Discriminating between Seasonal and Chemical Variation in Extracellular Enzyme Activities within Two Italian Beech Forests by Means of Multilevel Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Litter and Soil Sampling
2.3. Chemical Analyses
2.4. Enzyme Activities
2.5. Statistics
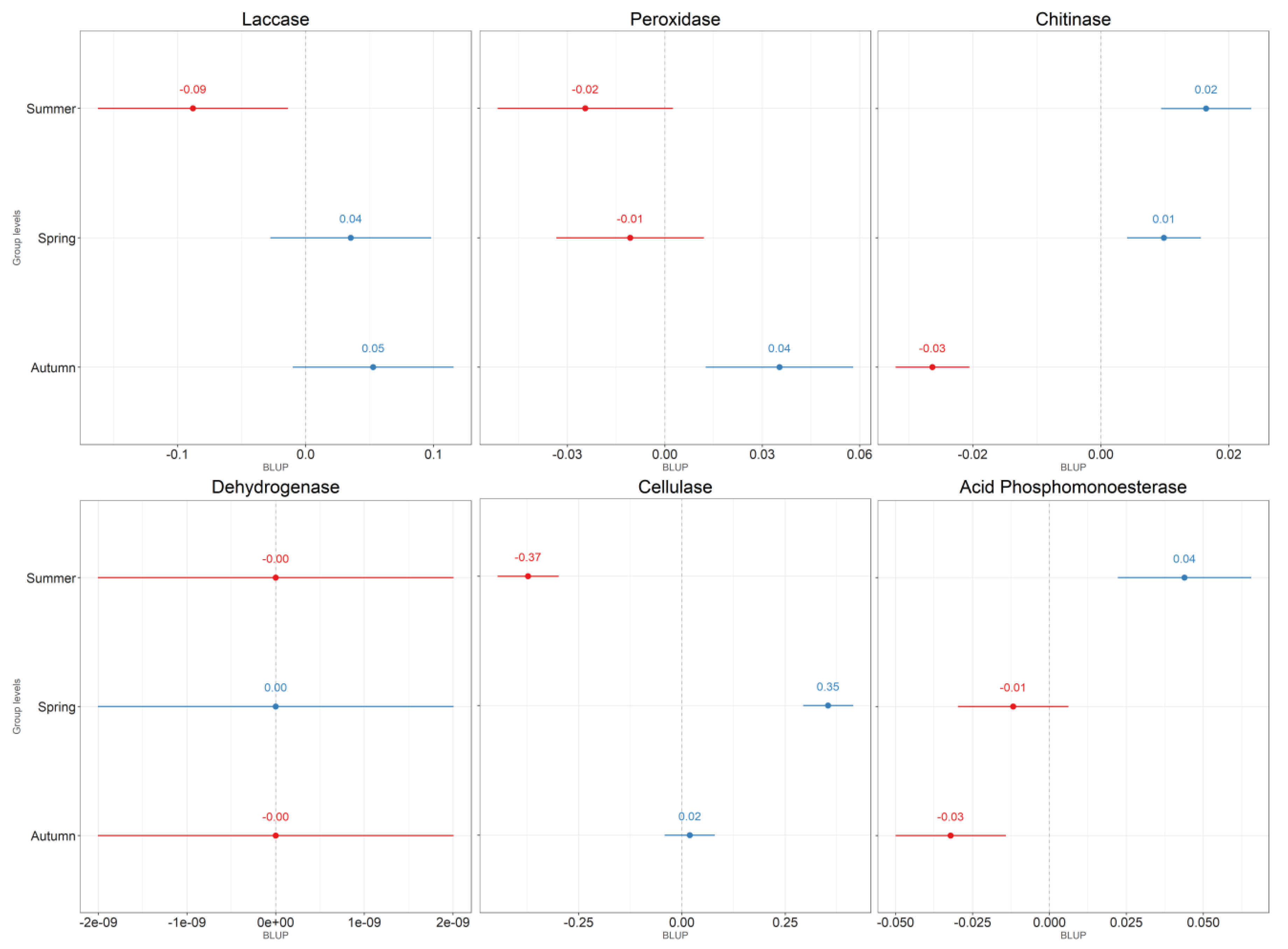
3. Results
3.1. Leaf Litter
3.2. Soil
4. Discussion
4.1. Leaf Litter
4.2. Soil
4.3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2010; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.A.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642388200. [Google Scholar]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; Virzo De Santo, A. C stocks in forest floor and mineral soil of two Mediterranean beech forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Rouifed, S.; Handa, I.T.; David, J.F.; Hättenschwiler, S. The importance of biotic factors in predicting global change effects on decomposition of temperate forest leaf litter. Oecologia 2010, 163, 247–256. [Google Scholar] [CrossRef] [PubMed]
- García-Palacios, P.; Shaw, E.A.; Wall, D.H.; Hättenschwiler, S. Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol. Lett. 2016, 19, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Menta, C.; García-Montero, L.G.; Pinto, S.; Conti, F.D.; Baroni, G.; Maresi, M. Does the natural “microcosm” created by Tuber aestivum affect soil microarthropods? A new hypothesis based on Collembola in truffle culture. Appl. Soil Ecol. 2014, 84, 31–37. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Brandstätter, C.; Keiblinger, K.; Wanek, W.; Zechmeister-Boltenstern, S. A closeup study of early beech litter decomposition: Potential drivers and microbial interactions on a changing substrate. Plant Soil 2013, 371, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Miller, M.; Zeller, B. Litter decomposition. In Carbon and Nitrogen Cycling in European Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 2000; pp. 276–296. [Google Scholar]
- Frankland, J.C. Fungal succession—Unravelling the unpredictable. Mycol. Res. 1998, 102, 1–15. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Moorhead, D.; Linkins, A. The enzymic basis of plant litter decomposition: Emergence of an ecological process. Appl. Soil Ecol. 1994, 1, 97–111. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.K.; Linkins, A.E. An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric. Ecosyst. Environ. 1991, 34, 43–54. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Kaiser, C.; Koranda, M.; Kitzler, B.; Fuchslueger, L.; Schnecker, J.; Schweiger, P.; Rasche, F.; Zechmeister-Boltenstern, S.; Sessitsch, A.; Richter, A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 2010, 187, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Šnajdr, J.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Valášková, V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol. Biochem. 2013, 56, 60–68. [Google Scholar] [CrossRef]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas Mit Den Alpen in Ökologischer, Dynamischer und Historischer Sicht; UTB: Stuttgart, Germany, 2010. [Google Scholar]
- Gasparini, P.; Tabacchi, G. L’Inventario Nazionale delle Foreste e dei Serbatoi Forestali di Carbonio INFC 2005; Secondo Inventario Forestale Nazionale Italiano; Metodi e Risultati. Ministero delle Politiche Agricole, Alimentari e Forestali; Corpo Forestale dello Stato; Edagricole-Il sole 24 Ore: Bologna, Italy, 2011. [Google Scholar]
- Innangi, M.; D’Alessandro, F.; Fioretto, A.; Di Febbraro, M. Modeling distribution of Mediterranean beech forests and soil carbon stock under climate change scenarios. Clim. Res. 2015, 66, 25–36. [Google Scholar] [CrossRef]
- Innangi, M.; Schenk, M.K.; d’Alessandro, F.; Pinto, S.; Menta, C.; Papa, S.; Fioretto, A. Field and microcosms decomposition dynamics of European beech leaf litter: Influence of climate, plant material and soil with focus on N and Mn. Appl. Soil Ecol. 2015, 93, 88–97. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Knelman, J.E.; Graham, E.B.; Ferrenberg, S.; Lecoeuvre, A.; Labrado, A.; Darcy, J.L.; Nemergut, D.R.; Schmidt, S.K. Rapid shifts in soil nutrients and decomposition enzyme activity in early succession following forest fire. Forests 2017, 8, 347. [Google Scholar] [CrossRef]
- Qian, S.S.; Cuffney, T.F.; Alameddine, I.; McMahon, G.; Reckhow, K.H. On the application of multilevel modeling in environmental and ecological studies. Ecology 2010, 91, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Innangi, M.; Niro, E.; D’Ascoli, R.; Danise, T.; Proietti, P.; Nasini, L.; Regni, L.; Castaldi, S.; Fioretto, A. Effects of olive pomace amendment on soil enzyme activities. Appl. Soil Ecol. 2017, 119, 242–249. [Google Scholar] [CrossRef]
- Leoni, A. Studio delle Biodiversità Vegetale e del Popolamento a Microartropodi Edafici nella Riserva Naturale “Guadine-Pradaccio”; Università degli Studi di Parma: Parma, Italy, 2008. [Google Scholar]
- Curcio, E.; Danise, T.; Innangi, M.; Alvarez Romero, M.; Coppola, E.; Fioretto, A.; Papa, S. Soil characterization and comparison of organic matter quality and quantity of two stands under different vegetation cover on Monte Faito Soil characterization and comparison of organic matter quality and quantity of two stands under different. Fresenius Environ. Bull. 2017, 26, 8–18. [Google Scholar]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Song, B.; Niu, S.; Zhang, Z.; Yang, H.; Li, L.; Wan, S. Light and heavy fractions of soil organic matter in response to climate warming and increased precipitation in a temperate steppe. PLoS ONE 2012, 7, e33217. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Horwàth, W.R.; Van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Bussotti, F.; Prancrazi, M.; Matteucci, G.; Gerosa, G. Leaf morphology and chemistry in Fagus sylvatica (beech) trees as affected by site factors and ozone: Results from CONECOFOR permanent monitoring plots in Italy. Tree Physiol. 2005, 25, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Wine, R.H. Determination of lignin and cellulose in acid-detergent fiber with permanganate. J. Assoc. Off. Anal. Chem. 1964, 51, 780–785. [Google Scholar]
- Schinner, F.; von Mersi, W. Xylanase-, CM-Cellulase and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Curcio, E.; Sorrentino, G.; Fuggi, A. Enzyme dynamics on decomposing leaf litter of Cistus incanus and Myrtus communis in a Mediterranean ecosystem. Soil Biol. Biochem. 2000, 32, 1847–1855. [Google Scholar] [CrossRef]
- Verchot, L.; Borelli, T. Application of para-nitrophenol (pNP) enzyme assays in degraded tropical soils. Soil Biol. Biochem. 2005, 37, 625–633. [Google Scholar] [CrossRef]
- Von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Leatham, G.F.; Stahmann, M.A. Studies on the laccase of Lentinus edodes: Specificity, localization and association with the development of fruiting bodies. J. Gen. Microbiol. 1981, 125, 147–157. [Google Scholar] [CrossRef]
- Di Nardo, C.; Cinquegrana, A.; Papa, S.; Fuggi, A.; Fioretto, A. Laccase and peroxidase isoenzymes during leaf litter decomposition of Quercus ilex in a Mediterranean ecosystem. Soil Biol. Biochem. 2004, 36, 1539–1544. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A.M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Nicolaus, M.; Tinbergen, J.M.; Ubels, R.; Both, C.; Dingemanse, N.J. Density fluctuations represent a key process maintaining personality variation in a wild passerine bird. Ecol. Lett. 2016, 19, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Green, P.J.; Silverman, B.W. Nonparametric Regression and Generalized Linear Models: A Roughness Penalty Approach; Chapman & Hall/CRC Monographs on Statistics & Applied Probability; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 1993; ISBN 9780412300400. [Google Scholar]
- Donnelly, P.K.; Entry, J.A.; Crawford, D.L.; Cromack, K. Cellulose and lignin degradation in forest soils: Response to moisture, temperature, and acidity. Microb. Ecol. 1990, 20, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, G.; Nilsson, S.I.; Persson, T.; Karlsson, P. Degradation of hemicellulose, cellulose and lignin in decomposing spruce needle litter in relation to N. Soil Biol. Biochem. 2004, 36, 1761–1768. [Google Scholar] [CrossRef]
- Romaní, A.M.; Fischer, H.; Mille-Lindblom, C.; Tranvik, L.J. Interactions of bacteria and fungi on decomposing litter: Differential extracellular enzyme activities. Ecology 2006, 87, 2559–2569. [Google Scholar] [CrossRef]
- Rihani, M.; Kiffer, E.; Botton, B. Decomposition of beech leaf litter by microflora and mesofauna. I: In vitro action of white-rot fungi on beech leaves and foliar components. Eur. J. Soil Biol. 1995, 31, 57–66. [Google Scholar]
- Heuck, C.; Spohn, M. Carbon, nitrogen and phosphorus net mineralization in organic horizons of temperate forests: Stoichiometry and relations to organic matter quality. Biogeochemistry 2016, 131, 1–14. [Google Scholar] [CrossRef]
- Albers, D.; Migge, S.; Schaefer, M.; Scheu, S. Decomposition of beech leaves (Fagus sylvatica) and spruce needles (Picea abies) in pure and mixed stands of beech and spruce. Soil Biol. Biochem. 2004, 36, 155–164. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Virzo De Santo, A.; Berg, B.; Alfani, A.; Fioretto, A. Lignin decomposition in decaying leaves of Fagus sylvatica L. and needles of Abies alba Mill. Soil Biol. Biochem. 1996, 28, 101–106. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Dise, N.B.; Matzner, E.; Armbruster, M.; Gundersen, P.; Forsius, M. Nitrogen input together with ecosystem nitrogen enrichment predict nitrate leaching from European forests. Glob. Chang. Biol. 2002, 8, 1028–1033. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.; Su, B.; Wan, S.; Luo, Y. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- De Nicola, C.; Zanella, A.; Testi, A.; Fanelli, G.; Pignatti, S. Humus forms in a Mediterranean area (Castelporziano Reserve, Rome, Italy): Classification, functioning and organic carbon storage. Geoderma 2014, 235–236, 90–99. [Google Scholar] [CrossRef]
- Michel, K.; Matzner, E. Nitrogen content of forest floor Oa layers affects carbon pathways and nitrogen mineralization. Soil Biol. Biochem. 2002, 34, 1807–1813. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Pellegrino, A.; Ferrigno, A. Microbial activities in soils of a Mediterranean ecosystem in different successional stages. Soil Biol. Biochem. 2009, 41, 2061–2068. [Google Scholar] [CrossRef]
- Papa, S.; Pellegrino, A.; Fioretto, A. Microbial activity and quality changes during decomposition of Quercus ilex leaf litter in three Mediterranean woods. Appl. Soil Ecol. 2008, 40, 401–410. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Palumbo, E. Laccase and Peroxidase Enzymes Activities along the Soil Profile in Two Beech Woods: Seasonal Variations and Isoforms Identification. M.Sc. Thesis, University of Campania “Luigi Vanvitelli”, Caserta, Italy, 2011. [Google Scholar]
- Papa, S.; Pellegrino, A.; Bartoli, G.; Ruosi, R.; Rianna, S.; Fuggi, A.; Fioretto, A. Soil organic matter, nutrient distribution, fungal and microbial biomass and enzyme activities in a forest beech stand on the Apennines of southern Italy. Plant Biosyst. 2014, 1–12. [Google Scholar] [CrossRef]
- Guckland, A.; Jacob, M.; Flessa, H.; Thomas, F.M.; Leuschner, C. Acidity, nutrient stocks, and organic-matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). J. Plant Nutr. Soil Sci. 2009, 172, 500–511. [Google Scholar] [CrossRef]
- Jobbágy, E.; Jackson, R. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Groffman, P.M.; Driscoll, C.D.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L.; Henry, K.S.; Welman, T.A.; Demers, J.D.; Nolan, S. Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 191–213. [Google Scholar] [CrossRef]
- Hogberg, M.N.; Briones, M.J.I.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Thornton, B.; Hurry, V.; Linder, S.; Näsholm, T.; et al. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Kjøller, A.; Struwe, S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
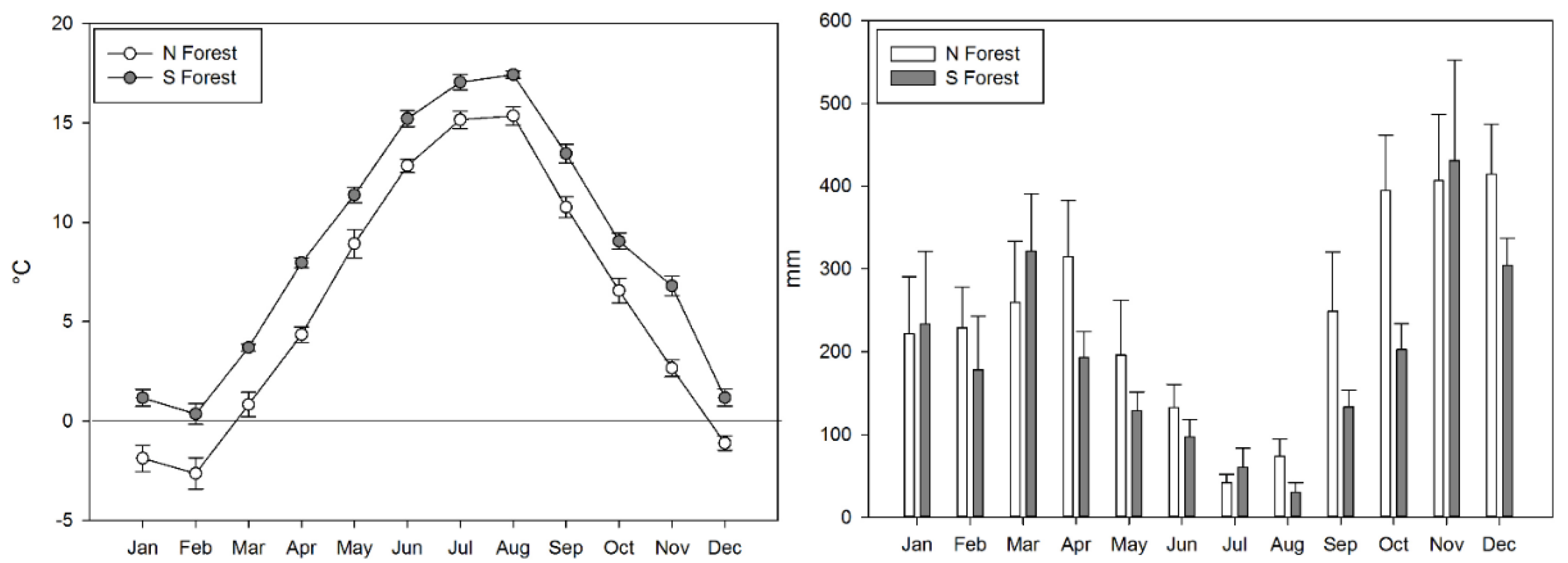



| Season | Location | Layer | O.M. | Cellulose | Lignin | N | Ca | P | K | Mg | Mn | Fe | C:N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autumn | N Forest | Li | 0.95 ± 0.004 | 526 ± 8 | 276 ± 9 | 13.21 ± 0.45 | 13.15 ± 0.16 | 0.41 ± 0.01 | 0.62 ± 0.001 | 0.85 ± 0.01 | 0.07 ± 0.001 | 0.10 ± 0.000 | 39.2 ± 1.1 |
| Lf | 0.94 ± 0.001 | 516 ± 12 | 277 ± 10 | 16.52 ± 0.71 | 13.24 ± 0.29 | 0.45 ± 0.007 | 0.63 ± 0.001 | 0.87 ± 0.01 | 0.088 ± 0.004 | 0.10 ± 0.000 | 27.96 ± 1.4 | ||
| Lhf | 0.91 ± 0.007 | 482 ± 13 | 320 ± 5 | 22.28 ± 0.16 | 12.61 ± 0.12 | 0.47 ± 0.004 | 0.63 ± 0.002 | 0.91 ± 0.02 | 0.101 ± 0.003 | 0.11 ± 0.000 | 22.0 ± 0.3 | ||
| S Forest | Li | 0.89 ± 0.005 | 537 ± 12 | 275 ± 11 | 14.06 ± 0.66 | 16.03 ± 0.13 | 0.49 ± 0.005 | 0.63 ± 0.001 | 0.96 ± 0.03 | 0.06 ± 0.002 | 0.10 ± 0.001 | 35.0 ± 1.2 | |
| Lf | 0.89 ± 0.008 | 510 ± 7 | 279 ± 10 | 13.07 ± 0.54 | 15.84 ± 0.12 | 0.49 ± 0.005 | 0.63 ± 0.002 | 0.94 ± 0.02 | 0.059 ± 0.002 | 0.10 ± 0.000 | 37.1 ± 1.4 | ||
| Lhf | 0.77 ± 0.017 | 466 ± 10 | 313 ± 12 | 17.69 ± 0.73 | 15.63 ± 0.32 | 0.56 ± 0.008 | 0.64 ± 0.004 | 1.04 ± 0.004 | 0.081 ± 0.003 | 0.12 ± 0.000 | 22.4 ± 0.45 | ||
| Spring | N Forest | Li | 0.93 ± 0.008 | 498 ± 11 | 306 ± 8 | 18.53 ± 0.32 | 13.02 ± 0.15 | 0.44 ± 0.005 | 0.62 ± 0.001 | 0.81 ± 0.006 | 0.074 ± 0.001 | 0.10 ± 0.000 | 28.1 ± 0.36 |
| Lf | 0.92 ± 0.010 | 505 ± 13 | 312 ± 11 | 21.35 ± 0.62 | 12.53 ± 0.23 | 0.45 ± 0.003 | 0.62 ± 0.001 | 0.85 ± 0.01 | 0.09 ± 0.004 | 0.10 ± 0.000 | 23.9 ± 0.8 | ||
| Lhf | 0.87 ± 0.021 | 492 ± 13 | 300 ± 7 | 19.75 ± 0.94 | 12.32 ± 0.23 | 0.46 ± 0.007 | 0.63 ± 0.003 | 0.95 ± 0.04 | 0.098 ± 0.005 | 0.11 ± 0.000 | 22.3 ± 0.4 | ||
| S Forest | Li | 0.89 ± 0.007 | 503 ± 6 | 292 ± 2 | 12.83 ± 0.65 | 15.79 ± 0.32 | 0.47 ± 0.006 | 0.62 ± 0.001 | 0.86 ± 0.01 | 0.05 ± 0.003 | 0.10 ± 0.000 | 39.4 ± 2.5 | |
| Lf | 0.85 ± 0.012 | 479 ± 18 | 313 ± 9 | 15.37 ± 0.67 | 16.27 ± 0.29 | 0.51 ± 0.007 | 0.62 ± 0.001 | 0.93 ± 0.03 | 0.06 ± 0.002 | 0.11 ± 0.000 | 31.3 ± 1.8 | ||
| Lhf | 0.75 ± 0.016 | 450 ± 13 | 338 ± 4 | 17.52 ± 0.61 | 16.38 ± 0.39 | 0.54 ± 0.013 | 0.63 ± 0.002 | 1.00 ± 0.02 | 0.067 ± 0.003 | 0.12 ± 0.000 | 24 ± 0.7 | ||
| Summer | N Forest | Li | 0.954 ± 0.002 | 426 ± 23 | 353 ± 20 | 28.87 ± 1.69 | 13.87 ± 0.23 | 0.57 ± 0.011 | 0.64 ± 0.027 | 0.92 ± 0.042 | 0.122 ± 0.007 | 0.13 ± 0.030 | 17.6 ± 1.13 |
| Lf | 0.940 ± 0.003 | 409 ± 20 | 383 ± 32 | 37.32 ± 2.43 | 13.67 ± 0.18 | 0.60 ± 0.023 | 0.64 ± 0.022 | 0.99 ± 0.04 | 0.150 ± 0.019 | 0.26 ± 0.070 | 13.37 ± 0.69 | ||
| Lhf | 0.906 ± 0.015 | 398 ± 15 | 429 ± 38 | 43.59 ± 2.67 | 12.55 ± 0.64 | 0.68 ± 0.017 | 0.71 ± 0.027 | 1.14 ± 0.06 | 0.0163 ± 0.015 | 0.50 ± 0.150 | 10.72 ± 0.46 | ||
| S Forest | Li | 0.932 ± 0.002 | 426 ± 8 | 341 ± 25 | 28.14 ± 1.48 | 16.33 ± 0.44 | 0.67 ± 0.014 | 0.67 ± 0.005 | 0.95 ± 0.016 | 0.086 ± 0.006 | 0.18 ± 0.03 | 17.7 ± 0.9 | |
| Lf | 0.916 ± 0.003 | 379 ± 11 | 338 ± 13 | 31.98 ± 1.12 | 17.74 ± 0.68 | 0.75 ± 0.030 | 0.69 ± 0.015 | 1.06 ± 0.027 | 0.111 ± 0.006 | 0.24 ± 0.04 | 15.1 ± 0.49 | ||
| Lhf | 0.830 ± 0.020 | 387 ± 11 | 387 ± 25 | 36.69 ± 0.89 | 17.11 ± 0.35 | 0.78 ± 0.014 | 0.704 ± 0.030 | 1.28 ± 0.05 | 0.128 ± 0.003 | 0.62 ± 0.15 | 12 ± 0.19 |
| Laccase | Peroxidase | Chitinase | Dehydrogenase | Cellulase | Acid Phosph. | |
|---|---|---|---|---|---|---|
| Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | |
| Fixed Parts | ||||||
| (Intercept) | 0.8 | 0.47 | 0.95 | 0.82 | 1.93 | 0.63 |
| (0.76 − 0.84) *** | (0.40 − 0.54) *** | (0.89 − 1.02) *** | (0.78 − 0.86) *** | (1.72 − 2.14) *** | (0.49 − 0.76) *** | |
| Lignin | −0.27 | −0.07 | 0.16 | 0.15 | −0.83 | 0.86 |
| (−0.50 − −0.04) * | (−0.60 − 0.46) | (−0.07 − 0.39) | (−0.07 − 0.37) | (−1.72 − 0.06) | (0.21 − 1.51) * | |
| Fe | 0.03 | 0.00 | −0.06 | 0.03 | −0.17 | −0.24 |
| (−0.03 − 0.10) | (−0.15 − 0.15) | (−0.13 − 0.00) | (−0.03 − 0.09) | (−0.42 − 0.07) | (−0.42 − −0.06) * | |
| K | 0.13 | −0.47 | 0.08 | −0.08 | 0.50 | 0.35 |
| (−0.18 − 0.43) | (−1.17 − 0.22) | (−0.22 − 0.38) | (−0.38 − 0.21) | (−0.66 − 1.66) | (−0.50 − 1.20) | |
| Location S Forest | 0.02 | 0.03 | −0.05 | −0.02 | −0.20 | −0.21 |
| (−0.01 − 0.06) | (−0.04 − 0.10) | (−0.08 − −0.02) * | (−0.05 − 0.01) | (−0.32 − −0.09) *** | (−0.29 − −0.12) *** | |
| Location N Forest: Layer Lf | −0.04 | 0.03 | 0.00 | −0.05 | −0.14 | 0.04 |
| (−0.07 − −0.01) * | (−0.03 − 0.10) | (−0.03 − 0.03) | (−0.08 − −0.02) ** | (−0.25 − −0.02) * | (−0.04 − 0.13) | |
| Location S Forest: Layer Lf | −0.03 | −0.07 | −0.01 | −0.01 | −0.03 | −0.01 |
| (−0.06 − −0.00) * | (−0.14 − 0.00) | (−0.03 − 0.02) | (−0.04 − 0.02) | (−0.15 − 0.08) | (−0.10 − 0.07) | |
| Location N Forest: Layer Lhf | −0.03 | −0.06 | −0.01 | −0.06 | −0.07 | 0.00 |
| (−0.06 − 0.01) | (−0.13 − 0.02) | (−0.04 − 0.02) | (−0.09 − −0.03) *** | (−0.19 − 0.06) | (−0.08 − 0.09) | |
| Location S Forest: Layer Lhf | 0.00 | −0.09 | −0.02 | −0.01 | −0.01 | 0.05 |
| (−0.03 − 0.03) | (−0.16 − −0.02) * | (−0.05 − 0.01) | (−0.04 − 0.03) | (−0.13 − 0.11) | (−0.04 − 0.14) | |
| Random Parts | ||||||
| σ2 | 0.002 | 0.011 | 0.002 | 0.002 | 0.031 | 0.016 |
| τ00, Season | 0.001 | 0.002 | 0.003 | 0.001 | 0.029 | 0.011 |
| ICCSeason | 0.354 | 0.148 | 0.611 | 0.284 | 0.490 | 0.406 |
| R2c/R2m | 0.192/0.478 | 0.158/0.283 | 0.160/0.673 | 0.126/0.374 | 0.149/0.566 | 0.349/0.613 |
| Season | Autumn | |||||||
| Location | N Forest | S Forest | ||||||
| Layer | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm |
| O.M. | 0.31 ± 0.06 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.27 ± 0.02 | 0.20 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| N | 6.64 ± 1.01 | 2.29 ± 0.14 | 1.42 ± 0.14 | 1.30 ± 0.10 | 8.58 ± 0.88 | 6.39 ± 0.57 | 3.97 ± 0.52 | 3.88 ± 0.15 |
| C:N | 17.05 ± 0.69 | 19.37 ± 1.86 | 21.21 ± 0.44 | 21.94 ± 0.32 | 13.11 ± 0.36 | 12.27 ± 0.16 | 12.82 ± 0.27 | 13.2 ± 0.27 |
| Season | Spring | |||||||
| Location | N Forest | S Forest | ||||||
| Layer | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm |
| O.M. | 0.42 ± 0.08 | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.32 ± 0.03 | 0.21 ± 0.01 | 0.16 ± 0.01 | 0.18 ± 0.00 |
| N | 10.14 ± 2.06 | 2.44 ± 0.51 | 1.53 ± 0.02 | 1.35 ± 0.12 | 7.81 ± 0.86 | 5.25 ± 0.45 | 3.8 ± 0.29 | 4.14 ± 0.35 |
| C:N | 14.01 ± 0.68 | 20.82 ± 2.57 | 21.99 ± 0.42 | 20.20 ± 0.32 | 13.20 ± 0.42 | 13.24 ± 0.42 | 12.94 ± 0.19 | 13.15 ± 0.37 |
| Season | Summer | |||||||
| Location | N Forest | S Forest | ||||||
| Layer | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm | 0–5 cm | 5–15 cm | 15–30 cm | 30–40 cm |
| O.M. | 0.42 ± 0.1 | 0.12 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.25 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 |
| N | 21.03 ± 4.21 | 5.19 ± 0.95 | 4.95 ± 0.69 | 4.77 ± 1.11 | 7.81 ± 0.86 | 5.25 ± 0.45 | 3.8 ± 0.29 | 4.14 ± 0.35 |
| C:N | 10.68 ± 0.24 | 9.68 ± 0.73 | 6.32 ± 1.39 | 6.93 ± 1.63 | 6.99 ± 0.28 | 6.39 ± 0.11 | 6.6 ± 0.21 | 6.51 ± 0.14 |
| Laccase | Peroxidase | Chitinase | Dehydrogenase | Cellulase | Acid Phosph. | |
|---|---|---|---|---|---|---|
| Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | Estimate (CI) | |
| Fixed Parts | ||||||
| (Intercept) | 0.69 | 0.16 | 0.95 | 0.81 | 2.37 | 0.62 |
| (0.50 − 0.88) *** | (0.09 − 0.23) *** | (0.92 − 0.98) *** | (0.74 − 0.88) *** | (1.93 − 2.81) *** | (0.56 − 0.69) *** | |
| OM | −0.74 | −0.19 | −0.14 | 0.68 | −1.07 | 0.44 |
| (−1.36 − −0.12) * | (−0.41 − 0.03) | (−0.19 − −0.09) ** | (0.43 − 0.94) *** | (−1.63 − −0.51) * | (0.28 − 0.61) *** | |
| Location S Forest | 0.01 | 0.18 | 0.00 | 0.09 | −0.43 | −0.04 |
| (−0.17 − 0.18) | (0.11 − 0.24) *** | (−0.01 − 0.02) | (0.01 − 0.16) * | (−0.59 − −0.27) ** | (−0.08 − 0.01) | |
| Location N Forest: Layer 5−15 cm | −0.07 | −0.02 | 0.00 | −0.01 | 0.16 | −0.08 |
| (−0.30 − 0.17) | (−0.11 − 0.06) | (−0.02 − 0.02) | (−0.11 − 0.09) | (−0.05 − 0.37) | (−0.15 − −0.02) * | |
| Location S Forest: Layer 5–15 cm | 0.23 | 0.01 | 0.01 | −0.1 | 0.15 | 0.02 |
| (0.06 − 0.40) * | (−0.05 − 0.08) | (−0.00 − 0.03) | (−0.17 − −0.03) ** | (−0.01 − 0.30) | (−0.03 − 0.06) | |
| Location N Forest: Layer 15–30 cm | 0.23 | 0.13 | 0.00 | −0.05 | 0.23 | −0.06 |
| (−0.02 − 0.47) | (0.04 − 0.22) ** | (−0.02 − 0.02) | (−0.15 − 0.05) | (0.01 − 0.44) | (−0.12 − 0.01) | |
| Location S Forest: Layer 15–30 cm | 0.45 | 0.07 | 0.03 | −0.17 | 0.3 | 0.03 |
| (0.27 − 0.63) *** | (0.01 − 0.14) * | (0.02 − 0.05) ** | (−0.25 − −0.10) *** | (0.14 − 0.46) * | (−0.01 − 0.08) | |
| Location N Forest: Layer 30–40 cm | 0.41 | 0.21 | 0.01 | −0.18 | 0.43 | −0.1 |
| (0.16 − 0.66) ** | (0.12 − 0.30) *** | (−0.01 − 0.03) | (−0.29 − −0.08) *** | (0.21 − 0.66) * | (−0.17 − −0.04) ** | |
| Location S Forest: Layer 30–40 cm | 0.46 | 0.06 | 0.03 | −0.28 | 0.38 | 0.03 |
| (0.28 − 0.64) *** | (−0.00 − 0.13) | (0.02 − 0.05) ** | (−0.35 − −0.20) *** | (0.21 − 0.54) ** | (−0.02 − 0.08) | |
| Random Parts | ||||||
| σ2 | 0.058 | 0.007 | 0.000 | 0.010 | 0.046 | 0.004 |
| τ00,Season | 0.007 | 0.001 | 0.001 | 0.000 | 0.133 | 0.002 |
| ICCSeason | 0.109 | 0.135 | 0.561 | 0.000 | 0.742 | 0.280 |
| R2c/R2m | 0.488/0.544 | 0.569/0.627 | 0.359/0.718 | 0.678/0.678 | 0.397/0.844 | 0.515/0.651 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioretto, A.; Innangi, M.; De Marco, A.; Menta, C.; Papa, S.; Pellegrino, A.; Virzo De Santo, A. Discriminating between Seasonal and Chemical Variation in Extracellular Enzyme Activities within Two Italian Beech Forests by Means of Multilevel Models. Forests 2018, 9, 219. https://doi.org/10.3390/f9040219
Fioretto A, Innangi M, De Marco A, Menta C, Papa S, Pellegrino A, Virzo De Santo A. Discriminating between Seasonal and Chemical Variation in Extracellular Enzyme Activities within Two Italian Beech Forests by Means of Multilevel Models. Forests. 2018; 9(4):219. https://doi.org/10.3390/f9040219
Chicago/Turabian StyleFioretto, Antonietta, Michele Innangi, Anna De Marco, Cristina Menta, Stefania Papa, Antonella Pellegrino, and Amalia Virzo De Santo. 2018. "Discriminating between Seasonal and Chemical Variation in Extracellular Enzyme Activities within Two Italian Beech Forests by Means of Multilevel Models" Forests 9, no. 4: 219. https://doi.org/10.3390/f9040219





