Resolving Variables Influencing the Residence Time of Biomass in the Old-Age Forest across Climate Gradients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Analytical Framework
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.J. Carbon dioxide concentration, photosynthesis, and dry matter production. Bioscience 1981, 31, 29–33. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. Lond. 2011, 366, 3225–3245. [Google Scholar] [CrossRef] [PubMed]
- Winjum, J.K.; Dixon, R.K.; Schroeder, P.E. Forest management and carbon storage: An analysis of 12 key forest nations. Water Air Soil Pollut. 1993, 70, 239–257. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Z.; Hu, H.; Kato, T.; Muraoka, H.; Son, Y. Forest biomass carbon sinks in east asia, with special reference to the relative contributions of forest expansion and forest growth. Glob. Chang. Biol. 2014, 20, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Creutzburg, M.K.; Scheller, R.M.; Lucash, M.S.; LeDuc, S.D.; Johnson, M.G. Forest management scenarios in a changing climate: Trade-offs between carbon, timber, and old forest. Ecol. Appl. 2017, 27, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Soloway, A.D.; Amiro, B.D.; Dunn, A.L.; Wofsy, S.C. Carbon neutral or a sink? Uncertainty caused by gap-filling long-term flux measurements for an old-growth boreal black spruce forest. Agric. For. Meteorol. 2017, 233, 110–121. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.Q.; Higuchi, N.; Tribuzy, E.S.; Trumbore, S.E. Carbon sink for a century. Nature 2001, 410, 429. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.A.; Shugart, H.H.; White, M.A. Sensitivity of russian forest timber harvest and carbon storage to temperature increase. Forestry 2013, 86, 283–293. [Google Scholar] [CrossRef]
- Lloyd, J.; Farquhar, G.D. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct. Ecol. 1996, 10, 4–32. [Google Scholar] [CrossRef]
- Galbraith, D.; Malhi, Y.; Affum-Baffoe, K.; Castanho, A.D.; Doughty, C.E.; Fisher, R.A.; Lewis, S.L.; Peh, K.S.-H.; Philips, O.L.; Quesada, C.A.; et al. Residence times of woody biomass in tropical forests. Trans. Bot. Soc. Edinb. 2013, 6, 139–157. [Google Scholar] [CrossRef]
- Coomes, D.A.; Flores, O.; Holdaway, R.; Jucker, T.; Lines, E.R.; Vanderwel, M.C. Wood production response to climate change will depend critically on forest composition and structure. Glob. Chang. Biol. 2015, 20, 3632–3645. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, H.Y. Climate change-associated tree mortality increases without decreasing water availability. Ecol. Lett. 2015, 18, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Moorcroft, P.R. Tree mortality in the eastern and central United States: Patterns and drivers. Glob. Chang. Biol. 2011, 17, 3312–3326. [Google Scholar] [CrossRef]
- Lines, E.R.; Coomes, D.A.; Purves, D.W. Influences of forest structure, climate and species composition on tree mortality across the eastern us. PLoS ONE 2010, 5, e13212. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Baker, T.R.; Phillips, O.L.; Almeida, S.; Alvarez, E.; Arroyo, L.; Chave, J.; Czimczik, C.I.; Fiore, A.D.; Higuchi, N. The above-ground coarse wood productivity of 104 neotropical forest plots. Glob. Chang. Biol. 2004, 10, 563–591. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in amazon forest structure and function are mediated by both soils and climate. Biogeosci. Discuss. 2012, 9, 2203–2246. [Google Scholar] [CrossRef] [Green Version]
- Michaletz, S.T.; Cheng, D.; Kerkhoff, A.J.; Enquist, B.J. Convergence of terrestrial plant production across global climate gradients. Nature 2014, 512, 39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wei, X.; Liu, Y.; Liu, G.; Wang, W.; Liu, W. Review of regional carbon counting methods for the chinese major ecological engineering programs. J. For. Res. 2016, 27, 727–738. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragcdo, L.E.O.C.; Lobo-Do-Vale, R.; Galbraith, D.; Fisher, R.A.; Chaves, M.M.; Maroco, J.P.; Costa, A.C.L.D.; De Almeida, S.S. Shifts in plant respiration and carbon use efficiency at a large-scale drought experiment in the eastern amazon. New Phytol. 2010, 187, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; Lachenbruch, B.; Dawson, T.E. Size- and Age-Related Changes in Tree Structure and Function; Springer: Dordrecht, The Netherlands, 2011; pp. 6891–6892. [Google Scholar]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Ruger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 50, 7490. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Turnbull, M.H.; Tissue, D.T.; Lewis, J.D.; Carson, R.; Schuster, W.S.F.; Whitehead, D.; Walcroft, A.S.; Li, J.; Griffin, K.L. Age-related decline of stand biomass accumulation is primarily due to mortality and not to reduction in NPP associated with individual tree physiology, tree growth or stand structure in a quercus-dominated forest. J. Ecol. 2012, 100, 428–440. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.D.; Lucht, W.; Rademacher, T.T.; Keribin, R.; Betts, R.; Cadule, P.; Ciais, P.; Clark, D.B.; Dankers, R.; Falloon, P.D. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric co2. Proc. Natl. Acad. Sci. USA 2014, 111, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Andreas, B.; Christian, A.; Magnus, L.; Palle, M.; Gertjan, N.; Peter, S.; Peter, S.; Joachim, R. Adaptive forest management in central europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar]


| Variable | Mean | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Age | 152.1 | 51.45 | 101 | 450 |
| MAT | 41.63 | 0.94 | 38.67 | 43.46 |
| MAP | 825.0 | 475.2 | 61.5 | 2784 |
| GSL | 6.2 | 2.64 | 1 | 12 |
| ATr | 27.46 | 12.69 | 6.26 | 100.8 |
| Models | P(M) | P(M|Data) | BFM | BF10 | Error % |
|---|---|---|---|---|---|
| Null model | 0.063 | 1.719 × 10−16 | 2.579 × 10−15 | 1 | |
| lgAge | 0.063 | 0.001 | 0.017 | 6.473 × 1012 | 0.004 |
| lgMAP | 0.063 | 2.413 × 10−17 | 3.620 × 10−16 | 0.140 | 0.002 |
| lgAge + lgMAP | 0.063 | 1.887 × 10−4 | 0.003 | 1.098 × 1012 | 5.165 × 10−4 |
| lgMAT | 0.063 | 2.340 × 10−16 | 3.510 × 10−15 | 1.361 | 0.004 |
| lgAge + lgMAT | 0.063 | 0.009 | 0.131 | 5.029 × 1013 | 0.001 |
| lgMAP + lgMAT | 0.063 | 1.643 × 10−16 | 2.464 × 10−15 | 0.956 | 0.01 |
| lgAge + lgMAP + lgMAT | 0.063 | 0.002 | 0.026 | 9.964 × 1012 | 0.004 |
| lgGSL | 0.063 | 3.975 × 10−15 | 5.962 × 10−14 | 23.121 | 0.004 |
| lgAge + lgGSL | 0.063 | 0.587 | 21.281 | 3.412 × 1015 | 0.002 |
| lgMAP + lgGSL | 0.063 | 5.525 × 10−15 | 8.288 × 10−14 | 32.141 | 0.001 |
| lgAge + lgMAP + lgGSL | 0.063 | 0.176 | 3.197 | 1.022 × 1015 | 0.003 |
| lgMAT + lgGSL | 0.063 | 1.503 × 10−15 | 2.255 × 10−14 | 8.746 | 0.004 |
| lgAge + lgMAT + lgGSL | 0.063 | 0.18 | 3.289 | 1.046 × 1015 | 0.003 |
| lgMAP + lgMAT + lgGSL | 0.063 | 1.593 × 10−15 | 2.390 × 10−14 | 9.268 | 0.004 |
| lgAge + lgMAP + lgMAT + lgGSL | 0.063 | 0.046 | 0.727 | 2.690 × 1014 | 0.002 |
| Covariates | Standardized Coefficients | t-Value | p-Value | 2.50% | 97.50% | VIF | Partial | Semipartial |
|---|---|---|---|---|---|---|---|---|
| Intercept | 0.641 | 0.522 | −307.512 | 604.639 | ||||
| lgAge | 0.473 | 8.853 | < 0.001 | 39.545 | 62.171 | 1.018 | 0.483 | 0.469 |
| lgMAP | 0.06 | 0.964 | 0.336 | −3.215 | 9.377 | 1.382 | 0.060 | 0.051 |
| lgMAT | −0.107 | −0.988 | 0.324 | −410.229 | 136.055 | 4.175 | −0.062 | −0.052 |
| lgGSL | −0.344 | −3.274 | 0.001 | −38.184 | −9.503 | 3.927 | −0.200 | −0.173 |
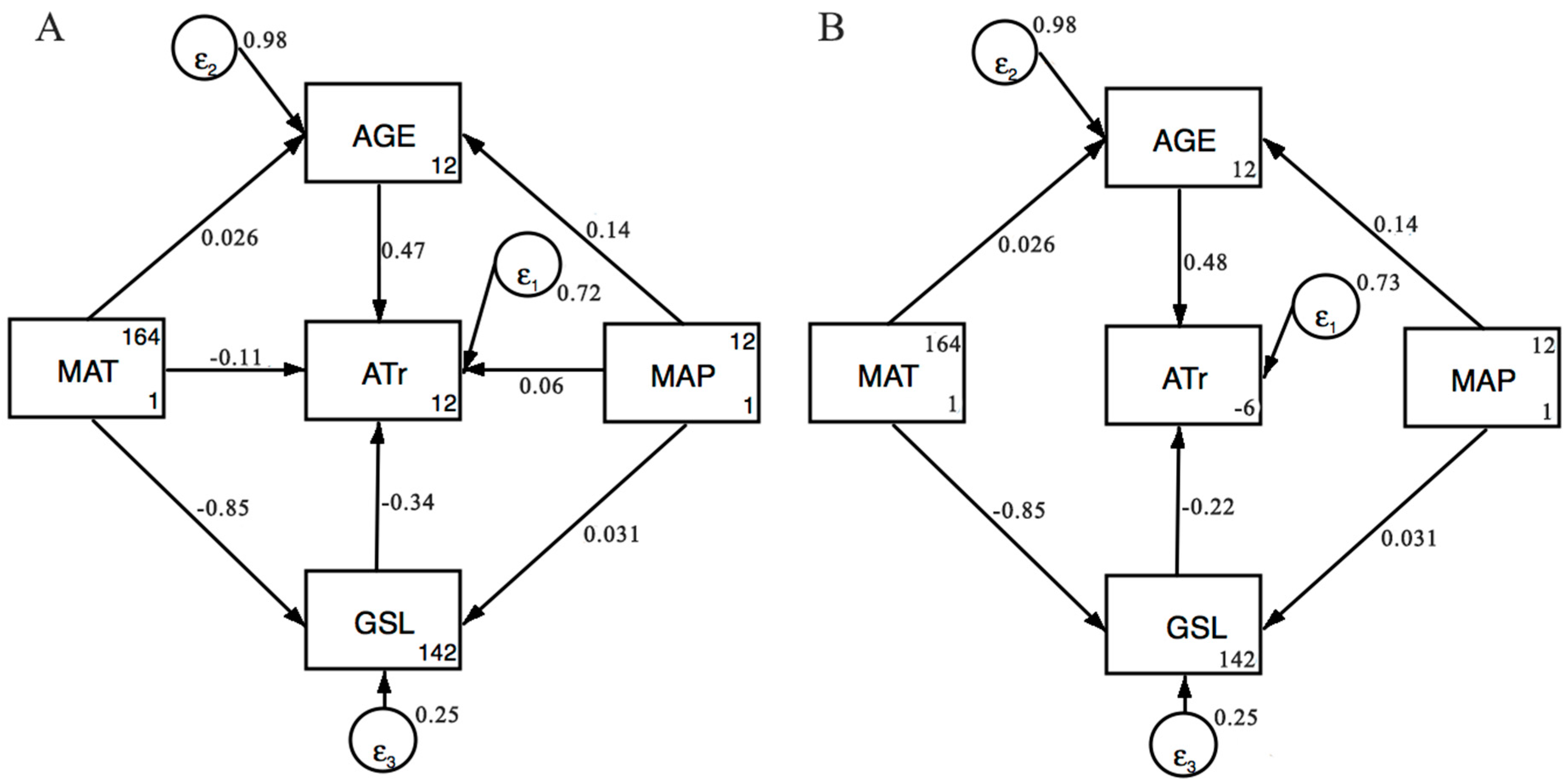
| SEM Model | Predictor | Pathway to ATr | Effect |
|---|---|---|---|
| Model in Figure 3A | AGE | direct | 0.473 *** |
| GSL | direct | −0.344 *** | |
| MAT | direct | −0.107 | |
| indirect | 0.303 *** | ||
| total | 0.196 *** | ||
| MAP | direct | 0.060 | |
| indirect | 0.058 | ||
| total | 0.118 | ||
| Model in Figure 3B | AGE | direct | 0.481 *** |
| GSL | direct | −0.224 *** | |
| MAT | indirect | 0.202 *** | |
| MAP | indirect | 0.063 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wang, W.; Zhang, W.; Zhang, J.; Shan, D. Resolving Variables Influencing the Residence Time of Biomass in the Old-Age Forest across Climate Gradients. Forests 2018, 9, 148. https://doi.org/10.3390/f9030148
Han Y, Wang W, Zhang W, Zhang J, Shan D. Resolving Variables Influencing the Residence Time of Biomass in the Old-Age Forest across Climate Gradients. Forests. 2018; 9(3):148. https://doi.org/10.3390/f9030148
Chicago/Turabian StyleHan, Yangrui, Weifeng Wang, Weiyan Zhang, Jun Zhang, and Dandan Shan. 2018. "Resolving Variables Influencing the Residence Time of Biomass in the Old-Age Forest across Climate Gradients" Forests 9, no. 3: 148. https://doi.org/10.3390/f9030148




