Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immunizations Prior to HD-Ad Vaccinations

| Macaque | Env peptides + FL3L + CpG 3X 12/2006 | Env peptides + CT2* 3X 12/2006 | Ad-EnvPep nasal 2X 12/2007 | Env Peptides + DCs 2X 3/2008 | HD-Ad5-Env 3X 9/2008 | HD-Ad6-Env HD-Ad1-EnvHD-Ad2-Env 9/2008 |
|---|---|---|---|---|---|---|
| Rh51 | + | + | + | + | ||
| Rh55 | + | + | + | + | ||
| Rh52 | + | + | + | + | ||
| Rh61 | + | + | + | + | ||
| Rh62 | + | + | + | + | ||
| Rh63 | + | + | + | + | ||
| Rh66 | + | + | + | + | ||
| Rh67 | + | + | + | + |
2.2. HD-Ad Vaccinations
2.3. Neutralizing Antibodies Generated Against HIV-1 Envelope
| ID50 in TZM-bl cells1 | |||
|---|---|---|---|
| Animal | Bleed day | SHIV-SF162P4 (ID#762) | SHIV-89.6P.18 (ID#767) |
| 51 | 0 | <20 | <20 |
| 24 | <20 | <20 | |
| 57 | <20 | <20 | |
| 83 | <20 | <20 | |
| 52 | 0 | 36 | 20 |
| 24 | 25 | <20 | |
| 57 | <20 | <20 | |
| 83 | 25 | <20 | |
| 55 | 0 | 31 | <20 |
| 24 | 23 | <20 | |
| 57 | <20 | <20 | |
| 83 | 55 | 23 | |
| 61 | 0 | 25 | <20 |
| 24 | <20 | <20 | |
| 57 | 22 | <20 | |
| 83 | <20 | <20 | |
| 62 | 0 | <20 | <20 |
| 24 | <20 | <20 | |
| 57 | <20 | <20 | |
| 83 | <20 | <20 | |
| 63 | 0 | 25 | <20 |
| 24 | <20 | <20 | |
| 57 | <20 | <20 | |
| 83 | 25 | <20 | |
| 66 | 0 | <20 | <20 |
| 24 | <20 | <20 | |
| 57 | <20 | <20 | |
| 83 | 22 | <20 | |
| 67 | 0 | <20 | <20 |
| 24 | <20 | <20 | |
| 57 | <20 | <20 | |
| 83 | 46 | <20 | |
2.4. Neutralizing Antibodies Against Adenovirus

2.5. T Cell Responses Generated by the HD-Ad Vaccines
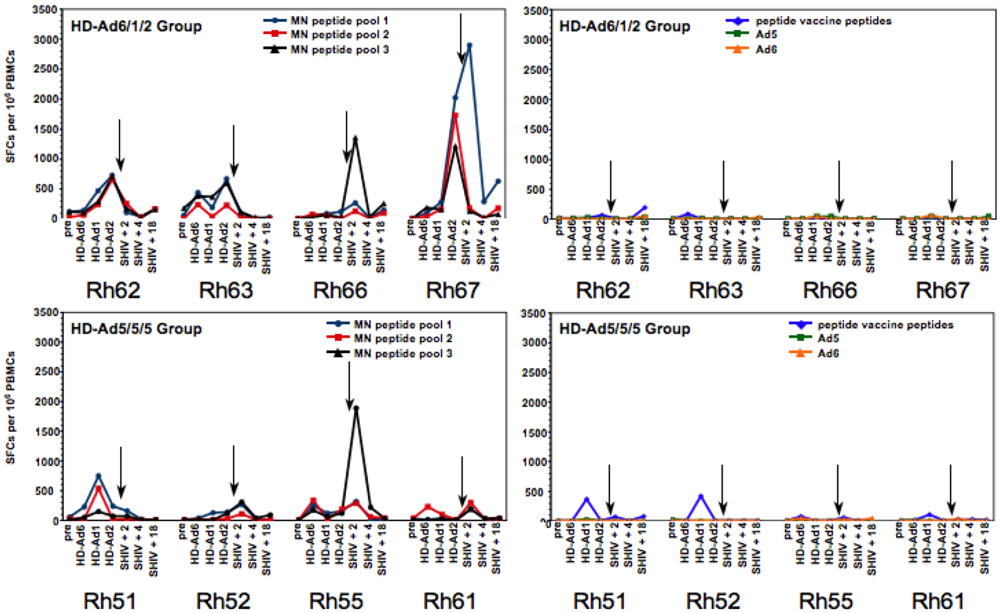
 |
2.6. Mucosal SHIV Challenge
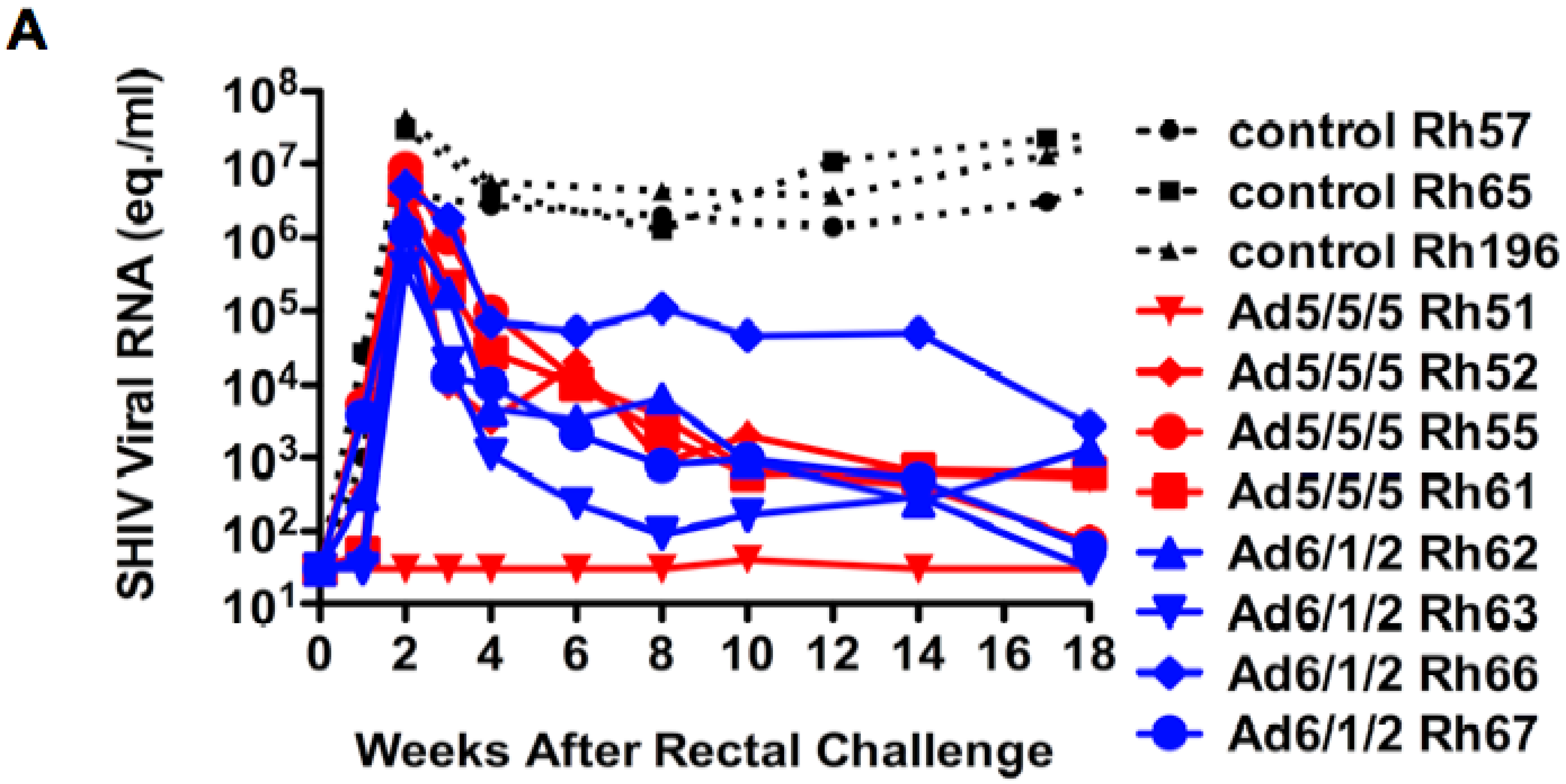
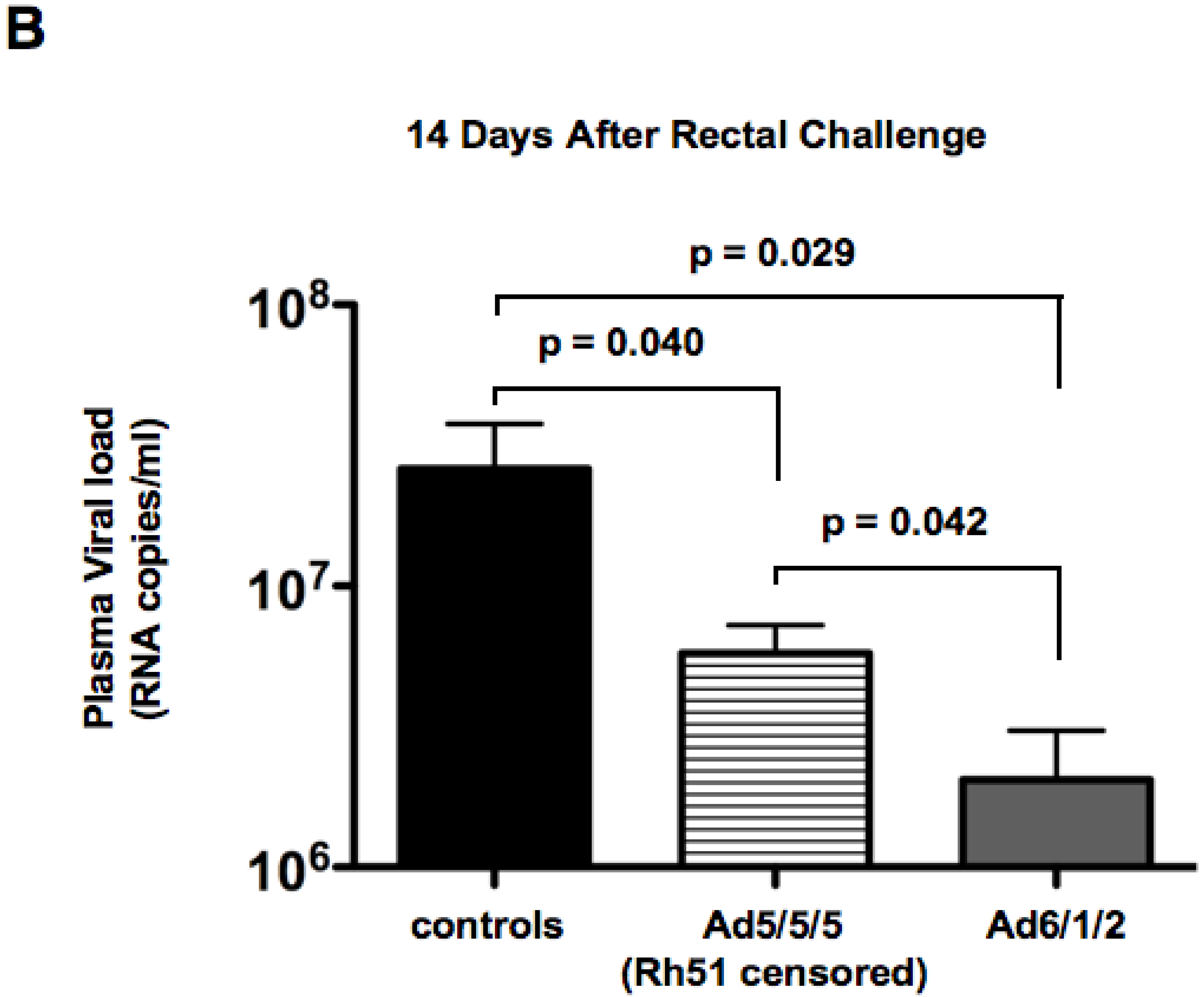
2.7. Discussion
3. Experimental Section
3.1. Adenoviruses
3.2. Animals
3.3. Immunizations Prior to HD-Ad Vaccinations
3.4. HD-Ad Vaccination
3.5. Collection of Samples
3.6. Assay for Neutralization of HIV and SHIV
3.7. Assay for Neutralization of Ad5
3.8. ELISPOT assay for detecting antigen-specific IFN-γ producing cells
3.9. Virus Challenge
3.10. Viral Load Determination
3.11. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Daniel, M.D.; Kirchhoff, F.; Czajak, S.C.; Sehgal, P.K.; Desrosiers, R.C. Protective effects of a live attenuated SIV vaccine with a deletion of the nef gene. Science 1992, 258, 1938–1941. [Google Scholar] [PubMed]
- Igarashi, T.; Ami, Y.; Yamamoto, H.; Shibata, R.; Kuwata, T.; Mukai, R.; Shinohara, K.; Komatsu, T.; Adachi, A.; Hayami, M. Protection of monkeys vaccinated with vpr- and/or nef-defective simian immunodeficiency virus strain mac/human immunodeficiency virus type 1 chimeric viruses: a potential candidate live-attenuated human AIDS vaccine. J. Gen. Virol. 1997, 78, 985–989. [Google Scholar] [PubMed]
- Benson, J.; Chougnet, C.; Robert-Guroff, M.; Montefiori, D.; Markham, P.; Shearer, G.; Gallo, R.C.; Cranage, M.; Paoletti, E.; Limbach, K.; Venzon, D.; Tartaglia, J.; Franchini, G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J. Virol. 1998, 72, 4170–4182. [Google Scholar] [PubMed]
- Ourmanov, I.; Brown, C.R.; Moss, B.; Carroll, M.; Wyatt, L.; Pletneva, L.; Goldstein, S.; Venzon, D.; Hirsch, V.M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 2000, 74, 2740–2751. [Google Scholar] [CrossRef] [PubMed]
- Mossman, S.P.; Bex, F.; Berglund, P.; Arthos, J.; O'Neil, S.P.; Riley, D.; Maul, D.H.; Bruck, C.; Momin, P.; Burny, A.; Fultz, P.N.; Mullins, J.I.; Liljestrom, P.; Hoover, E.A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 1996, 70, 1953–1960. [Google Scholar] [PubMed]
- Buge, S.L.; Murty, L.; Arora, K.; Kalyanaraman, V.S.; Markham, P.D.; Richardson, E.S.; Aldrich, K.; Patterson, L.J.; Miller, C.J.; Cheng, S.M.; Robert-Guroff, M. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251. J. Virol. 1999, 73, 7430–7440. [Google Scholar] [PubMed]
- Robert-Guroff, M.; Kaur, H.; Patterson, L.J.; Leno, M.; Conley, A.J.; McKenna, P.M.; Markham, P.D.; Richardson, E.; Aldrich, K.; Arora, K.; Murty, L.; Carter, L.; Zolla-Pazner, S.; Sinangil, F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 1998, 72, 10275–10280. [Google Scholar] [PubMed]
- Wang, B.; Ugen, K.E.; Srikantan, V.; Agadjanyan, M.G.; Dang, K.; Refaeli, Y.; Sato, A.I.; Boyer, J.; Williams, W.V.; Weiner, D.B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1993, 90, 4156–4160. [Google Scholar] [CrossRef]
- Wang, B.; Boyer, J.; Srikantan, V.; Ugen, K.; Gilbert, L.; Phan, C.; Dang, K.; Merva, M.; Agadjanyan, M.G.; Newman, M.; Carrano, R.; McCallus, D.; Coney, L.; Williams, W.V.; Weiner, D.B. Induction of humoral and cellular immune responses to the human immunodeficiency type 1 virus in nonhuman primates by in vivo DNA inoculation. Virology 1995, 211, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Santoro, J.C.; Fuller, D.H.; Haynes, J.R.; Robinson, H.L. Use of DNAs expressing HIV-1 env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology 1995, 209, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Schapiro, S.J.; Johnson, P.C.; Murthy, K.K.; Satterfield, W.C.; Sastry, K.J. A synthetic peptide from the first conserved region in the envelope protein gp160 is a strong T-cell epitope in HIV-infected chimpanzees and humans. Viral Immunol. 1998, 11, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Nehete, B.P.; Hill, L.; Manuri, P.R.; Baladandayuthapani, V.; Feng, L.; Simmons, J.; Sastry, K.J. Selective induction of cell-mediated immunity and protection of rhesus macaques from chronic SHIV(KU2) infection by prophylactic vaccination with a conserved HIV-1 envelope peptide-cocktail. Virology 2008, 370, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, G.V.; Ritter, G.D.; Vey, M.; Compans, R.W. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology 1995, 214, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Deml, L.; Notka, F.; Wolf, H.; Schirmbeck, R.; Reimann, J.; Teeuwsen, V.; Heeney, J. Safety and immunogenicity of recombinant human immunodeficiency virus-like particles in rodents and rhesus macaques. Intervirology 1996, 39, 93–103. [Google Scholar] [PubMed]
- Yao, Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Res. Initiat. Treat. Action 2003, 8, 20–21. [Google Scholar] [PubMed]
- Dale, C.J.; Liu, X.S.; De Rose, R.; Purcell, D.F.; Anderson, J.; Xu, Y.; Leggatt, G.R.; Frazer, I.H.; Kent, S.J. Chimeric human papilloma virus-simian/human immunodeficiency virus virus-like-particle vaccines: immunogenicity and protective efficacy in macaques. Virology 2002, 301, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lehner, T.; Anton, P.A. Mucosal immunity and vaccination against HIV. Aids 2002, 16, S125–S132. [Google Scholar] [PubMed]
- Simecka, J.W. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv. Drug Deliv. Rev. 1998, 34, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Santra, S.; Schmitz, J.E.; Kuroda, M.J.; Fu, T.M.; Wagner, W.; Bilska, M.; Craiu, A.; Zheng, X.X.; Krivulka, G.R.; Beaudry, K.; Lifton, M.A.; Nickerson, C.E.; Trigona, W.L.; Punt, K.; Freed, D.C.; Guan, L.; Dubey, S.; Casimiro, D.; Simon, A.; Davies, M.E.; Chastain, M.; Strom, T.B.; Gelman, R.S.; Montefiori, D.C.; Lewis, M.G.; Emini, E.A.; Shiver, J.W.; Letvin, N.L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000, 290, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, D.R.; Chen, L.; Fu, T.M.; Evans, R.K.; Caulfield, M.J.; Davies, M.E.; Tang, A.; Chen, M.; Huang, L.; Harris, V.; Freed, D.C.; Wilson, K.A.; Dubey, S.; Zhu, D.M.; Nawrocki, D.; Mach, H.; Troutman, R.; Isopi, L.; Williams, D.; Hurni, W.; Xu, Z.; Smith, J.G.; Wang, S.; Liu, X.; Guan, L.; Long, R.; Trigona, W.; Heidecker, G.J.; Perry, H.C.; Persaud, N.; Toner, T.J.; Su, Q.; Liang, X.; Youil, R.; Chastain, M.; Bett, A.J.; Volkin, D.B.; Emini, E.A.; Shiver, J.W. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003, 77, 6305–6313. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, D.R.; Tang, A.; Chen, L.; Fu, T.M.; Evans, R.K.; Davies, M.E.; Freed, D.C.; Hurni, W.; Aste-Amezaga, J.M.; Guan, L.; Long, R.; Huang, L.; Harris, V.; Nawrocki, D.K.; Mach, H.; Troutman, R.D.; Isopi, L.A.; Murthy, K.K.; Rice, K.; Wilson, K.A.; Volkin, D.B.; Emini, E.A.; Shiver, J.W. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003, 77, 7663–7668. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Wang, S.; Smith, J.G.; Tobery, T.W.; Liu, X.; Davies, M.E.; Casimiro, D.R.; Fu, T.M.; Simon, A.; Evans, R.K.; Emini, E.A.; Shiver, J. Sustained peptide-specific gamma interferon T-cell response in rhesus macaques immunized with human immunodeficiency virus gag DNA vaccines. J. Virol. 2002, 76, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Shiver, J. A non-replicating adenoviral vector as a potential HIV vaccine. Res. Initiat. Treat. Action 2003, 8, 14–16. [Google Scholar] [PubMed]
- Shiver, J.W.; Fu, T.M.; Chen, L.; Casimiro, D.R.; Davies, M.E.; Evans, R.K.; Zhang, Z.Q.; Simon, A.J.; Trigona, W.L.; Dubey, S.A.; Huang, L.; Harris, V.A.; Long, R.S.; Liang, X.; Handt, L.; Schleif, W.A.; Zhu, L.; Freed, D.C.; Persaud, N.V.; Guan, L.; Punt, K.S.; Tang, A.; Chen, M.; Wilson, K.A.; Collins, K.B.; Heidecker, G.J.; Fernandez, V.R.; Perry, H.C.; Joyce, J.G.; Grimm, K.M.; Cook, J.C.; Keller, P.M.; Kresock, D.S.; Mach, H.; Troutman, R.D.; Isopi, L.A.; Williams, D.M.; Xu, Z.; Bohannon, K.E.; Volkin, D.B.; Montefiori, D.C.; Miura, A.; Krivulka, G.R.; Lifton, M.A.; Kuroda, M.J.; Schmitz, J.E.; Letvin, N.L.; Caulfield, M.J.; Bett, A.J.; Youil, R.; Kaslow, D.C.; Emini, E.A. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002, 415, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Piedra, P.A.; Poveda, G.A.; Ramsey, B.; McCoy, K.; Hiatt, P.W. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 1998, 101, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.; Evelegh, C.; Graham, F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999, 6, 1565–1573. [Google Scholar] [CrossRef]
- Morral, N.; O'Neal, W.; Rice, K.; Leland, M. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. U S A 1999, 96, 12816–12821. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Fitzgerald, J.C.; Giles-Davis, W.; Gao, G.P.; Wilson, J.M.; Ertl, H.C. Induction of CD8(+) T Cells to an HIV-1 Antigen through a Prime Boost Regimen with Heterologous E1-Deleted Adenoviral Vaccine Carriers. J. Immunol. 2003, 171, 6774–6779. [Google Scholar] [PubMed]
- Lemckert, A.A.; Sumida, S.M.; Holterman, L.; Vogels, R.; Truitt, D.M.; Lynch, D.M.; Nanda, A.; Ewald, B.A.; Gorgone, D.A.; Lifton, M.A.; Goudsmit, J.; Havenga, M.J.; Barouch, D.H. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J. Virol. 2005, 79, 9694–9701. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Pau, M.G.; Custers, J.H.; Koudstaal, W.; Kostense, S.; Havenga, M.J.; Truitt, D.M.; Sumida, S.M.; Kishko, M.G.; Arthur, J.C.; Korioth-Schmitz, B.; Newberg, M.H.; Gorgone, D.A.; Lifton, M.A.; Panicali, D.L.; Nabel, G.J.; Letvin, N.L.; Goudsmit, J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004, 172, 6290–6297. [Google Scholar] [PubMed]
- McCoy, K.; Tatsis, N.; Korioth-Schmitz, B.; Lasaro, M.O.; Hensley, S.E.; Lin, S.W.; Li, Y.; Giles-Davis, W.; Cun, A.; Zhou, D.; Xiang, Z.; Letvin, N.L.; Ertl, H.C. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 2007, 81, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; O'Brien, K.L.; Lynch, D.M.; Simmons, N.L.; La Porte, A.; Riggs, A.M.; Abbink, P.; Coffey, R.T.; Grandpre, L.E.; Seaman, M.S.; Landucci, G.; Forthal, D.N.; Montefiori, D.C.; Carville, A.; Mansfield, K.G.; Havenga, M.J.; Pau, M.G.; Goudsmit, J.; Barouch, D.H. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 2008, 457, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef]
- Hardy, S.; Kitamura, M.; Harris-Stansil, T.; Dai, Y.; Phipps, M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997, 71, 1842–1849. [Google Scholar] [PubMed]
- Mitani, K.; Graham, F.L.; Caskey, C.T.; Kochanek, S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc. Natl. Acad. Sci. USA 1995, 92, 3854–3858. [Google Scholar] [CrossRef]
- Clemens, P.R.; Kochanek, S.; Sunada, Y.; Chan, S.; Chen, H.H.; Campbell, K.P.; Caskey, C.T. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996, 3, 965–972. [Google Scholar] [PubMed]
- Fisher KJ, C.H. Burda J, Chen SJ, Wilson JM, Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology 1996, 217, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Balfe, P.; Shapiro, S.; Hsu, M.; Buckner, C.; Harouse, J.M.; Cheng-Mayer, C. Expansion of quasispecies diversity but no evidence for adaptive evolution of SHIV during rapid serial transfers among seronegative macaques. Virology 2004, 318, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.J.; Arlinghaus, R.B. Identification of T-cell epitopes without B-cell activity in the first and second conserved regions of the HIV Env protein. Aids 1991, 5, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Satterfield, W.C.; Matherne, C.M.; Arlinghaus, R.B.; Sastry, K.J. Induction of human immunodeficiency virus-specific T cell responses in rhesus monkeys by synthetic peptides from gp160. AIDS Res. Hum. Retroviruses 1993, 9, 235–240. [Google Scholar] [CrossRef]
- Nehete, P.N.; Lewis, D.E.; Tang, D.N.; Pollack, M.S.; Sastry, K.J. Presence of HLA-C-restricted cytotoxic T-lymphocyte responses in long-term nonprogressors infected with human immunodeficiency virus. Viral Immunol. 1998, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Chitta, S.; Hossain, M.M.; Hill, L.; Bernacky, B.J.; Baze, W.; Arlinghaus, R.B.; Sastry, K.J. Protection against chronic infection and AIDS by an HIV envelope peptide-cocktail vaccine in a pathogenic SHIV-rhesus model. Vaccine 2001, 20, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Nehete, B.P.; Manuri, P.; Hill, L.; Palmer, J.L.; Sastry, K.J. Protection by dendritic cells-based HIV synthetic peptide cocktail vaccine: preclinical studies in the SHIV-rhesus model. Vaccine 2005, 23, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Mercier, G.T.; Nehete, P.N.; Passeri, M.F.; Nehete, B.N.; Weaver, E.A.; Templeton, N.S.; Schluns, K.; Buchl, S.S.; Sastry, K.J.; Barry, M.A. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine 2007, 25, 8687–8701. [Google Scholar] [PubMed]
- Hidajat, R.; Xiao, P.; Zhou, Q.; Venzon, D.; Summers, L.E.; Kalyanaraman, V.S.; Montefiori, D.C.; Robert-Guroff, M. Correlation of vaccine-elicited systemic and mucosal non-neutralizing antibody activities with reduced acute viremia following intrarectal SIVmac251 challenge of rhesus macaques. J. Virol. 2008. [Google Scholar]
- Hsu, M.; Harouse, J.M.; Gettie, A.; Buckner, C.; Blanchard, J.; Cheng-Mayer, C. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J. Virol. 2003, 77, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Buckner, C.; Harouse, J.; Gettie, A.; Blanchard, J.; Robinson, J.E.; Cheng-Mayer, C. Antigenic variations in the CD4 induced sites of the CCR5-tropic, pathogenic SHIVsf162p3 gp120 variants. J. Med. Primatol. 2003, 32, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Ng, P. Improved System for Helper-dependent adenoviral vector production. Mol. Ther. 2003, 8, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, F.; Mascola, J.R.; Stamatatos, L.; Polonis, V.R.; Koutsoukos, M.; Voss, G.; Goepfert, P.; Gilbert, P.; Greene, K.M.; Bilska, M.; Kothe, D.L.; Salazar-Gonzalez, J.F.; Wei, X.; Decker, J.M.; Hahn, B.H.; Montefiori, D.C. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005, 79, 10108–10125. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. 2005, 12 Unit 12, 11. [Google Scholar] [PubMed]
- Nehete, P.N.; Gambhira, R.; Nehete, B.P.; Sastry, K.J. Dendritic cells enhance detection of antigen-specific cellular immune responses by lymphocytes from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine. J. Med. Primatol. 2003, 32, 67–73. [Google Scholar] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Weaver, E.A.; Nehete, P.N.; Nehete, B.P.; Buchl, S.J.; Palmer, D.; Montefiori, D.C.; Ng, P.; Sastry, K.J.; Barry, M.A. Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines. Viruses 2009, 1, 920-938. https://doi.org/10.3390/v1030920
Weaver EA, Nehete PN, Nehete BP, Buchl SJ, Palmer D, Montefiori DC, Ng P, Sastry KJ, Barry MA. Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines. Viruses. 2009; 1(3):920-938. https://doi.org/10.3390/v1030920
Chicago/Turabian StyleWeaver, Eric A., Pramod N. Nehete, Bharti P. Nehete, Stephanie J. Buchl, Donna Palmer, David C. Montefiori, Philip Ng, K. Jagannadha Sastry, and Michael A. Barry. 2009. "Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines" Viruses 1, no. 3: 920-938. https://doi.org/10.3390/v1030920




