynamics in forest ecosystems of the eastern USA. Following extensive harvesting, conversion to agriculture or intensive forestry, and then subsequent abandonment, large areas of forest throughout the northeastern USA are currently 80–140 years old [
1,
2]. Since abandonment, these regenerating forests have been moderate to strong sinks for atmospheric carbon dioxide (CO
2) [
3,
4,
5,
6,
7]. In the mid-Atlantic region, estimates of net primary production (NPP) and net ecosystem production (NEP) for intermediate-age forests are highest for oak–hickory forests and lowest for pine-dominated forests [
8,
9,
10]. Overall NEP has been projected to decline slowly as forests age across the region [
1,
9,
11,
12], with productivity declining more slowly for hardwood-dominated forests than conifer-dominated forests [
5,
9,
10]. These intermediate-age forests now experience disturbance regimes dominated by wind and ice storms, insect damage, and managed wildland fire that differ in temporal and spatial scales and intensity compared to past stand-replacing disturbance such as intensive timber management or large wildfires [
6,
13,
14,
15,
16]. Following disturbance, they are also likely to have different long-term trajectories of NEP compared to patterns characterizing recovery following stand-replacing disturbances [
17,
18]. Syntheses of NEP estimated from eddy covariance measurements following forest harvesting or wildfires in a wide range of forest types indicate that gross ecosystem productivity (GEP) is a strong function of the recovery of leaf area [
17,
19]. Recently, it has been hypothesized that relatively high rates of GEP and NEP in intermediate-age forests can be maintained by frequent low-intensity disturbances which delay the onset of steady-state conditions [
18,
20,
21]. Two primary mechanisms are thought to be involved: increased canopy heterogeneity following disturbance allows greater light penetration into the sub-canopy and results in greater light use efficiency and compensatory photosynthesis by the remaining trees, saplings, and understory vegetation [
22,
23,
24]; and nutrients released during and following disturbance are rapidly reabsorbed by recovering vegetation, stimulating foliage production and enhancing photosynthetic rates [
18,
25].
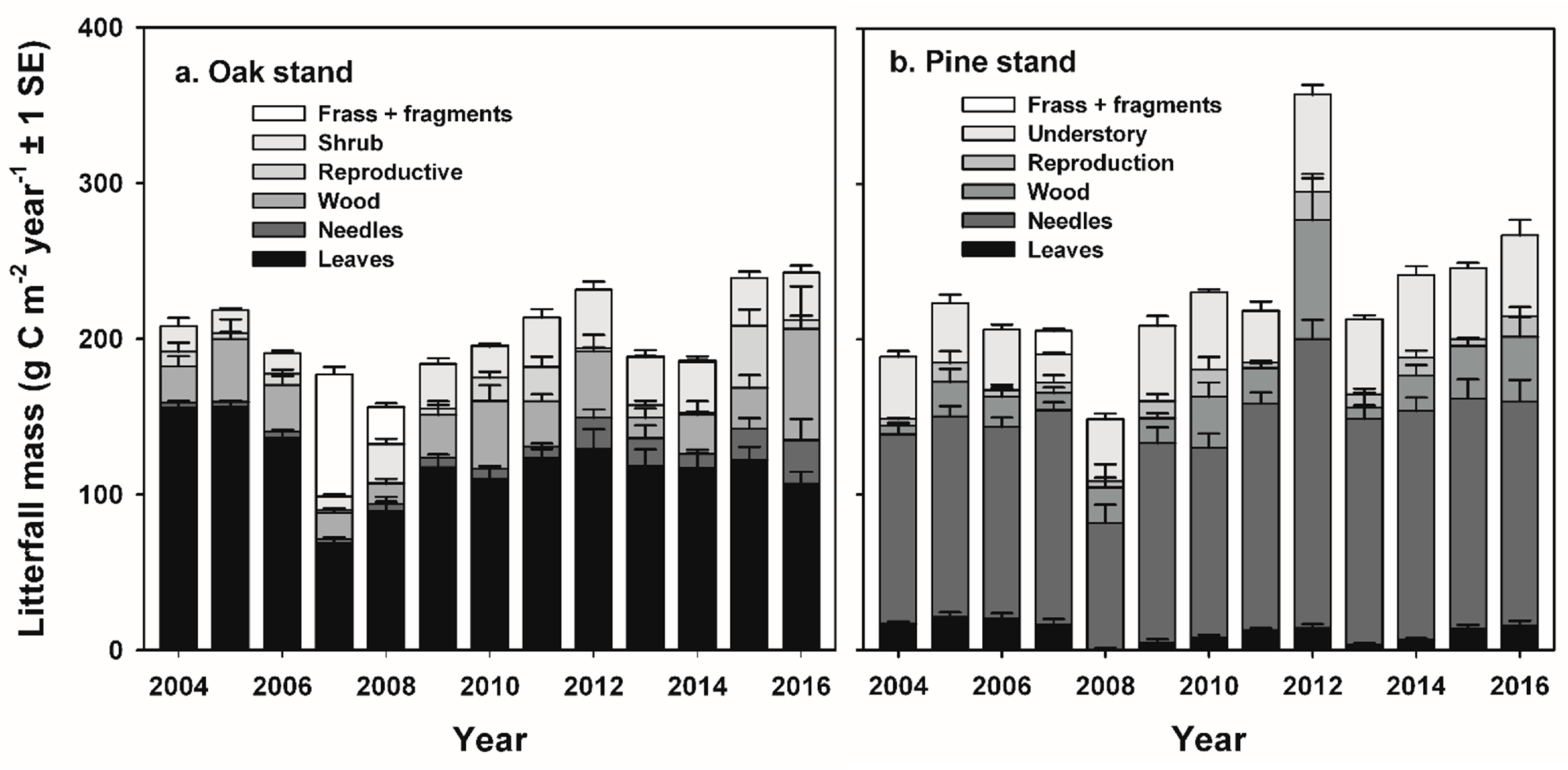
The fate of increased detrital mass following non-stand-replacing disturbance in intermediate-age forests remains less clear, but potentially results in greater ecosystem respiration (R
e) by enhancing rates of heterotrophic respiration (R
h) [
26,
27,
28,
29,
30]. Eddy covariance measurements of NEP from a limited number of eastern forests where tree mortality and increased standing dead and coarse woody debris (CWD) have resulted in increased R
h have either reported little effect on R
e and NEP was similar to pre-disturbance levels [
30], or increased R
e and reduced NEP [
27]. These results contrast somewhat with a number of studies that have documented little change to R
e and reduced soil respiration following infestations of mountain pine beetle (
Dendroctonus ponderosae Hopkins) and significant tree mortality in the Rocky Mountains of the western USA [
22,
31,
32], leading to the conclusion that these forests are more resilient to insect damage than previously predicted (e.g., [
26,
29]). Because few long-term measurements of NEP following non-stand-replacing disturbance in eastern forests exist to evaluate process-based models, considerable uncertainty continues to exist in our ability to accurately predict future C dynamics of forests of the mid-Atlantic region.
In this study, we quantified and contrasted the response of two intermediate-age forests to two of the predominant non-stand-replacing disturbances on the mid-Atlantic coastal plain USA: invasive insect infestations and planned wildland fires. Building upon two related studies that employed repeated light detection and ranging (LiDAR) acquisitions to demonstrate an increase in heterogeneity of canopy structure following defoliation by gypsy moth (
Lymantria dispar L.) and subsequent tree mortality in an oak-dominated stand [
33], and crown scorch and needle and stem consumption during a relatively intense prescribed fire in a pine-dominated stand [
34,
35], we evaluated two hypotheses: (1) Rapid recovery of foliage results in the maintenance of GEP and aboveground net primary productivity (ANPP) following non-stand-replacing disturbance; and (2) changes to detrital mass that occur during and following disturbance alter R
h and R
e, and thus NEP. We predicted that increased detrital mass following defoliation and tree mortality at the oak stand would increase R
h and R
e, resulting in reduced NEP, and in contrast, consumption of detritus on the forest floor during fires would reduce R
h and R
e at the pine stand, resulting in increased NEP following recovery of leaf area. We used near-continuous eddy flux and meteorological measurements to estimate NEP, GEP, and R
e before and following each disturbance [
35,
36,
37]. We used biometric measurements made in and around permanent forest census plots to estimate leaf area and its correspondence with GEP, and to estimate ANPP. We also characterized fine litterfall and decomposition rates, as well as changes in standing dead and coarse woody debris mass to assess the correspondence of detrital dynamics with R
e and estimated R
h in each stand [
27,
38].
4. Discussion
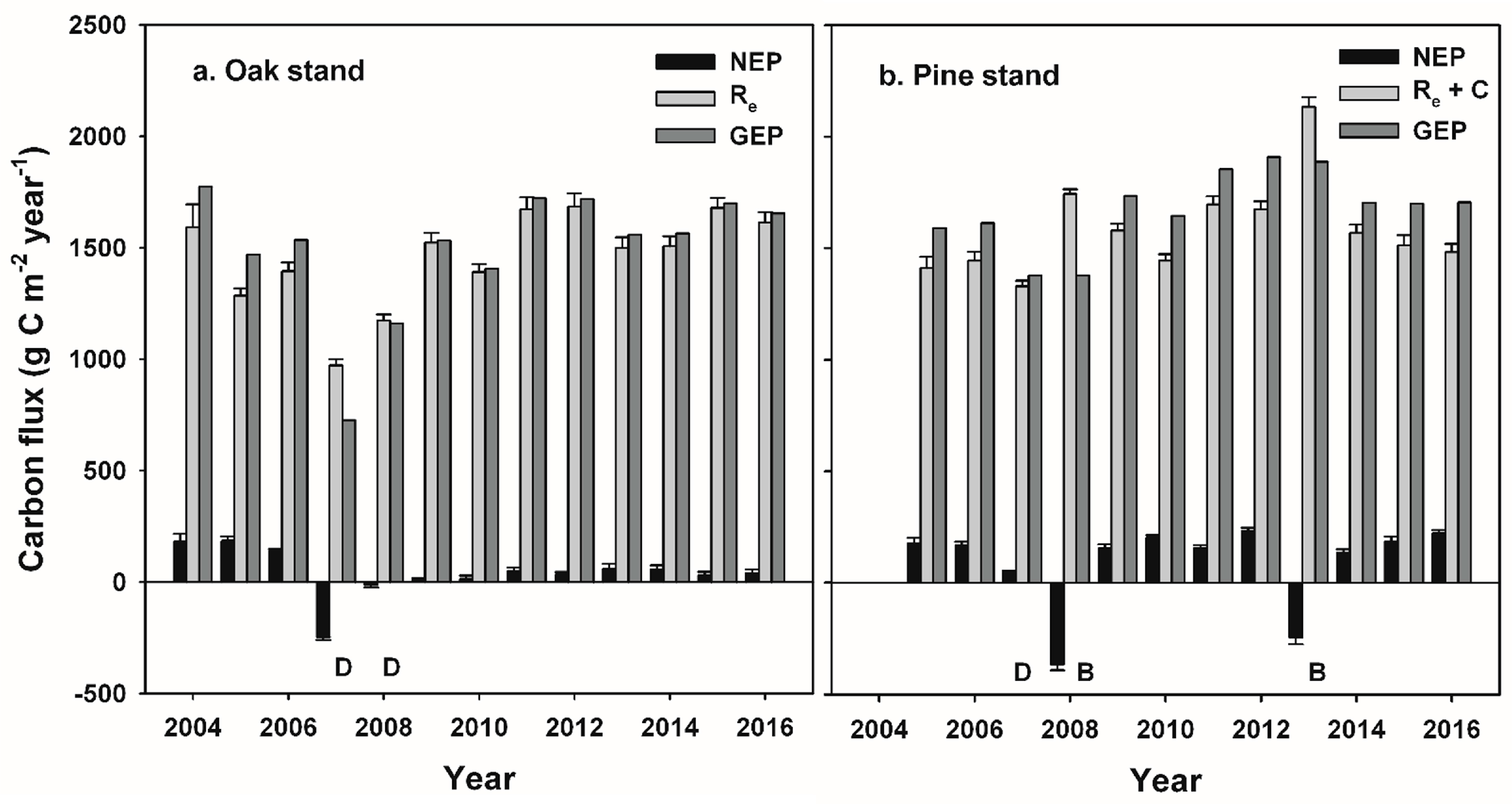
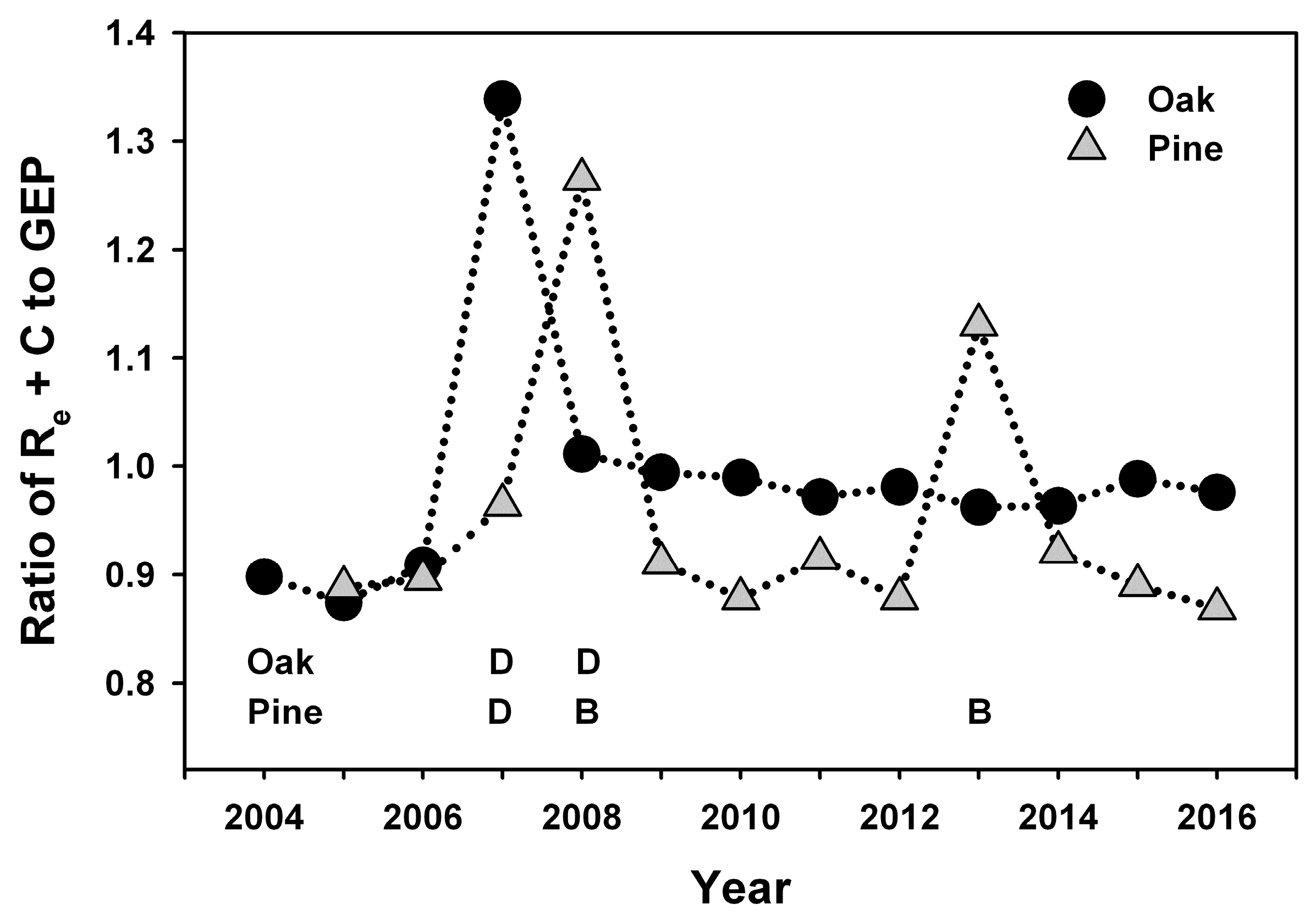
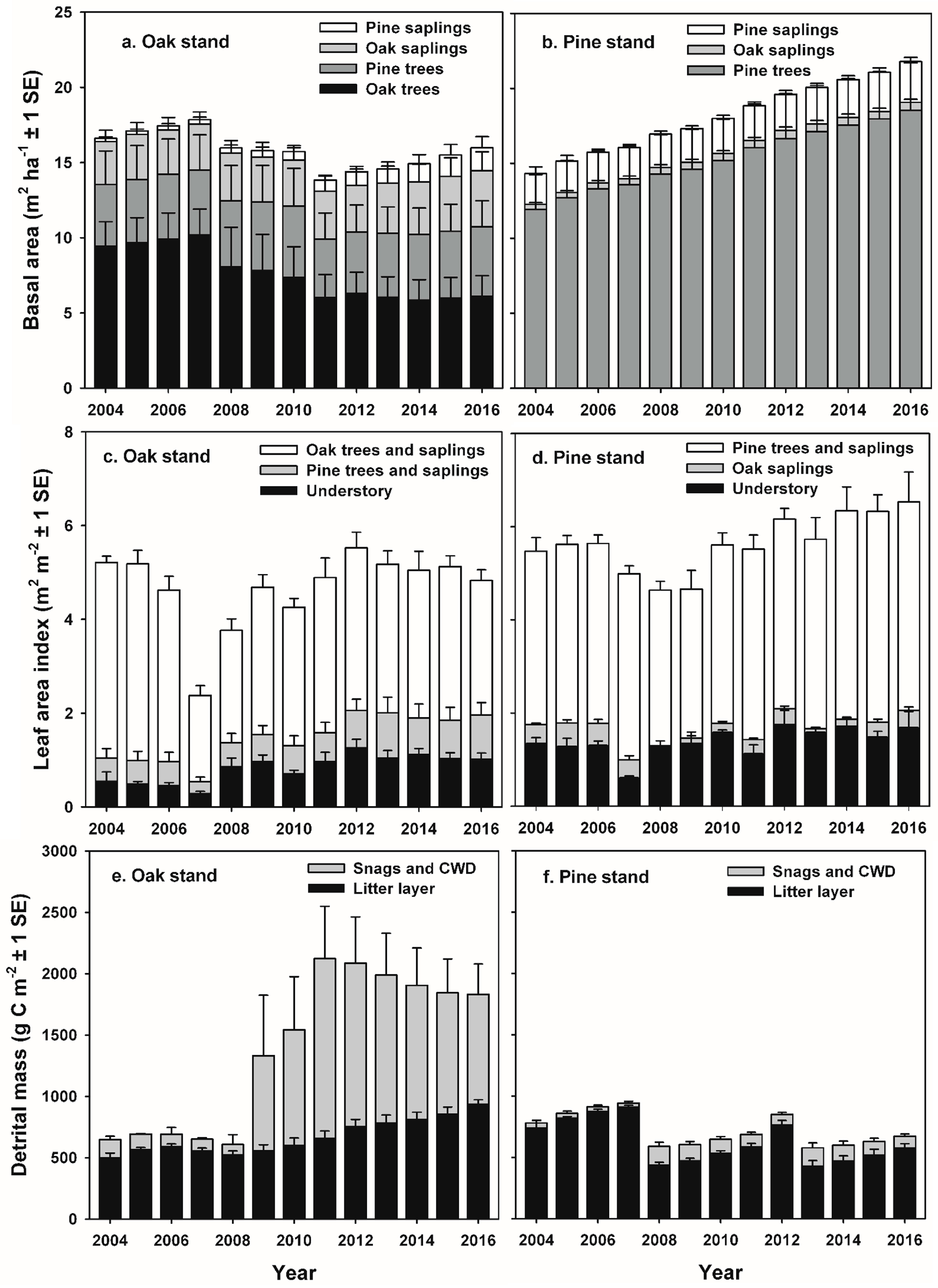
Our study indicates that infestations of defoliator insects can result in a decadal-scale reduction in NEP in oak-dominated forests of the mid-Atlantic region. In contrast, NEP following planned wildland fires in pine-dominated forests recovered to pre-disturbance values during the following year. Leaf area and GEP recovered rapidly following disturbance at both stands, and ANPP was nearly unaffected at both stands, consistent with patterns of recovery following non-stand-replacing disturbance in other intermediate-age forests [
17,
18]. At the oak stand, oak tree and sapling mortality following gypsy moth defoliation increased snag density and coarse woody debris on the forest floor, and corresponded to prolonged elevated rates of R
e and R
h, and relatively low annual NEP values during the decade following defoliation [
27,
30]. At the pine stand, consumption of the forest floor and understory during each prescribed burn was equivalent to annual NEP over two to three years, but had little effect on R
e or estimated R
h, and NEP was similar to pre-disturbance values in the years following each fire [
35]. When integrated over multiple years, cumulative NEP and net C accumulation by major C pools indicate that patterns of recovery following these two disturbances were divergent, with annual NEP averaging only 22% of pre-disturbance values following insect defoliation, and 106% of pre-disturbance NEP values during years following prescribed burns. Divergent outcomes were related to the fate of detritus following each disturbance and its effect on R
h and R
e. Over the course of our study, the area of forest impacted by insect damage greatly exceeded that affected by wildland fires at landscape and regional scales [
15,
16,
46,
47,
48]. Explicitly simulating C release from detritus following tree and sapling mortality in process-based models will lead to more accurate estimates of the magnitude of the long-term C sink in mid-Atlantic forests.
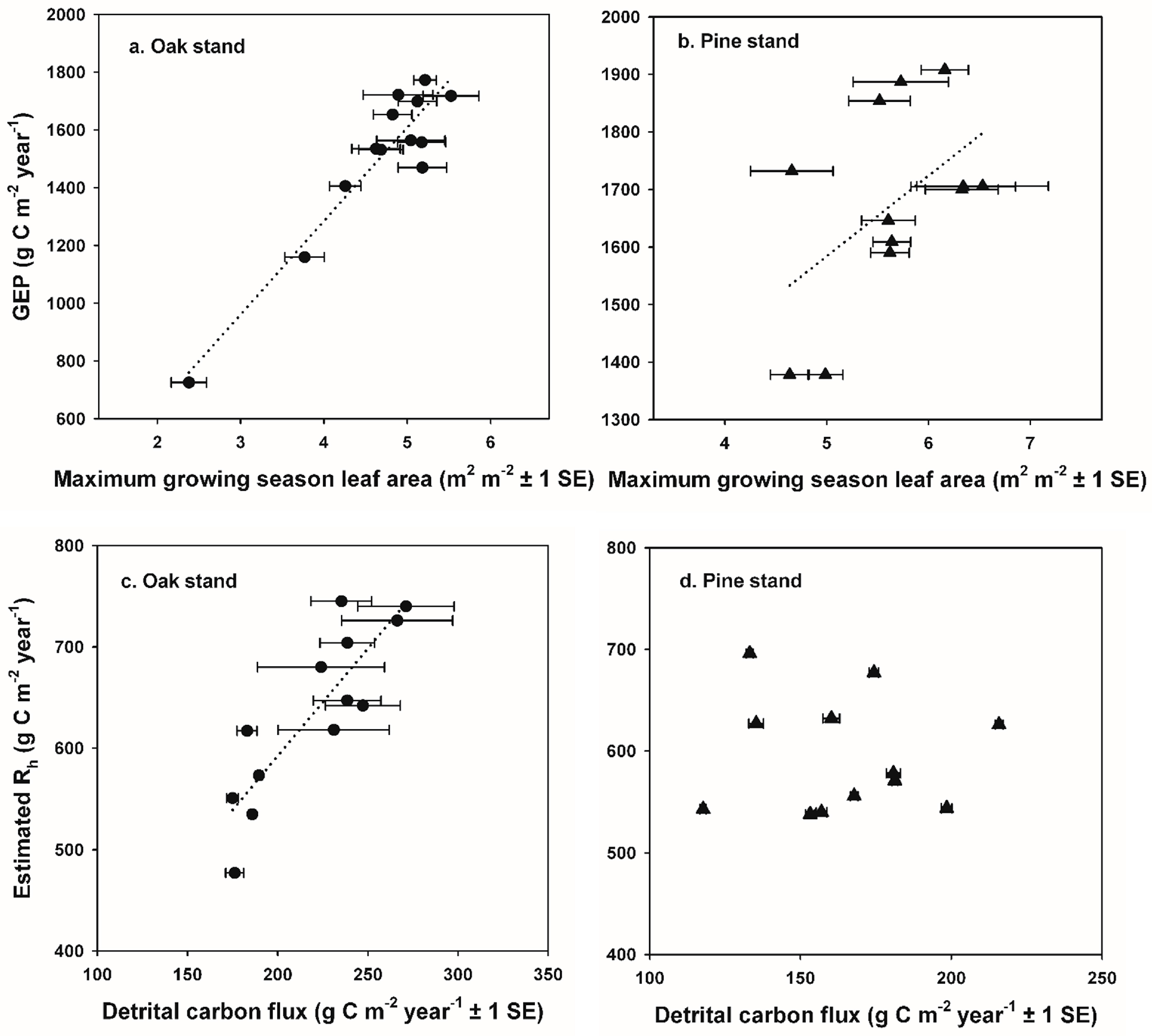
GEP returned to pre-disturbance values soon after each disturbance, corresponding to the rapid recovery of leaf area at each stand [
11,
17,
21,
54,
56]. Following the cessation of herbivory or other disturbances that damage foliage early in the growing season (including experimental defoliation), canopy and understory oak species typically produce a second flush of leaves driven by the reallocation of nonstructural stored carbon (NSC) [
36,
37,
54,
55]. In late spring in 2007 at the oak stand, the second leaf flush following complete defoliation totaled approximately half of spring leaf production and had a lower mean nitrogen content than pre- or post- disturbance periods (1.7% vs. 1.9% nitrogen (N) in canopy foliage) [
36,
37,
54,
57]. Both pitch and shortleaf pines readily produce new shoots and needles from epicormic meristems following insect defoliation or fires [
25,
58]. Resprouting and recovery of ericaceous shrubs following wildland fires or other disturbance is well documented, and is facilitated by extensive belowground stems and root systems [
35,
59,
60]. Our study suggests that compensatory photosynthesis contributed to the maintenance of GEP and ANPP following disturbance [
18,
23]. At the oak stand, partial defoliation of the canopy in 2008 allowed greater penetration of light lower into the sub-canopy and understory, and understory shrubs and scrub oaks responded with a doubling of leaf area that was stable through the end of the study [
33,
37,
49]. Differential mortality of black and white oaks in the canopy following defoliation resulted in large reductions in tree basal area and live biomass, and damaged individuals had reduced stem increments for up to three years following defoliation [
54]. As oak mortality progressed and canopy gaps expanded, compensatory growth of undamaged canopy trees—primarily chestnut and scarlet oaks as well as pitch and shortleaf pines—were important in maintaining GEP and ANPP. Stomatal conductance and whole-tree transpiration measured using sap flux gauges increased for many of the remaining canopy oaks, suggesting that light use efficiency and photosynthetic rates also increased [
23,
61]. As canopy gaps persisted, increased productivity of sub-canopy oaks and pine seedlings and saplings became increasingly important in maintaining GEP and ANPP at the oak stand, similar to results reported for sub-canopy species following non-stand-replacing disturbance in other forests (e.g., [
18,
22,
23,
24,
62]). Although canopy oak species in the PNR have twice the average foliar N concentrations, higher maximum stomatal conductance, and greater water use efficiencies (WUEs) compared to pitch and shortleaf pines, pines and oaks have similar light compensation points, quantum yields, and maximum assimilation rates on a leaf area basis [
61]. Pines also maintain greater leaf area through the dormant season, and are photosynthetically active earlier in the spring and later in the fall compared to oaks [
37,
61]. At the pine stand, rapid recovery of understory foliage likely compensated for canopy foliage that was scorched and abscised prematurely during the relatively intense prescribed fire in 2008 [
25,
34,
35]. Redistribution of nutrients released following disturbance also likely contributed to the maintenance of GEP and ANPP, with mechanisms driving nutrient redistribution related to the type of disturbance. At the oak stand, complete defoliation of the canopy and understory transferred 6.5 g N m
−2 in frass and green leaf fragments to the forest floor by mid-summer, approximately twice the amount of N that would normally occur in litterfall during the fall months [
36,
37]. Canopy foliar N concentrations and total N content were lower in the second flush of foliage following defoliation, and ecosystem WUE was reduced by approximately 42% during the growing season compared to pre-disturbance years [
37,
57]. During the growing seasons following insect defoliation, little difference in foliar N levels or assimilation rates of canopy oak foliage occurred, but overall canopy N content was lower following oak mortality compared to the pre-disturbance period [
61,
63]. However, foliar N content of sub-canopy and understory species increased following defoliation, and thus little net change in the total amount of N in foliage occurred and ecosystem WUE was not significantly different pre- and post-disturbance [
37,
57,
63]. Increased foliar N levels and enhanced maximum assimilation rates in the remaining pine needles were observed following relatively intense prescribed burns in the PNR, but not during low-intensity burns [
25,
64]. Increased N levels and photosynthetic rates following wildland fires are likely related to the transient pulse of inorganic N associated with consumption of forest floor material, in addition to pyro-mineralization and release of phosphorus and basic cations [
65,
66]. Enhanced photosynthetic rates in pine needles did not persist beyond the first growing season following each fire [
25,
64].
Non-stand-replacing disturbances can initially lead to reduced R
e, R
a and soil respiration, because reduction in leaf area and canopy photosynthesis results in reduced C supply to fine roots and the rhizosphere [
22,
32,
67,
68], and alters the allocation of NSC to storage pools [
54,
55]. Simultaneously, an increase or decrease in detrital pools can occur, and respiration of previously-fixed C can lead to increased R
h [
27,
28,
29,
30,
69]. The initial reduction in R
e during defoliation by gypsy moth in 2007 and 2008 at the oak stand is consistent with patterns of reduced R
e and soil CO
2 flux reported following mortality by mountain pine beetle in the Rocky Mountains of the western USA [
22,
31,
32,
68], and with girdling treatments in a variety of forests (e.g., [
67,
70,
71]). Although we lack long-term measurements of soil respiration, fine root productivity, and changes in O horizon and soil C pools, estimated partitioning of R
e following [
17,
51] indicates that R
a was relatively small in magnitude while R
h was similar to pre-disturbance values, suggesting that the supply of labile C to fine roots and associated mycorrhizal fungi in the rhizosphere was limited during and immediately following insect defoliation at the oak stand.
The reduction in R
e that we observed was relatively short-lived and limited to the two years when defoliation occurred at the oak stand, and the transition to increased R
h occurred during the year following defoliation, as mortality of oak trees and saplings increased standing dead and forest floor detritus. Our results are consistent with experimental girdling treatments in an intermediate-age oak-dominated forest in New York, USA that resulted in a 50% mortality of red (
Quercus rubra L.), black, white, and chestnut oaks, where soil respiration returned to pre-disturbance levels after two years [
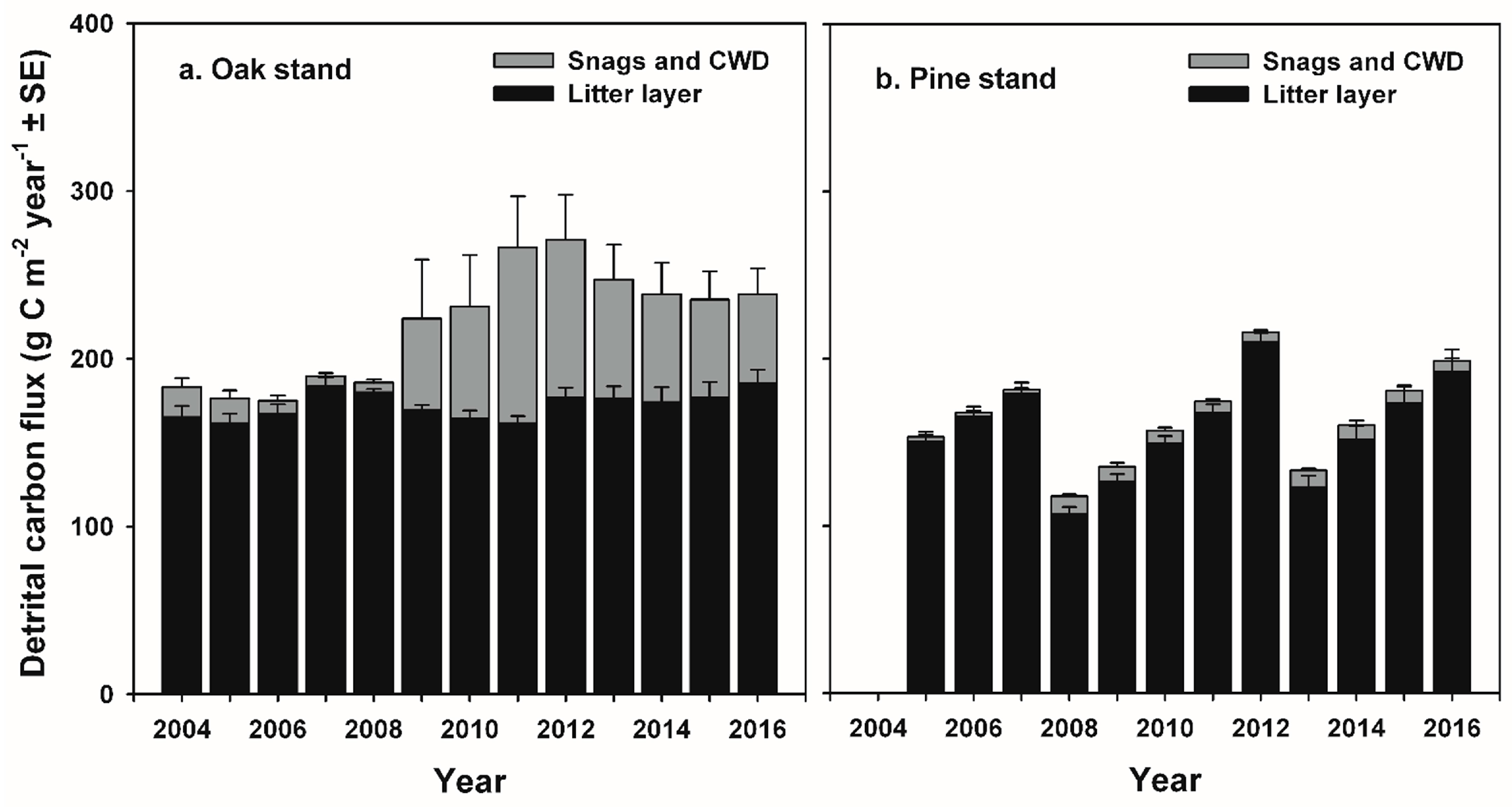
71]. At the oak stand, estimated respiration from additional snags and CWD ≥ 7 cm diameter in the FIA-type plots contributed 30 g C m
−2 year
−1 and 46 g C m
−2 year
−1 to annual R
e at the oak stand in 2009 and 2011, representing approximately 2 and 4% of R
e, and 18% and 27% of pre-disturbance NEP values, respectively [
27]. Our estimates of C fluxes from CWD are somewhat higher (up to 7% of R
e and 68% of pre-disturbance NEP) than those reported in [
27] because we estimated CO
2 fluxes from smaller-diameter stems, and tree mortality continued through 2012. The increase in respiration from detritus following non stand-replacing disturbance is consistent with experimental girdling and overstory mortality in an intermediate-age aspen (
Populus gradidentata Michaux) and birch (
Betula papyrifera Marshall) stand that resulted in an increase of approximately 3500 g C m
−2 as snags and CWD, which enhanced R
h from snags and CWD from 110 to 210 g C m
−2 year
−1, and accounted for 12 to 24% of R
e six years following girdling [
30]. In the long-term, our results contrast with the persistent reduction in R
e and soil respiration reported following stand damage by mountain pine beetle [
22,
31,
32,
68], and in girdling experiments documented in [
21,
30]. Much of the insect damage in oak-dominated forests in the PNR resulted in mortality of <40% of canopy trees, and not to the extent of canopy tree mortality observed in stands impacted by mountain pine beetle (40% to >80% mortality) [
13,
22,
26,
72] or of southern pine beetle in the PNR (>90% pine mortality) [
47]. In comparison to other stands impacted by insect infestations, a threshold likely exists where low levels of mortality are insufficient to reduce components of R
e—including respiration from live fine roots and mycorrhizal fungi in the rhizosphere—for long periods of time, but still severe enough to increase detrital mass and R
h [
27,
30,
73]. Enhanced dynamics of snags and CWD in our study are also likely related to climatic differences, as CO
2 release from detritus had a strong temperature dependence early in the decomposition process, with r
2 values of 0.59 and 0.81 for the relationship between substrate temperature and CO
2 emissions for standing dead and CWD, respectively [
27], as well as the presence of abundant fungal decomposers [
74] and macroinvertebrates such as termites in the PNR.
Our study indicates that infestations of defoliating insects will have significant consequences for the NEP of oak-dominated forests at landscape to regional scales in the future. We previously used aerial survey data to estimate that severe gypsy moth defoliation of approximately 20% of the upland oak-, mixed, and pine-dominated stands in the 400,000 ha Pinelands National Reserve in 2007 reduced landscape-scale NEP by approximately 41% [
36,
75]. During the course of our study (2004–2016), moderate-to-severe defoliation and tree mortality caused by gypsy moth totaled 503,000 ha, and damage by southern pine beetle resulting in >90% pine tree mortality totaled 19,550 ha in New Jersey alone, equivalent to approximately 65% of the forested area in the state [
46,
47,
76]. The proportion of forest area impacted by insects in New Jersey during some years of our study exceeded 12% year
−1, one of the highest rates of forest disturbance in the mid-Atlantic or northeastern USA, which averaged 2 to 4% year
−1 over the same period [
15,
16,
76].
Aerial surveys and remotely sensed information derived from Landsat, MODIS, and other satellite products are highly effective at detecting changes in leaf area resulting from insect defoliation or wildland fire [
8,
15,
16,
45,
76]. However, changes in tree and sapling biomass, and the increase in standing dead and CWD that we quantified using a much denser network of FIA-type forest inventory plots that was sampled more frequently than standard FIA plots distributed across the region are more difficult to detect remotely. Repeated LiDAR acquisitions have successfully detected changes in canopy and sub-canopy biomass following insect defoliation and tree mortality as well as wildland fires, but accurately sampling changes in detrital mass on the forest floor is more difficult [
33,
34,
71]. Similarly, predicting the impact of invasive insects on long-term forest C dynamics using process-based simulation models has been only partially successful. Although process-based models have accurately captured the effects of gypsy moth defoliation on tree biomass, stand species composition, and the dynamics of GEP and ANPP, they have generally underestimated the long-term reduction in NEP in forests of the PNR following disturbance [
9,
10,
11]. For example, the Ecosystem Demography 2 Model parameterized for xeric hardwoods and conifers and to represent defoliation events and tree mortality at oak-dominated stands in the PNR over 200-year simulations indicated that defoliation intensity was linearly related to decreases in annual NEP, but also predicted that post-disturbance NEP exceeded 80 to 90 g C m
−2 year
−1 at all levels of defoliation [
11]. Simulations with LANDIS II coupled with the CENTURY succession extension [
10] predicted changes in species composition and reduced live tree biomass accurately in the PNR, but also predicted relatively little effect on long-term NEP following periodic gypsy moth outbreaks over 100-year simulations [
10]. Neither of these models accurately capture the dynamics of detritus resulting from tree and sapling mortality, which our study demonstrates is important in the decadal-scale reduction in NEP. More recently, parameterization of PnET-CN [
12]) using extensive forest inventory data and tree mortality rates to explicitly simulate wood turnover in mid-Atlantic forests where oak mortality was significant resulted in relatively low estimates of annual NEP that are similar in magnitude to those measured in our study [
12,
77].
In comparison to insect damage, wildfires and prescribed burns have disturbed much less area in New Jersey, with 36,656 ha affected by wildfires and 76,829 ha affected by prescribed burning over the course of our study [
45,
78]. Consumption of the forest floor and understory across a range of prescribed burns conducted in oak-, mixed-, and pine- dominated forests is equivalent to approximately two to four years annual NEP [
35], but prescribed fires apparently have little effect on the ratio of GEP to R
e or to estimated R
h, in contrast to simulations using BiomBGC (Terrestrial Ecosystem Process Model) [
19] that predicted litter consumption by prescribed fires would reduce R
h and increase NEP (e.g., [
79,
80]). Future improvements to process-based models simulating forest carbon cycling and the impacts of insects and wildland fires should focus on a better representation of the dynamics of snags, CWD, and the forest floor following disturbance, such as the approach employed by [
12].
Forests in the mid-Atlantic and northeastern USA are exposed to a relatively large and increasing number of invasive insects and pathogens that can result in increased rates of tree and sapling mortality, including gypsy moth, southern pine beetle, emerald ash borer (
Agrilus planipennis), hemlock wooly adelgid (
Adelges tsugae), and spruce budworm (
Choristoneura spp.) [
13,
14,
15,
16,
47,
48,
81]. If our results can be extended to intermediate-age forests across these regions that will be impacted by insect damage in the future, the potential for long-term reductions in NEP dwarfs C losses from all other disturbances other than forest harvesting [
15,
16,
81]. Accurately detecting and accounting for the impacts of invasive and native insects on rates of tree mortality and enhanced losses of C via respiratory pathways in process-based simulation models will greatly improve our estimates of the magnitude of the long-term CO
2 sink in forests in the mid-Atlantic and northeastern USA.