Methods to Improve Survival and Growth of Planted Alternative Species Seedlings in Black Ash Ecosystems Threatened by Emerald Ash Borer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ottawa National Forest Site Description
2.2. Ottawa National Forest Study Design
2.3. Superior Municipal Forest Site Description
2.4. Superior Municipal Forest Study Design
2.5. Field and Laboratory Procedures
2.6. Analysis
3. Results
3.1. Ottawa National Forest
3.2. Superior Municipal Forest
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haack, R.; Jendek, E.; Liu, H.; Marchant, K.; Petrice, T.; Poland, T.; Ye, H. The emerald ash borer: A new exotic Pest in North America. Newslett. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Siegert, N.; McCullough, D.; Liebhold, A.; Telewski, F. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- MacFarlane, D.; Meyer, S. Characteristics and distribution of potential ash tree hosts for emerald ash borer. For. Ecol. Manag. 2005, 213, 15–24. [Google Scholar] [CrossRef]
- Marshall, J.; Smith, E.; Mech, R.; Storer, A. Estimates of Agrilus planipennis infestation rates and potential survival of ash. Am. Midl. Nat. 2013, 169, 179–193. [Google Scholar] [CrossRef]
- Herms, D.; McCullough, D. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, G.; Crow, T.; Ralph, M., Jr.; Wilson, C. Managing black ash in the Lake States. In General Technical Report NC-115; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1987. [Google Scholar]
- Wright, J.; Rauscher, H. Fraxinus nigra marsh. Black ash. Silv. N. Am. 1990, 2, 344–347. [Google Scholar]
- Hewlett, J.; Fortson, J. Stream temperature under an inadequate buffer strip in the southeast piedmont. J. Am. Water Resour. Assoc. 1982, 18, 983–988. [Google Scholar] [CrossRef]
- Bourque, C.A.; Pomeroy, J.H. Effects of forest harvesting on summer stream temperatures in New Brunswick, Canada: An inter-catchment, multiple-year comparison. Hydrol. Earth Syst. Sci. Discuss. 2001, 5, 599–614. [Google Scholar] [CrossRef]
- Sheridan, J.; Lowrance, R.; Bosch, D. Management effects on runoff and sediment transport in riparian forest buffers. Trans. Am. Soc. Agric. Eng. 1999, 42, 55–64. [Google Scholar] [CrossRef]
- Lowrance, R.; Altier, L.; Newbold, J.; Schnabel, R.; Groffman, P.; Denver, J.; Correll, D.; Gilliam, J.; Robinson, J.; Brinsfield, R. Water quality functions of riparian forest buffers in Chesapeake Bay watersheds. Environ. Manag. 1997, 21, 687–712. [Google Scholar] [CrossRef]
- Davis, J.; Shannon, J.; Bolton, N.; Kolka, R.; Pypker, T. Vegetation responses to simulated emerald ash borer infestation in Fraxinus nigra-dominated wetlands of Upper Michigan, USA. Can. J. For. Res. 2017, 47, 319–330. [Google Scholar] [CrossRef]
- Palik, B.; Ostry, M.; Venette, R.; Abdela, E. Fraxinus nigra (black ash) dieback in Minnesota: Regional variation and potential contributing factors. For. Ecol. Manag. 2011, 261, 128–135. [Google Scholar] [CrossRef]
- Palik, B.; Ostry, M.; Venette, R.; Abdela, E. Tree regeneration in black ash (Fraxinus nigra) stands exhibiting crown dieback in Minnesota. For. Ecol. Manag. 2012, 269, 26–30. [Google Scholar] [CrossRef]
- Ponnamperuma, F. Effects of flooding on soils. In Flooding and Plant Growth; Academic Press, Inc.: New York, NY, USA, 1984; pp. 9–45. [Google Scholar]
- Roy, V.; Bernier, P.; Plamondon, A.; Ruel, J. Effect of drainage and microtopography in forested wetlands on the microenvironment and growth of planted black spruce seedlings. Can. J. For. Res. 1999, 29, 563–574. [Google Scholar] [CrossRef]
- Looney, C.; D’Amato, A.; Palik, B.; Slesak, R. Overstory treatment and planting season affect survival of replacement tree species in emerald ash borer threatened Fraxinus nigra forests in Minnesota, USA. Can. J. For. Res. 2015, 45, 1728–1738. [Google Scholar] [CrossRef]
- Staff, S.S. Natural Resources Conservation Service Web Soil Survey, United States Department of Agriculture. 2017. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 26 April 2017).
- Burns, R.; Honkala, B. Silvics of North America: 1. Conifers; 2. Hardwoods; United States Department of Agriculture: Washington, DC, USA, 1990.
- WinSCANOPY, Pro Version ed; Regent Instruments Inc.: Quebec, QC, Canada, 2010.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Van Grinsven, M.; Shannon, J.; Davis, J.; Bolton, N.; Wagenbrenner, J.; Kolka, R.; Pypker, T. Source water contributions and hydrologic responses to simulated emerald ash borer infestations in depressional black ash wetlands. Ecohydrology 2017, 10, e1862. [Google Scholar] [CrossRef]
- Londo, A.; Mroz, G. Bucket mounding as a mechanical site preparation technique in wetlands. North. J. Appl. For. 2001, 18, 7–13. [Google Scholar]
- Van Grinsven, M. Implications of Emerald Ash Borer Disturbance on Black Ash Wetland Watershed Hydrology, Soil Carbon Efflux, and Dissolved Organic Matter. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2015. [Google Scholar]
- Slesak, R.A.; Lenhart, C.F.; Brooks, K.N.; D’Amato, A.W.; Palik, B.J. Water table response to harvesting and simulated emerald ash borer mortality in black ash wetlands in Minnesota, USA. Can. J. For. Res. 2014, 44, 961–968. [Google Scholar] [CrossRef]
- Shannon, J.; Van Grinsven, M.; Davis, J.; Bolton, N.; Noh, N.; Pypker, T.; Kolka, R. Water level controls on sap flux of canopy species in black ash wetlands. Forests 2018. accepted. [Google Scholar]
- Williams, M.; Dumroese, R. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Iverson, L.; Knight, K.S.; Prasad, A.; Herms, D.A.; Matthews, S.; Peters, M.; Smith, A.; Hartzler, D.M.; Long, R.; Almendinger, J. Potential species replacements for black ash (Fraxinus nigra) at the confluence of two threats: Emerald ash borer and a changing climate. Ecosystems 2016, 19, 248–270. [Google Scholar] [CrossRef]
- Janowiak, M.; Iverson, L.; Mladenoff, D.; Peters, E.; Wythers, K.; Xi, W.; Brandt, L.; Butler, P.; Handler, S.; Shannon, P.; et al. Forest Ecosystem Vulnerability Assessment and Synthesis for Northern Wisconsin and Western Upper Michigan: A Report from the Northwoods Climate Change Response Framework Project; General Technical Report NRS-136; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014; Volume 247.
- Chimner, R.; Hart, J. Hydrology and microtopography effects on northern white-cedar regeneration in michigan’s Upper Peninsula. Can. J. For. Res. 1996, 26, 389–393. [Google Scholar] [CrossRef]
- Cornett, M.; Frelich, L.; Puettmann, K.; Reich, P. Conservation implications of browsing by Odocoileus virginianus in remnant upland Thuja occidentalis forests. Biol. Conserv. 2000, 93, 359–369. [Google Scholar] [CrossRef]
- Rooney, T.; Waller, D. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Russell, F.; Zippin, D.; Fowler, N. Effects of white-tailed deer (Odocoileus virginianus) on plants, plant populations and communities: A review. Am. Midl. Nat. 2001, 146, 1–26. [Google Scholar] [CrossRef]
- Abrams, M.D. The red maple paradox. BioScience 1998, 48, 355–364. [Google Scholar] [CrossRef]
- Kobe, R.; Pacala, S.; Silander, J.; Canham, C. Juvenile tree survivorship as a component of shade tolerance. Ecol. Appl. 1995, 5, 517–532. [Google Scholar] [CrossRef]
- Krajicek, J.; Williams, R. Celtis occidentalis L. Hackberry. Silv. N. Am. 1990, 2, 262. [Google Scholar]
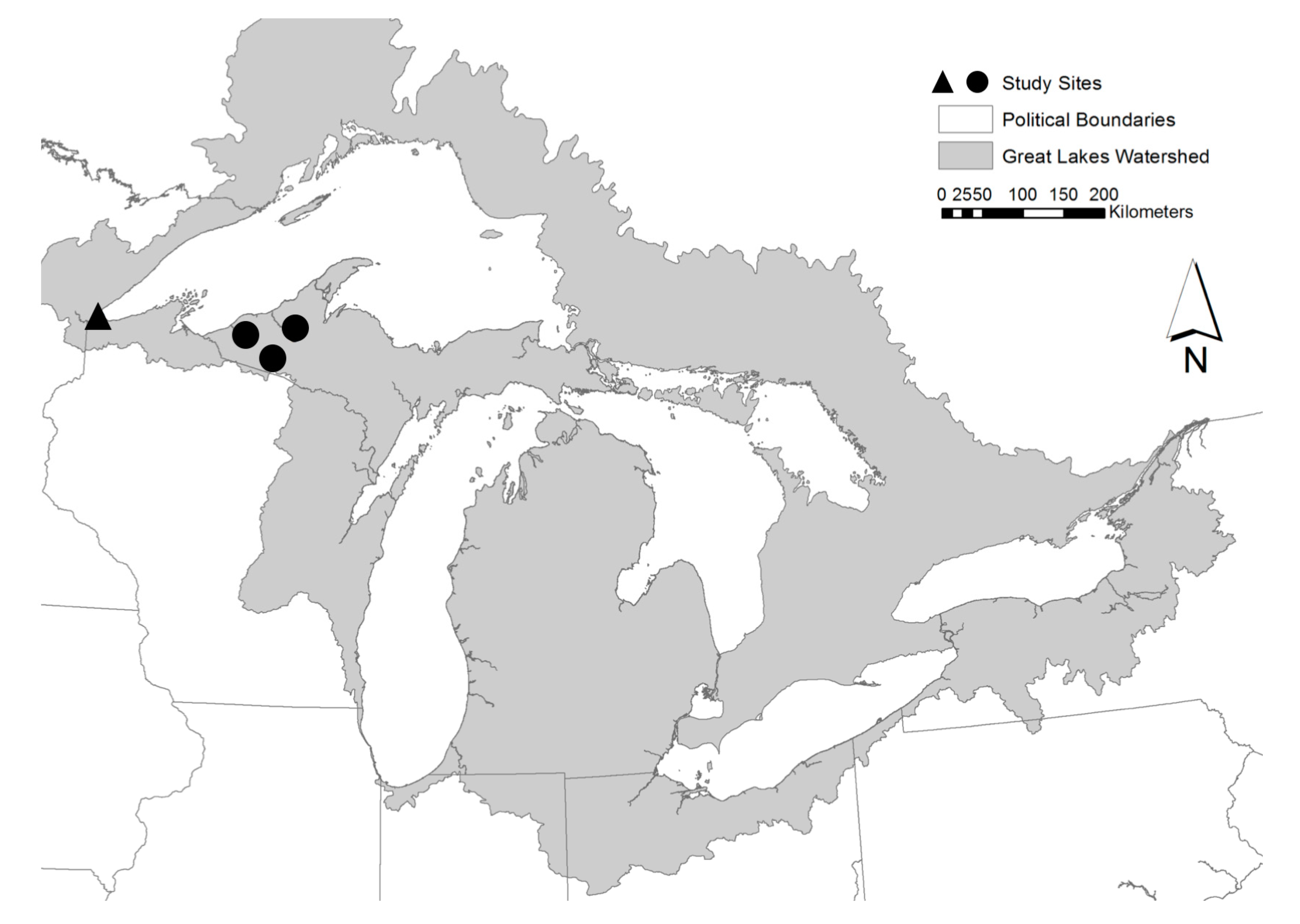
| Site | Percent Canopy Black Ash (%) | Planting Year | Soil Type | Elevation (m) | Canopy Openness (%) |
|---|---|---|---|---|---|
| ONF Control | 48 | 2013 | Woody peat Histosol | 507 | 19.7 |
| ONF Girdle | 88 | 2013 | Woody peat Histosol | 499 | 16.5 |
| ONF Ash-Cut | 38 | 2013 | Woody peat Histosol | 371 | 6.6 |
| SMF | 90 | 2015 | Arnheim mucky silt loam or Udifluvents | 183 | Closed–open |
| Common Name | Scientific Name | Age (Years) | Stock Type |
|---|---|---|---|
| American elm | Ulmus Americana L. | 2 | P |
| basswood (linden) | Tilia americana L. | 3 | BR |
| burr oak | Quercus macrocarpa Michx. | 3 | BR |
| red maple | Acer rubrum L. | 2 | BR |
| silver maple | Acer saccharinum L. | 4 | BR |
| yellow birch | Betula alleghaniensis Britton | 2 | P |
| balsam fir | Abies balsamea (L.) Mill | 2 | BR |
| black spruce | Picea marina (Mill.) Britton | 2 | P |
| northern white cedar | Thuja occidentalis L. | 2 | P |
| tamarack | Larix larcinia K. Koch | 2 | BR |
| Common Name | Scientific Name | Age (Years) | Stock Type |
|---|---|---|---|
| hackberry | Celtis occidentalis L. | 2 | BR |
| red maple | Acer rubrum L. | 2 | BR |
| northern white cedar | Thuja occidentalis L. | 2 | BR |
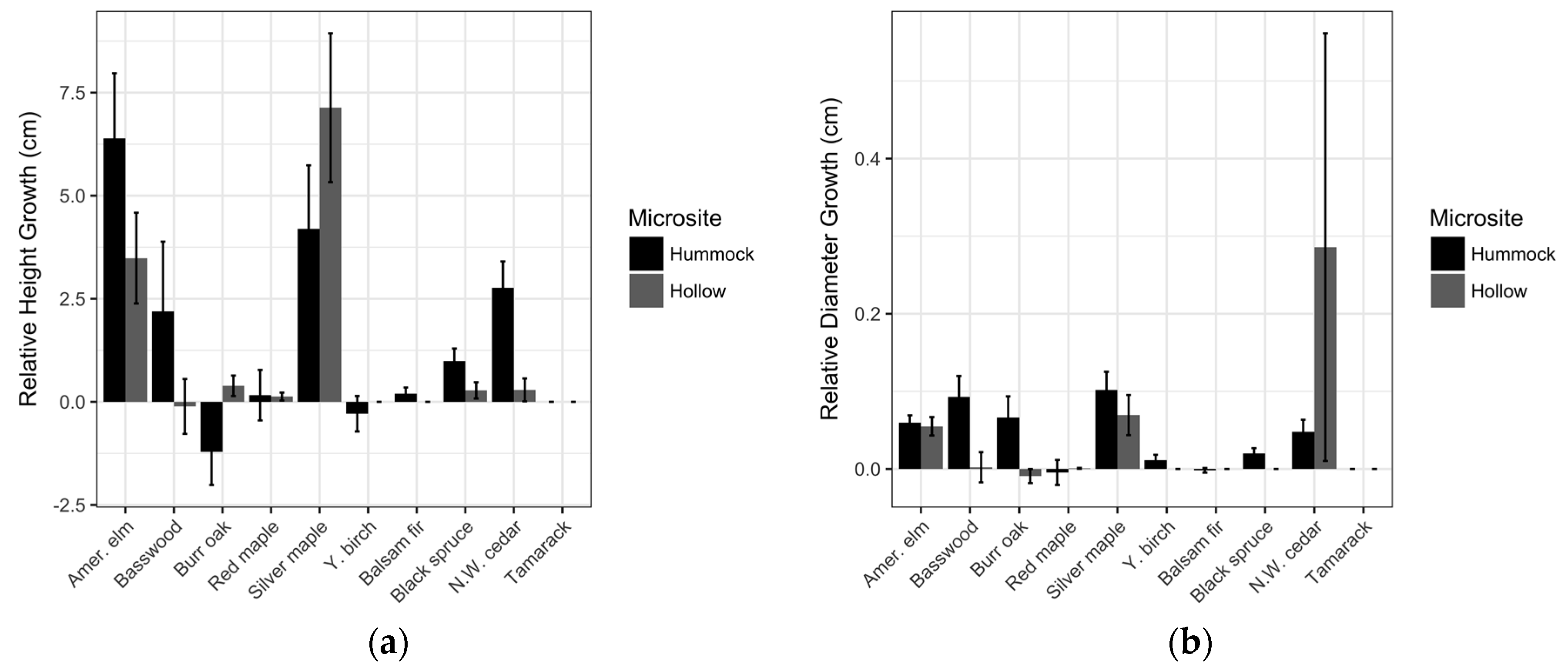
| Species | Microsite | Survival (%) | Relative Height Growth (cm) | Relative Diameter Growth (cm) |
|---|---|---|---|---|
| American elm | Hummock | 68 * | 6.4 ± 15.0 | 0.1 ± 0.1 |
| Hollow | 38 | 3.5 ± 10.4 | 0.1 ± 0.1 | |
| Basswood (linden) | Hummock | 64 * | 2.2 ± 16.0 | 0.1 ± 0.3 |
| Hollow | 17 | −0.1 ± 6.3 | 0.0 ± 0.2 | |
| burr oak | Hummock | 38 * | −1.2 ± 7.6 | 0.0 ± 0.3 |
| Hollow | 11 | 0.4 ± 2.4 | 0.0 ± 0.1 | |
| red maple | Hummock | 11 * | 0.2 ± 5.8 | 0.0 ± 0.2 |
| Hollow | 2 | 0.1 ± 1.0 | 0.0 ± 0.0 | |
| silver maple | Hummock | 76 | 4.2 ± 14.7 | 0.1 ± 0.2 |
| Hollow | 72 | 7.1 ± 17.1 | 0.1 ± 0.3 | |
| yellow birch | Hummock | 8 * | −0.3 ± 4.0 | 0.0 ± 0.1 |
| Hollow | 0 | - | - | |
| balsam fir | Hummock | 7 * | 0.2 ± 1.4 | 0.0 ± 0.0 |
| Hollow | 0 | - | - | |
| black spruce | Hummock | 13 * | 0.9 ± 2.9 | 0.0 ± 0.1 |
| Hollow | 2 | 0.3 ± 1.9 | 0.0 ± 0.0 | |
| northern white cedar | Hummock | 39 * | 2.8 ± 6.1 | 0.1 ± 0.2 |
| Hollow | 8 | 0.3 ± 2.6 | 0.3 ± 2.6 | |
| tamarack | Hummock | 0 | - | - |
| Hollow | 0 | - | - |
| Species | Microsite | Survival (%) | Relative Height Growth (cm) | Relative Diameter Growth (cm) |
|---|---|---|---|---|
| hackberry | CH | 66 | −0.1 ± 14.6 | 0.4 ± 5.6 |
| N | 60 | −0.9 ± 11.9 | −0.6 ± 1.8 | |
| S | 58 | −1.0 ± 10.4 | −0.6 ± 1.7 | |
| red maple | CH | 68 | 12.2 ± 20.9 | 0.2 ± 1.8 |
| N | 57 | 10.9 ± 19.9 | −0.5 ± 1.8 | |
| S | 63 | 6.4 ± 14.1 | −0.6 ± 1.2 | |
| northern white cedar | CH | 39 | −1.2 ± 7.9 | 0 ± 1.4 |
| N | 43 | 0.2 ± 5.6 | −0.1 ± 1.3 | |
| S | 32 | 0.3 ± 9.7 | −0.1 ± 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolton, N.; Shannon, J.; Davis, J.; Grinsven, M.V.; Noh, N.J.; Schooler, S.; Kolka, R.; Pypker, T.; Wagenbrenner, J. Methods to Improve Survival and Growth of Planted Alternative Species Seedlings in Black Ash Ecosystems Threatened by Emerald Ash Borer. Forests 2018, 9, 146. https://doi.org/10.3390/f9030146
Bolton N, Shannon J, Davis J, Grinsven MV, Noh NJ, Schooler S, Kolka R, Pypker T, Wagenbrenner J. Methods to Improve Survival and Growth of Planted Alternative Species Seedlings in Black Ash Ecosystems Threatened by Emerald Ash Borer. Forests. 2018; 9(3):146. https://doi.org/10.3390/f9030146
Chicago/Turabian StyleBolton, Nicholas, Joseph Shannon, Joshua Davis, Matthew Van Grinsven, Nam Jin Noh, Shon Schooler, Randall Kolka, Thomas Pypker, and Joseph Wagenbrenner. 2018. "Methods to Improve Survival and Growth of Planted Alternative Species Seedlings in Black Ash Ecosystems Threatened by Emerald Ash Borer" Forests 9, no. 3: 146. https://doi.org/10.3390/f9030146






