How Forest Gap and Elevation Shaped Abies faxoniana Rehd. et Wils. Regeneration in a Subalpine Coniferous Forest, Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Data Processing and Analysis
2.4. Structural Equation Modeling
3. Results
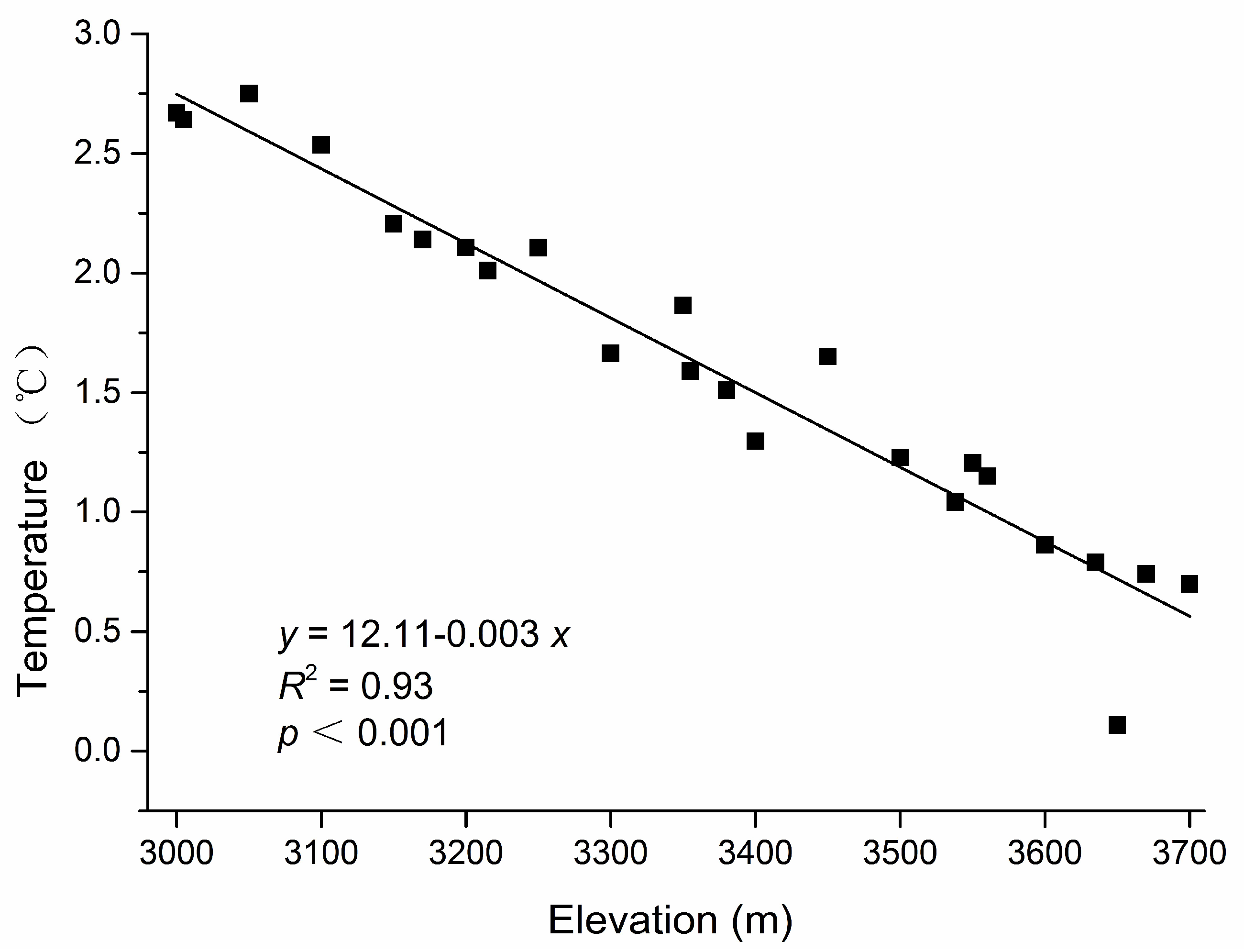
3.1. Gap Heterogeneity, Elevation Gradient and Independent Variables
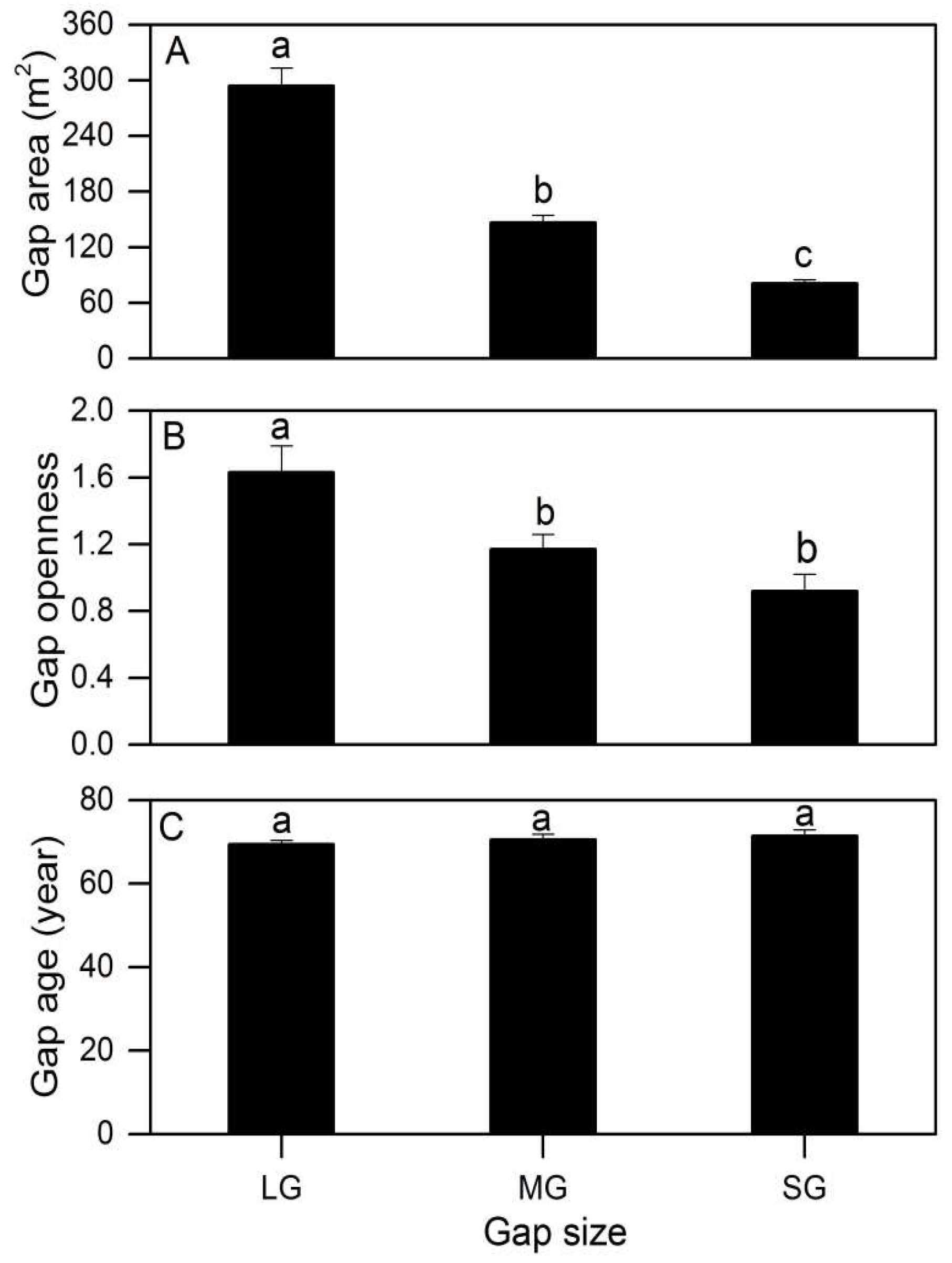
3.2. Gap Size, Elevation Gradient and Independent Variables
3.3. Relationship between Indicators and Abies faxoniana Regeneration in SEM
4. Discussion
4.1. Effect of Biotic Factors on Abies faxoniana Regeneration
4.2. Effect of Abiotic Factors on Abies faxoniana Regeneration
4.3. Direct and Indirect Effects of Forest Gap and Elevation on Abies faxoniana Regeneration in the SEM
4.4. Implications for Management and Gaps
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Denslow, J.S. Gap partitioning among tropical rainforest trees. Biotropica 1980, 12, 47–55. [Google Scholar] [CrossRef]
- Zhang, C.; Zou, C.J.; Peltola, H.; Wang, K.Y.; Xu, W.D. The effects of gap size and age on natural regeneration of Picea mongolica in the semi-arid region of Northern China. New. For. 2013, 44, 297–310. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J.G. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Nakamura, A.; Morimoto, Y.; Mizutani, Y. Adaptive management approach to increasing the diversity of a 30-year-old planted forest in an urban area of Japan. Landsc. Urban Plan. 2005, 70, 291–300. [Google Scholar] [CrossRef]
- Adamic, M.; Diaci, J.; Rozman, A.; Hladnik, D. Long-term use of uneven-aged silviculture in mixed mountain Dinaric forests: A comparison of old-growth and managed stands. Forestry 2017, 90, 279–291. [Google Scholar] [CrossRef]
- Christensen, N.L.; Bartuska, A.M.; Brown, J.H.; Carpenter, S.; D’Antonio, C.; Francis, R.; Franklin, J.F.; MacMahon, J.A.; Noss, R.F.; Parsons, D.J.; et al. The report of the ecological society of America committee on the scientific basis for ecosystem management. Ecol. Appl. 1996, 6, 665–691. [Google Scholar] [CrossRef]
- Vajari, A.K.; Jalilvand, H.; Pourmajidian, M.R.; Espahbodi, K.; Moshki, A. Effect of canopy gap size and ecological factors on species diversity and beech seedlings in managed beech stands in Hyrcanian forests. J. For. Res. 2012, 23, 217–222. [Google Scholar] [CrossRef]
- Muscolo, A.; Mallamaci, C.; Sidari, M.; Mercurio, R. Effects of gap size and soil chemical properties on the natural regeneration in black pine (Pinus nigra Arn.) Stands. Tree For. Sci. Biotechnol. 2011, 5, 65–71. [Google Scholar]
- Buettel, J.C.; Ondei, S.; Brook, B.W. Look down to see what’s up: A systematic overview of treefall dynamics in forests. Forests 2017, 8, 123. [Google Scholar] [CrossRef]
- Sapkota, I.P.; Oden, P.C. Gap characteristics and their effects on regeneration, dominance and early growth of woody species. J. Plant Ecol. 2009, 2, 21–29. [Google Scholar] [CrossRef]
- Malcom, D.C.; Mason, W.L.; Clarke, G.C. The transformation of conifer forests in Britain- regeneration, gap size and silvicultural systems. For. Ecol. Manag. 2001, 151, 7–23. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Effects of gap size, light and herbivory on the herb layer vegetation in European beech forest gaps. For. Ecol. Manag. 2007, 244, 141–149. [Google Scholar] [CrossRef]
- Wangchuk, K.; Darabant, A.; Rai, P.B.; Wurzinger, M.; Zollitsch, W.; Gratzer, G. Species richness, diversity and density of vegetation along disturbance gradients in the Himalayan conifer forest. J. Mt. Sci. 2014, 11, 1182–1191. [Google Scholar] [CrossRef]
- Lima, R.A.F.D.; Moura, L.C. Gap disturbance regime and composition in the Atlantic Montane Rain Forest: The influence of topography. Plant Ecol. 2008, 197, 239–253. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- De Montigny, L.E.; Smith, N.J. The effects of gap size in a group selection silvicultural system on the growth response of young, planted Douglas-fir: A sector plot analysis. Forestry 2017, 90, 426–435. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.Z.; Yang, W.Q.; Xu, L.Y.; Ni, X.Y.; He, J.; Tan, B.; Hu, Y. Effects of forest gaps on litter lignin and cellulose dynamics vary seasonally in an alpine forest. Forests 2016, 7, 27. [Google Scholar] [CrossRef]
- Sapkota, I.P.; Tigabu, M.; Oden, P.C. Species diversity and regeneration of old-growth seasonally dry Shorea robusta forests following gap formation. J. For. Res. 2009, 20, 7–14. [Google Scholar] [CrossRef]
- Kang, W.; Tian, C.; Kang, D.W.; Wang, M.J.; Li, Y.X.; Wang, X.R.; Li, J.Q. Effects of gap size, gap age, and bamboo Fargesia denudata on Abies faxoniana recruitment in South-western China. For. Syst. 2015, 24. [Google Scholar] [CrossRef]
- Gale, N. The Relationship between canopy gaps and topography in a western Ecuadorian rain forest. Biotropica 2000, 32, 653–661. [Google Scholar] [CrossRef]
- Runkle, J.R.; Yetter, T.C. Treefalls revisited—Gap dynamics in the southern Appalachians. Ecology 1987, 68, 417–424. [Google Scholar] [CrossRef]
- Taylor, A.H.; Jiang, S.W.; Zhu, L.J.; Liang, C.P.; Miao, C.J.; Huang, J.Y. Regeneration patterns and tree species coexistence in old-growth Abies–Picea forests in southwestern China. For. Ecol. Manag. 2006, 223, 303–317. [Google Scholar] [CrossRef]
- Bossard, C.C.; Cao, Y.; Wang, J.; Rose, A.; Tang, Y. New patterns of establishment and growth of Picea, Abies and Betula tree species in subalpine forest gaps of Jiuzhaigou National Nature Reserve, Sichuan, southwestern China in a changing environment. For. Ecol. Manag. 2015, 356, 84–92. [Google Scholar] [CrossRef]
- Dee, J.R.; Menges, E.S. Gap ecology in the Florida scrubby flatwoods: Effects of time-since-fire, gap area, gap aggregation and microhabitat on gap species diversity. J. Veg. Sci. 2014, 25, 1235–1246. [Google Scholar] [CrossRef]
- Klopcic, M.; Simoncic, T.; Boncina, A. Comparison of regeneration and recruitment of shade-tolerant and light-demanding tree species in mixed uneven-aged forests: Experiences from the Dinaric region. Forestry 2015, 88, 552–563. [Google Scholar] [CrossRef]
- Zhu, J.J.; Matsuzaki, T.; Lee, F.Q.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Dekker, M.; van Breugel, M.; Sterck, F.J. Effective height development of four co-occurring species in the gap-phase regeneration of Douglas fir monocultures under nature-oriented conversion. For. Ecol. Manag. 2007, 238, 189–198. [Google Scholar] [CrossRef]
- Zhu, J.J.; Lu, D.L.; Zhang, W.D. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Kubota, Y.; Murata, H.; Kikuzawa, K. Effects of topographic heterogeneity on tree species richness and stand dynamics in a subtropical forest in Okinawa Island, southern Japan. J. Ecol. 2004, 92, 230–240. [Google Scholar] [CrossRef]
- Grace, J.B.; Pugesek, B.H. A structural equation model of plant species richness and its application to a coastal wetland. Am. Nat. 1997, 149, 436–460. [Google Scholar] [CrossRef]
- Gough, L.; Grace, J.B. Effects of Environmental Change on Plant Species Density: Comparing Predictions with Experiments. Ecology 1999, 80, 882. [Google Scholar] [CrossRef]
- Kern, C.C.; Burton, J.I.; Raymond, P.; D’Amato, A.W.; Keeton, W.S.; Royo, A.A.; Walters, M.B.; Webster, C.R.; Willis, J.L. Challenges facing gap-based silviculture and possible solutions for mesic northern forests in North America. Forestry 2017, 90, 4–17. [Google Scholar] [CrossRef]
- Runkle, J.R. Gap regeneration in some old-growth forests of the Eastern-United-States. Ecology 1981, 62, 1041–1051. [Google Scholar] [CrossRef]
- Sefidi, K.; Mohadjer, M.R.M.; Mosandl, R.; Copenheaver, C.A. Canopy gaps and regeneration in old-growth Oriental beech (Fagus orientalis Lipsky) stands, northern Iran. For. Ecol. Manag. 2011, 262, 1094–1099. [Google Scholar] [CrossRef]
- Beck, J.; Chey, V.K. Explaining the elevational diversity pattern of geometrid moths from Borneo: A test of five hypotheses. J. Biogeogr. 2008, 35, 1452–1464. [Google Scholar] [CrossRef]
- Parent, S.; Messier, C. A simple and efficient method to estimate microsite light availability under a forest canopy. Can. J. Forest. Res. 1996, 26, 151–154. [Google Scholar] [CrossRef]
- Gendron, F.; Messier, C.; Comeau, P.G. Comparison of various methods for estimating the mean growing season percent photosynthetic photon flux density in forests. Agric. For. Meteorol. 1998, 92, 55–70. [Google Scholar] [CrossRef]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Morante-Filho, J.C.; Arroyo-Rodriguez, V.; Lohbeck, M.; Tscharntke, T.; Faria, D. Tropical forest loss and its multitrophic effects on insect herbivory. Ecology 2016, 97, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kissling, W.D.; He, F.L. Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. J. Biogeogr. 2013, 40, 1131–1142. [Google Scholar] [CrossRef]
- Huth, F.; Wagner, S. Gap structure and establishment of Silver birch regeneration (Betula pendula Roth.) in Norway spruce stands (Picea abies L. Karst.). For. Ecol. Manag. 2006, 229, 314–324. [Google Scholar] [CrossRef]
- Kramer, K.; Brang, P.; Bachofen, H.; Bugmann, H.; Wohlgemuth, T. Site factors are more important than salvage logging for tree regeneration after wind disturbance in Central European forests. For. Ecol. Manag. 2014, 331, 116–128. [Google Scholar] [CrossRef]
- Tirado, R.; Pugnaire, F.I. Community structure and positive interactions in constraining environments. Oikos 2005, 111, 437–444. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends. Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol. 2005, 93, 748–757. [Google Scholar] [CrossRef]
- Denslow, J.S. Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- Taylor, A.H.; Qin, Z.S. Regeneration patterns in old growth Abies-Betula forests in the Wolong Natural Reserve, Sichuan, China. J. Ecol. 1998, 76, 1204–1218. [Google Scholar] [CrossRef]
- Abd Latif, Z.; Blackburn, G.A. The effects of gap size on some microclimate variables during late summer and autumn in a temperate broadleaved deciduous forest. Int. J. Biometeorol. 2010, 54, 119–129. [Google Scholar] [CrossRef] [PubMed]
- He, Z.S.; Liu, J.F.; Wu, C.T.; Zheng, S.Q.; Hong, W.; Su, S.J.; Wu, C.Z. Effects of forest gaps on some microclimate variables in Castanopsis kawakamii natural forest. J. Mt. Sci. 2012, 9, 706–714. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y.J.; Zhang, J.; Wang, X.L. Latitudinal gradients in phylogenetic relatedness of angiosperm trees in North America. Glob. Ecol. Biogeogr. 2013, 22, 1183–1191. [Google Scholar] [CrossRef]
- Aiba, S.I.; Kohyama, T. Tree species stratification in relation to allometry and demography in a warm-temperate rain forest. J. Ecol. 1996, 84, 207–218. [Google Scholar] [CrossRef]
- D’Oliveira, M.V.N.; Ribas, L.A. Forest regeneration in artificial gaps twelve years after canopy opening in Acre State Western Amazon. For. Ecol. Manag. 2011, 261, 1722–1731. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef]
- Bongjoh, C.A.; Mama, N. Early regeneration of commercial timber species in a logged-over forest of southern Cameroon. In Seminar FORAFRI Libreville-Session 2: Knowledge Ecosystem; CIRAD: Montpellier, QC, Canada, 1999; pp. 1–9. [Google Scholar]
- Yin, H.J.; Liu, Q.; Lai, T. Warming effects on growth and physiology in the seedlings of the two conifers Picea asperata and Abies faxoniana under two contrasting light conditions. Ecol. Res. 2008, 23, 459–469. [Google Scholar] [CrossRef]
- Poulson, T.L.; Platt, W.J. Replacement patterns of beech and sugar maple in Warren Woods, Michigan. Ecology 1996, 77, 1234–1253. [Google Scholar] [CrossRef]
- Ritter, E.; Starr, M.; Vesterdal, L. Losses of nitrate from gaps of different sizes in a managed beech (Fagus sylvatica) forest. Can. J. Forest. Res. 2005, 35, 308–319. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Mercurio, R. Influence of gap size on organic matter decomposition, microbial biomass and nutrient cycle in Calabrian pine (Pinus laricio, Poiret) stands. For. Ecol. Manag. 2007, 242, 412–418. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parsons, W.F.J.; Parkinson, D. Decomposition of Populus-Tremuloides Leaf litter accelerated by addition of Alnus-Crispa Litter. Can. J. For. Res. 1989, 19, 674–679. [Google Scholar] [CrossRef]
- Denslow, J.S.; Ellison, A.M.; Sanford, R.E. Treefall gap size effects on above- and below-ground processes in a tropical wet forest. J. Ecol. 1998, 86, 597–609. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Keeley, J.E. Forest reproduction along a climatic gradient in the Sierra Nevada, California. For. Ecol. Manag. 2006, 225, 391–399. [Google Scholar] [CrossRef]






| Variables | Gap + CK | CK | Gap | ||
|---|---|---|---|---|---|
| Large Gap | Medium Gap | Small Gap | |||
| N | 34 | 8 | 9 | 9 | 8 |
| Elevation * (m) | 3356.0 ± 36.6 | 3383.1 ± 75.7 | 3339.0 ± 80.1 | 3379.0 ± 71.1 | 3322.0 ± 77.0 |
| Habitat characteristic * | |||||
| Area (m2) | 161.20 ± 15.50 | 100.00 ± 0.00 | 294.07 ± 19.13 | 149.64 ± 8.12 | 85.94 ± 5.84 |
| Gap openness | 1.63 ± 0.16 | 1.15 ± 0.09 | 0.97 ± 0.10 | ||
| Gap age (year) | 69.4 ± 1.0 | 71.1 ± 1.4 | 71.3 ± 1.3 | ||
| Biotic factor # | |||||
| Cover of shrub layer (%) | 36.3 ± 4.4 | 25.3 ± 6.5 | 47.3 ± 10.2 | 35.9 ± 8.7 | 35.4 ± 8.8 |
| Cover of herb layer (%) | 42.9 ± 4.6 | 49.6 ± 4.1 | 46.1 ± 7.2 | 39.4 ± 7.3 | 36.5 ± 9.7 |
| Moss layer thickness (cm) | 6.70 ± 0.44 | 6.75 ± 0.31 | 7.22 ± 0.89 | 6.11 ± 1.01 | 6.63 ± 1.20 |
| Abiotic factor # | |||||
| Temperature (°C) | 1.59 ± 0.11 | 1.50 ± 0.23 | 1.64 ± 0.24 | 1.55 ± 0.22 | 1.68 ± 0.23 |
| PPFD (μmol m−2 s−1) | 216.44 ± 15.87 | 54.75 ± 2.80 | 264.89 ± 8.64 | 262.67 ± 1.13 | 271.63 ± 5.54 |
| Soil total C (%) | 7.45 ± 0.52 | 6.74 ± 0.74 | 6.14 ± 0.96 | 8.40 ± 1.37 | 6.62 ± 0.92 |
| Soil total N (%) | 0.45 ± 0.02 | 0.43 ± 0.30 | 0.44 ± 0.06 | 0.52 ± 0.05 | 0.39 ± 0.03 |
| Abies faxoniana regeneration # | |||||
| DS (N m−2) | 0.63 ± 0.05 | 0.32 ± 0.05 | 0.61 ± 0.08 | 0.73 ± 0.09 | 0.82 ± 0.13 |
| DST (N m−2) | 0.37 ± 0.05 | 0.07 ± 0.01 | 0.64 ± 0.09 | 0.37 ± 0.06 | 0.36 ± 0.06 |
| HDR | 90.10 ± 2.44 | 80.13 ± 3.02 | 97.54 ± 6.02 | 92.20 ± 3.87 | 89.344.56 |
| Variables | Gap vs. CK | Elevation | Gap Size | Elevation | ||||
|---|---|---|---|---|---|---|---|---|
| (Ngap = 26, NCK = 8) | N = 34 | (Nlarge = 9, NMedium = 9, NSmall = 7) | N = 26 | |||||
| F | p | F | p | F | p | F | p | |
| gap openness | 5.985 | 0.044 | 0.874 | 0.570 | ||||
| gap age | 0.540 | 0.613 | 0.984 | 0.518 | ||||
| PPFD | 406.434 | <0.001 | 2.43 | 0.174 | 0.839 | 0.485 | 3.165 | 0.113 |
| MAT | 0.745 | 0.570 | 140.654 | <0.001 | 0.789 | 0.504 | 157.861 | <0.001 |
| soil total N | 0.409 | 0.754 | 0.273 | 0.927 | 0.613 | 0.578 | 0.419 | 0.840 |
| soil total C | 0.253 | 0.857 | 1.185 | 0.436 | 0.377 | 0.704 | 1.295 | 0.397 |
| soil water content | 0.216 | 0.881 | 0.788 | 0.615 | 0.041 | 0.960 | 1.369 | 0.374 |
| moss thickness | 0.593 | 0.646 | 3.049 | 0.050 | 0.879 | 0.471 | 5.536 | 0.028 |
| cover of shrub | 4.772 | 0.063 | 17.961 | 0.003 | 3.888 | 0.096 | 20.784 | 0.002 |
| cover of herb | 3.262 | 0.118 | 15.694 | 0.004 | 3.05 | 0.136 | 21.057 | 0.002 |
| DS | 30.819 | 0.001 | 21.803 | 0.002 | 0.779 | 0.510 | 25.887 | 0.001 |
| DST | 82.595 | <0.001 | 20.281 | 0.002 | 38.494 | 0.001 | 27.657 | 0.001 |
| HD ratio of TSD | 5.962 | 0.042 | 10.154 | 0.011 | 1.999 | 0.23 | 10.443 | 0.010 |
| Models | Model Fit Indices | ||||||
|---|---|---|---|---|---|---|---|
| χ2 | DF | p | RMSEA | GFI | CFI | AIC | |
| A | 4.727 | 10 | 0.909 | <0.001 | 0.959 | 1 | 40.727 |
| B | 3.675 | 6 | 0.721 | <0.001 | 0.963 | 1 | 33.675 |
| C | 8.505 | 11 | 0.668 | <0.001 | 0.915 | 1 | 58.505 |
| D | 3.689 | 8 | 0.869 | <0.001 | 0.948 | 1 | 29.869 |
| Regeneration Indexes | Gap + CK | Gap | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elevation | HY | T | PPFD | PA | SC | DS | Elevation | GA | T | STC | GO | HC | MLT | DS | DST | ||
| Total effect | |||||||||||||||||
| DS | −0.74 | 0.47 | 0.79 | −0.71 | −0.27 | - | 0 | −0.82 | −0.36 | 0.61 | 0.10 | 0.09 | 0.27 | - | 0 | - | |
| DST | −0.53 | 0.60 | 0.56 | −0.51 | 0.46 | - | 0.71 | −0.59 | 0.65 | 0.44 | 0.07 | 0.06 | 0.19 | - | 0.72 | - | |
| HDR ratio | −0.43 | 0.36 | 0.43 | 0.27 | - | 0.53 | - | −0.63 | 0.29 | 0.43 | - | - | - | 0.35 | - | 0.56 | |
| Direct effect | |||||||||||||||||
| DS | 0 | 1.28 | 0.79 | −0.71 | −0.27 | 0 | 0 | −0.43 | 0.61 | 0 | 0 | 0.27 | - | 0 | - | ||
| DST | 0 | 0 | 0 | 0 | 0.65 | 0.71 | 0 | 0.91 | 0 | 0 | 0 | 0 | - | 0.72 | - | ||
| HDR ratio | 0 | 0 | 0 | 0.27 | 0.53 | 0 | 0 | 0 | - | - | - | 0.35 | - | 0.56 | |||
| Indirect effect | |||||||||||||||||
| DS | −0.74 | −0.81 | 0 | 0 | 0 | - | 0 | −0.82 | 0.07 | 0 | 0.10 | 0.09 | 0 | - | - | - | |
| DST | −0.53 | 0.60 | 0.56 | −0.51 | −0.19 | - | 0 | −0.59 | −0.26 | 0.44 | 0.07 | 0.06 | 0.19 | - | 0 | - | |
| HDR | −0.43 | 0.36 | 0.43 | 0 | - | 0 | - | −0.63 | 0.29 | 0.43 | - | - | - | 0 | - | 0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, G.; Liu, D. How Forest Gap and Elevation Shaped Abies faxoniana Rehd. et Wils. Regeneration in a Subalpine Coniferous Forest, Southwestern China. Forests 2018, 9, 271. https://doi.org/10.3390/f9050271
Chen L, Liu G, Liu D. How Forest Gap and Elevation Shaped Abies faxoniana Rehd. et Wils. Regeneration in a Subalpine Coniferous Forest, Southwestern China. Forests. 2018; 9(5):271. https://doi.org/10.3390/f9050271
Chicago/Turabian StyleChen, Li, Guohua Liu, and Dan Liu. 2018. "How Forest Gap and Elevation Shaped Abies faxoniana Rehd. et Wils. Regeneration in a Subalpine Coniferous Forest, Southwestern China" Forests 9, no. 5: 271. https://doi.org/10.3390/f9050271




