Stem Photosynthesis of Twig and Its Contribution to New Organ Development in Cutting Seedlings of Salix Matsudana Koidz.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Methods
2.3.1. Response of Stem Photosynthesis to Light Intensity
2.3.2. Effective Photochemical Efficiency Determination
2.3.3. Twig Photosynthetic Pigment Content
2.3.4. Soluble Sugar and Starch Analyses
2.3.5. Carbon Isotope Analysis
2.4. Statistical Analysis
3. Results
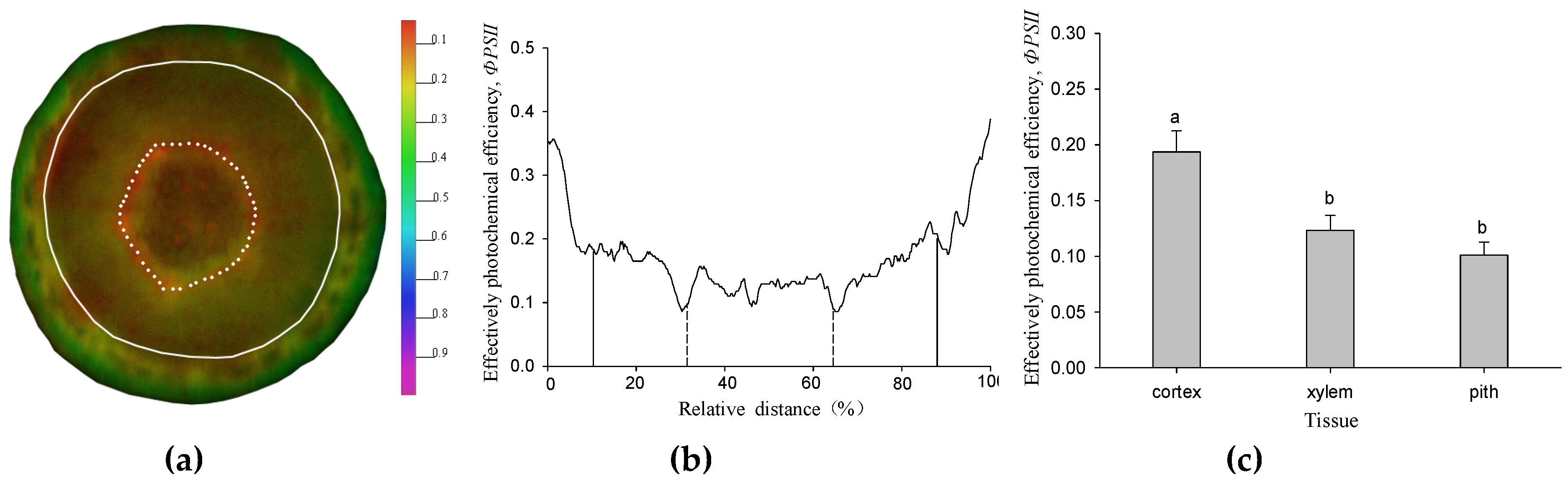
3.1. Radial Heterogeneity of ΦPSⅡ in the Twig Cross Section
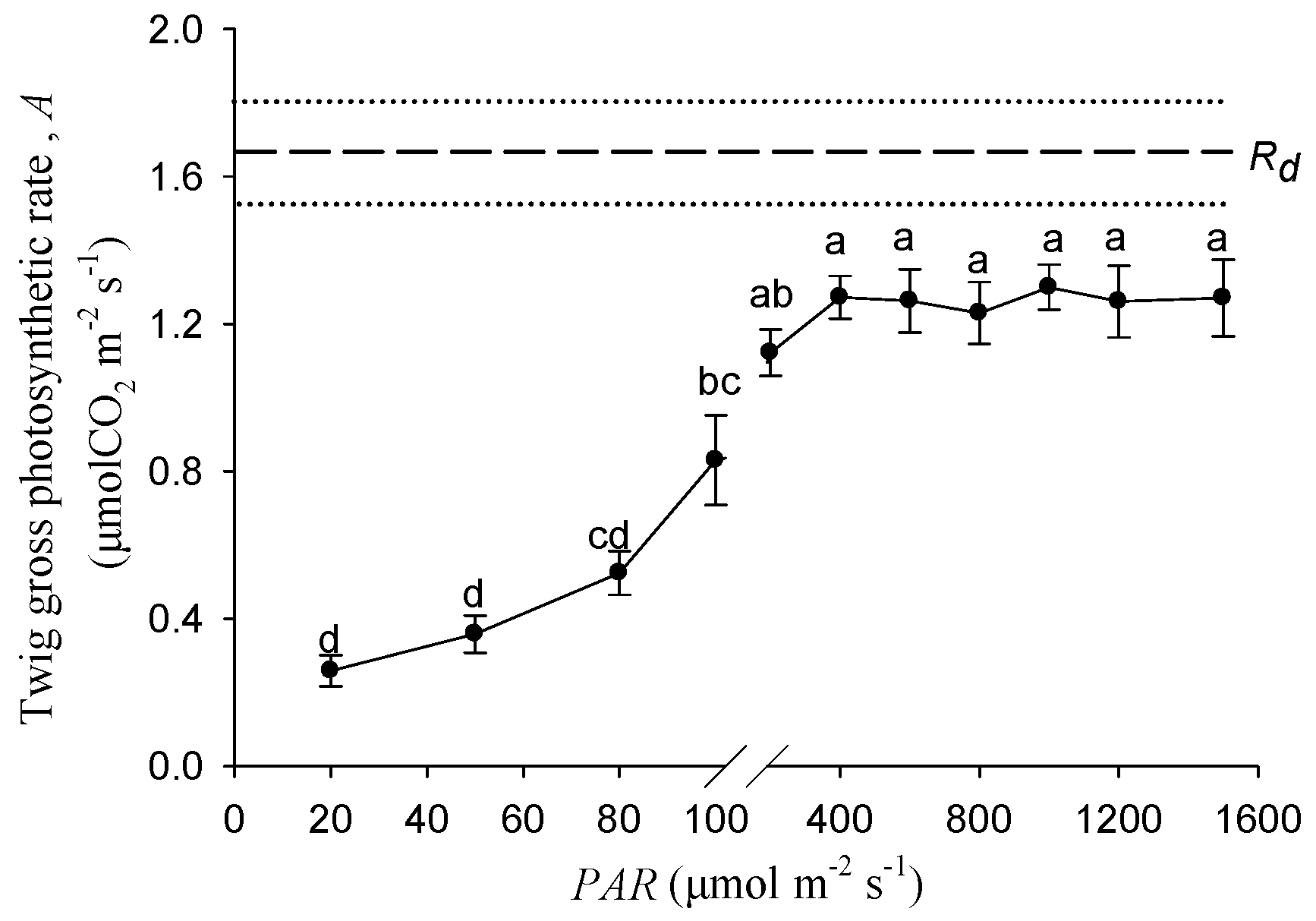
3.2. Response of Gross Photosynthesis Rate with Increasing Light Intensity
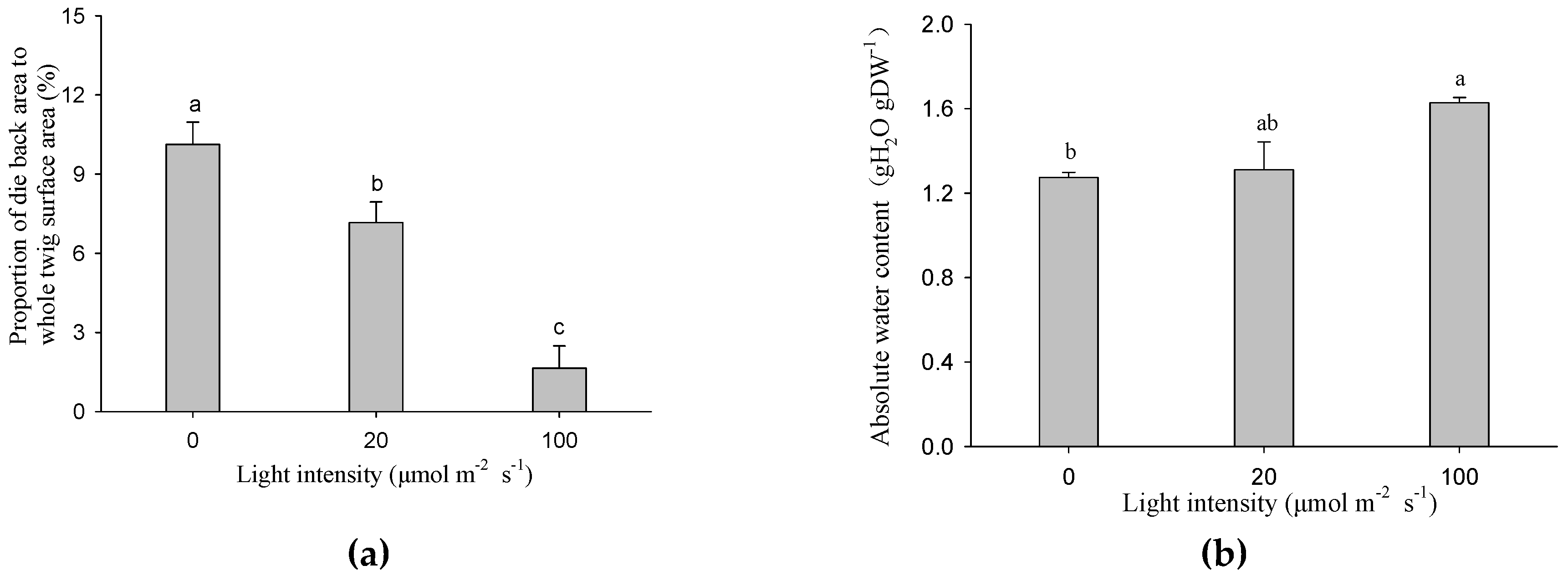
3.3. The Responses of Stem Photosynthetic Pigments and New Organ Development to Different Light Intensities
3.4. Effects of Light Intensity on the Water Content of Stem
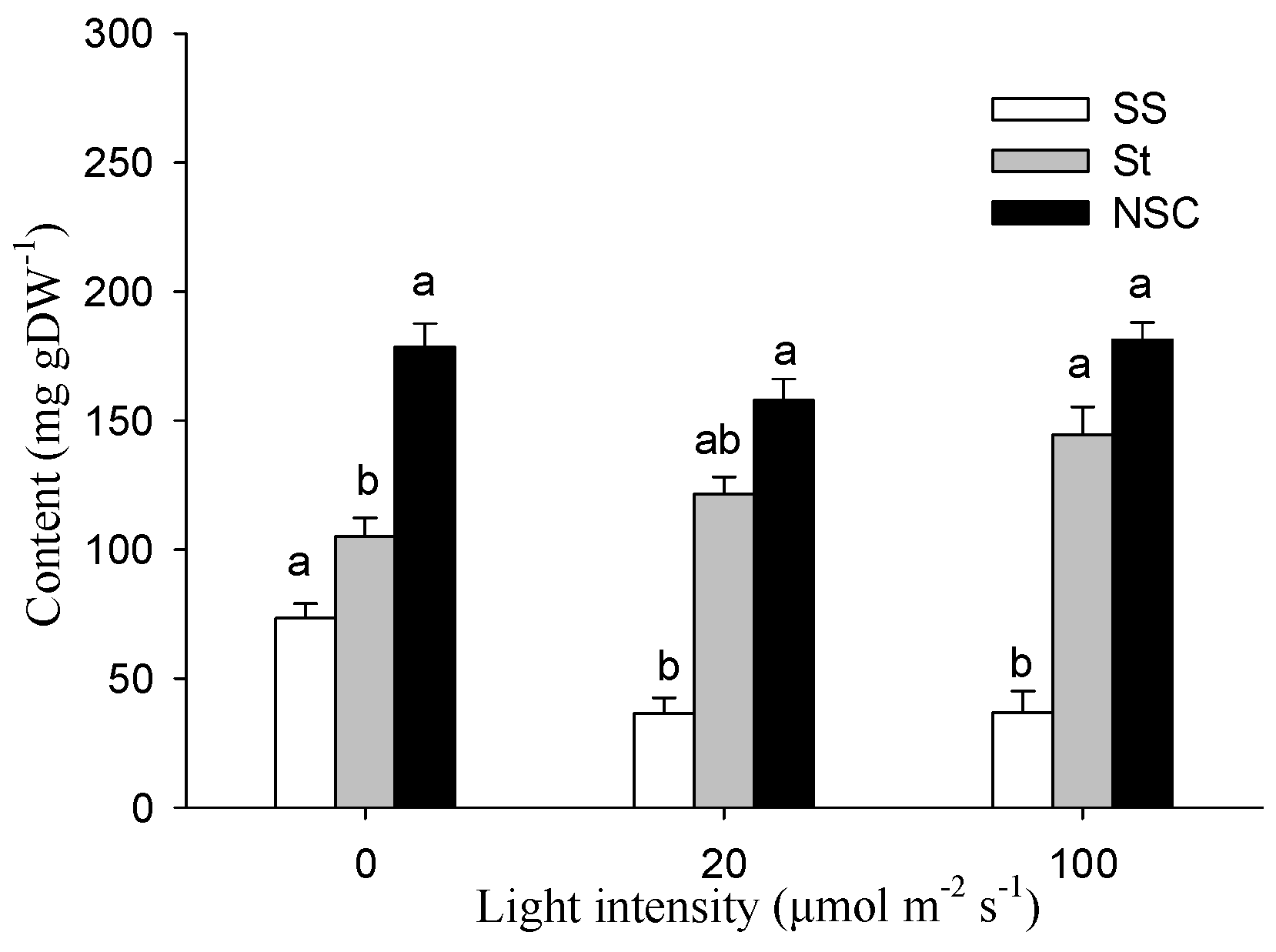
3.5. Effects of Light Intensity on the SS, St, and Non-Structural Carbohydrate Contents of Stem
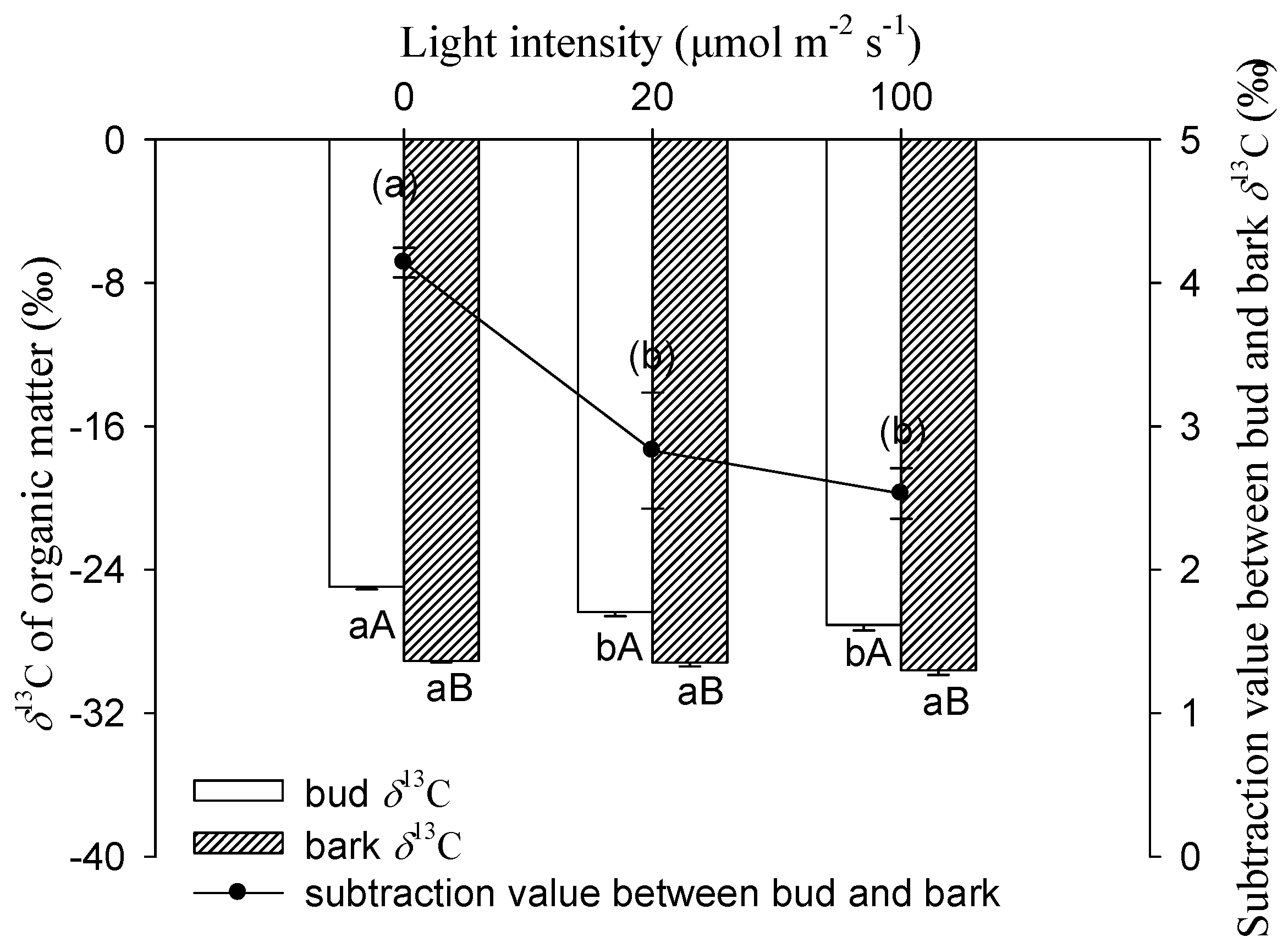
3.6. Effects of Light Intensity on δ13C of Bud and Bark in Cutting Seedlings
4. Discussion
4.1. Stem Photosynthesis of Twig and Its Response to Different Light Treatment
4.2. Larger Bud and Root Productions Dependent on More Water and Carbohydrates Supply from Stem Determined by Stem Photosynthesis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lendzian, K.J. Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J. Exp. Bot. 2006, 57, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Saveyn, A.; McGuire, M.A.; Lemeur, R.; Teskey, R.O. Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct. Plant Biol. 2007, 34, 785–792. [Google Scholar] [CrossRef]
- Pfanz, H.; Aschan, G. The existence of bark photosynthesis in woody plants and its significance for the overall carbon gain. An eco-physiological approach. Prog. Bot. 2001, 62, 477–510. [Google Scholar]
- Manetas, Y. Probing corticular photosynthesis through the in vivo chlorophyll fluorescence measurements: Evidence that high internal CO2 levels suppress electron flow and increase the risk of photoinhibition. Physiol. Plant. 2004, 120, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Pfanz, H. Bark photosynthesis. Trees 2008, 22, 137–138. [Google Scholar] [CrossRef]
- Pilarski, J.; Tokarz, K.; Kocurek, M. Comparison of photosynthetic pigment contents in stems and leaves of fruit trees: Cherry, sweet cherry, common plum, and walnut tree. Folia Hortic. 2007, 19, 53–65. [Google Scholar]
- Rentzou, A.; Psaras, G.K. Green plastids, maximal PSH photochemical efficiency and starch content of inner stem tissues of three Mediterranean woody species during the year. Flora 2008, 203, 350–357. [Google Scholar] [CrossRef]
- Berveiller, D.; Kierzkowski, D.; Damesin, C. Interspecific variability of stem photosynthesis among tree species. Tree Physiol. 2007, 27, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yiotis, C.; Petropoulou, Y.; Manetas, Y. Evidence for light-independent and steeply decreasing PSII efficiency along twig depth in four tree species. Photosynthetica 2009, 47, 223–231. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. The optical, absorptive and chlorophyll fluorescence properties of young stems of five woody species. Environ. Exp. Bot. 2016, 121, 83–93. [Google Scholar] [CrossRef]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and ecophysiology of tree stems: Corticular and wood photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar] [PubMed]
- Wittmann, C.; Pfanz, H. Temperature dependency of bark photosynthesis in beech (Fagus sylvatica L.) and birch (Betula pendula Roth.) trees. J. Exp. Bot. 2007, 58, 4293–4306. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, L.; Wallin, G.; Lim, H.; Linder, S.; Oren, R.; Ottosson, L.M.; Rantfors, M.; Tor-Nqern, P.; Marshall, J. Photosynthetic refixation varies along the stem and reduces CO2 efflux in mature boreal Pinus sylvestris trees. Tree Physiol. 2018, 38, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.; Pilarski, J. Optical properties and the content of photosynthetic pigments in the stems and leaves of the apple-tree. Acta Physiol. Plant. 2005, 27, 183–191. [Google Scholar] [CrossRef]
- Wittmann, C.; Aschan, G.; Pfanz, H. Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. Basic Appl. Ecol. 2001, 2, 145–154. [Google Scholar] [CrossRef]
- Damesin, C. Respiration and photosynthesis characteristics of current-year stems of Fagus sylvatica: From the seasonal pattern to an estimation over the year. New Phytol. 2003, 158, 465–475. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. Bark and woody tissue photosynthesis: A means to avoid hypoxia or anoxia in developing stem tissues. Funct. Plant Biol. 2014, 41, 940–953. [Google Scholar] [CrossRef]
- Saveyn, A.; Steppe, K.; Ubierna, N.; Dawson, T.E. Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ. 2010, 33, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Simbo, D.J.; Bilcke, N.V.; Samson, R. Contribution of corticular photosynthesis to bud development in African baobab (Adansonia digitata L.) and Castor bean (Ricinus communis L.) seedlings. Environ. Exp. Bot. 2013, 9, 1–5. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 178, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, E.; Farquhar, G.D. Photosynthetic fractionation of carbon isotopes. In Photosynthesis, Physiology and Metabolism; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 399–434. [Google Scholar]
- Aschan, G.; Pfanz, H. Non-foliar photosynthesis—A strategy of additional carbon acquisition. Flora 2003, 198, 81–97. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Marshall, J.D. Photosynthetic refixation in branches of Western white pine. Funct. Ecol. 2000, 14, 300–311. [Google Scholar]
- Cernusak, L.A.; Marshall, J.D.; Comstock, J.P.; Balster, N.J. Carbon isotope discrimination in photosynthetic bark. Oecologia 2001, 128, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Demo, M.; Bako, A.; Huska, D.; Hauptvogl, M. Biomass production potential of different willow varieties (Salix spp.) grown in soilclimatic conditions of south-western Slovakia. Wood Res. 2013, 58, 651–662. [Google Scholar]
- Bernardini, A.; Salvatori, E.; Di Re, S.; Fusaro, L.; Nervo, G.; Manes, F. Natural and commercial Salix clones differ in their ecophysiological response to Zn stress. Photosynthetica 2016, 54, 56–64. [Google Scholar] [CrossRef]
- Mitchell1, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarova, L.; Strelcova, K.; Brestic, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica). Environ. Exp. Bot. 2017. [Google Scholar] [CrossRef]
- Schmitz, N.; Egerton, J.J.G.; Lovelock, C.E.; Ball, M.C. Light-dependent maintenance of hydraulic function in mangrove branches: Do xylary chloroplasts play a role in embolism repair? New Phytol. 2012, 195, 40–46. [Google Scholar] [CrossRef] [PubMed]
- ÁvilaLovera, E.; Zerpa, A.J.; Santiago, L.S. Stem photosynthesis and hydraulics are coordinated in desert plant species. New Phytol. 2017, 216, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Niels, J.F.; Baerdemaeker, D.; Salomon, R.L.; Roo, L.D.; Steppe, K. Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytol. 2017, 261, 720–727. [Google Scholar] [CrossRef]
- Hoch, G. Carbon reserves as indicators for carbon limitation in trees. In Progress in Botany; Lüttge, U., Beyschlag, W., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 76, pp. 321–346. [Google Scholar]
- Piper, F.I.; Fajardo, A. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiol. 2016, 36, 1400–1408. [Google Scholar] [PubMed]
- Ocheltree, T.W.; Marshall, J.D. Apparent respiratory discrimination is correlated with growth rate in the shoot apex of sunflower (Helianthus annuus). J. Exp. Bot. 2004, 55, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
| Indicators (mean ± SE) | Light Intensity (μmol m−2 s−1) | ||
|---|---|---|---|
| 0 | 20 | 100 | |
| Chlorophyll a content (mg gDW−1) | 0.30 ± 0.01 b | 0.40 ± 0.01 a | 0.35 ± 0.02 ab |
| Chlorophyll b content (mg gDW−1) | 0.07 ± 0.00 a | 0.08 ± 0.00 a | 0.08 ± 0.00 a |
| Total chlorophyll content (mg gDW−1) | 0.37 ± 0.01 b | 0.48 ± 0.01 a | 0.43 ± 0.03 ab |
| Carotenoid content (mg gDW−1) | 0.05 ± 0.00 b | 0.06 ± 0.00 a | 0.05 ± 0.00 ab |
| Bud number | 5.33 ± 0.60 b | 7.17 ± 0.73 b | 14.17 ± 0.17 a |
| Bud biomass (mg) | 6.00 ± 1.76 b | 9.28 ± 2.75 b | 27.92 ± 2.18 a |
| Bud DW: total DW (mg g−1) | 4.39 ± 1.13 b | 5.99 ± 2.37 b | 21.60 ± 2.26 a |
| Root length (cm) | 23.90 ± 3.23 a | 26.85 ± 3.65 a | 40.00 ± 5.52 a |
| Root DW (mg) | 4.82 ± 1.21 b | 6.38 ± 0.86 ab | 8.97 ± 0.47 a |
| Root DW:total DW (mg g−1) | 3.55 ± 0.62 b | 3.76 ± 0.28 ab | 8.01 ± 1.61 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gu, L.; Yu, Y.; Ju, G.; Sun, Z. Stem Photosynthesis of Twig and Its Contribution to New Organ Development in Cutting Seedlings of Salix Matsudana Koidz. Forests 2018, 9, 207. https://doi.org/10.3390/f9040207
Liu J, Gu L, Yu Y, Ju G, Sun Z. Stem Photosynthesis of Twig and Its Contribution to New Organ Development in Cutting Seedlings of Salix Matsudana Koidz. Forests. 2018; 9(4):207. https://doi.org/10.3390/f9040207
Chicago/Turabian StyleLiu, Junxiang, Lin Gu, Yongchang Yu, Guansheng Ju, and Zhenyuan Sun. 2018. "Stem Photosynthesis of Twig and Its Contribution to New Organ Development in Cutting Seedlings of Salix Matsudana Koidz." Forests 9, no. 4: 207. https://doi.org/10.3390/f9040207




