Myelin Oligodendrocyte Glycoprotein-Independent Rubella Infection of Keratinocytes and Resistance of First-Trimester Trophoblast Cells to Rubella Virus In Vitro
Abstract
:1. Introduction
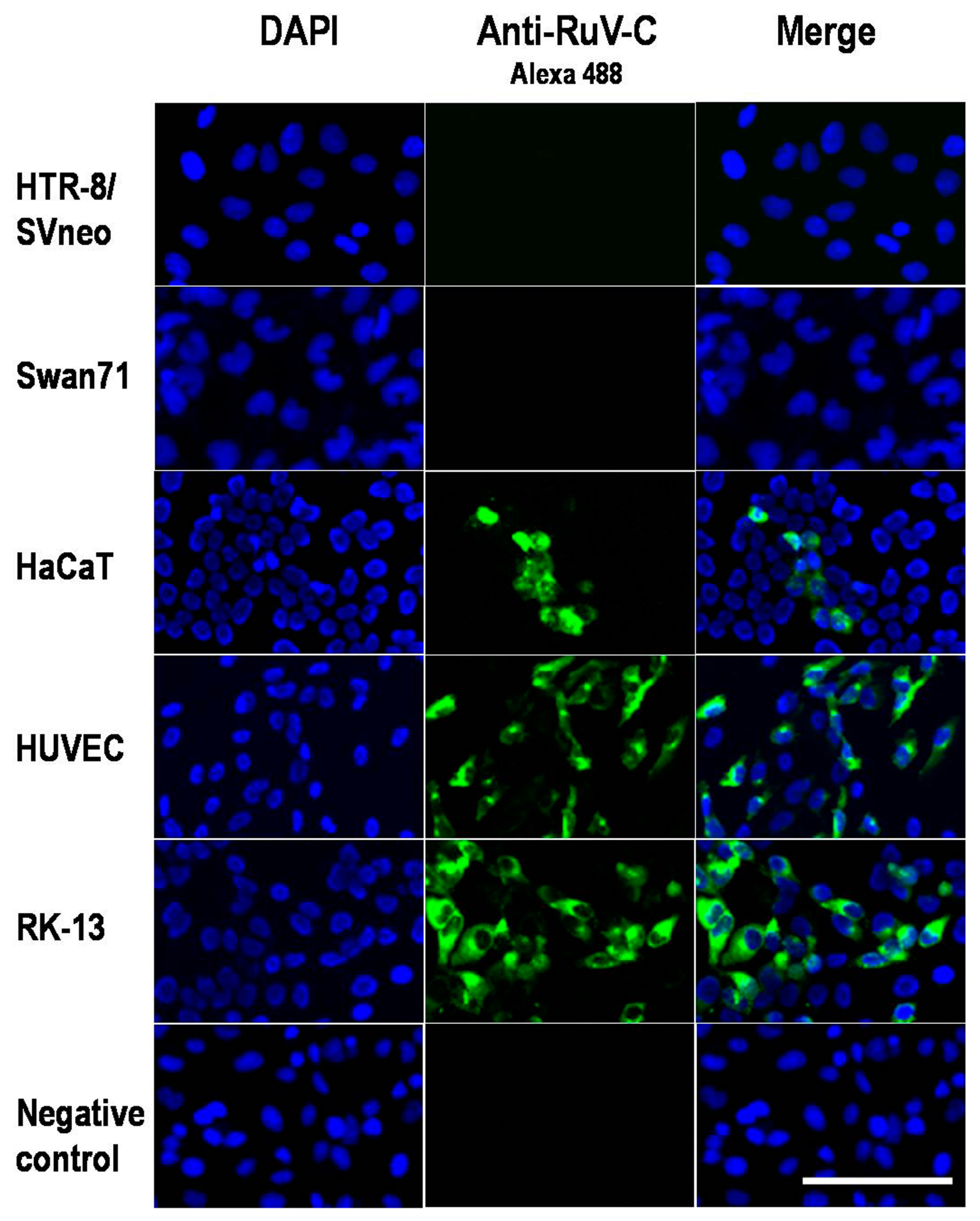
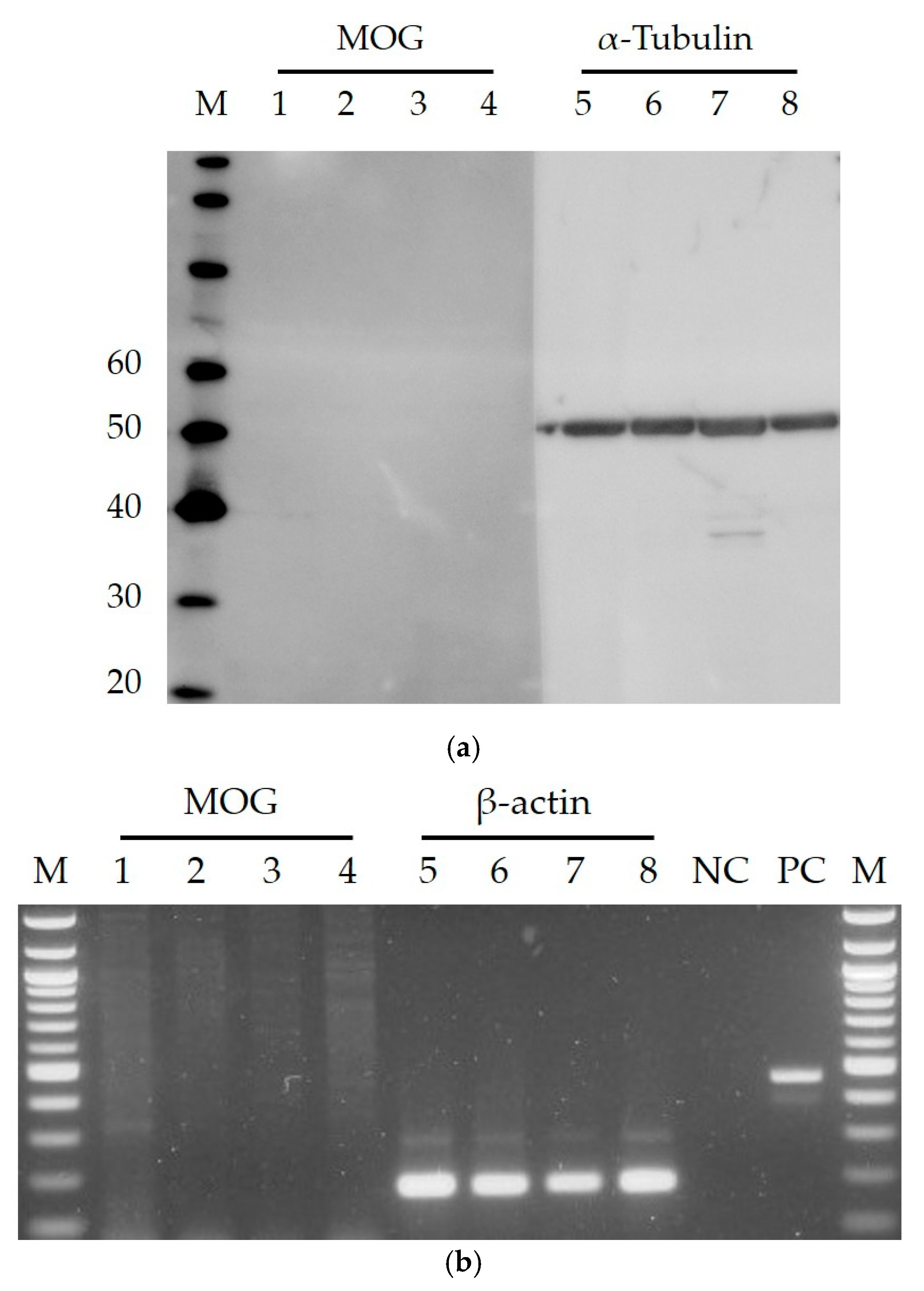
2. Results and Discussion
3. Materials and Methods
3.1. Cells and Viruses
3.1.1. Cells and Cell Cultures
3.1.2. Virus and Virus Infection
3.2. Immunofluorescence Assay
3.3. Western Blotting
3.4. Reverse Transcriptase PCR
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cong, H.; Jiang, Y.; Tien, P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J. Virol. 2011, 85, 11038–11047. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Lambert, N.D.; Ovsyannikova, I.G.; Kennedy, R.B.; Larrabee, B.R.; Pankratz, V.S.; Poland, G.A. Associations between single nucleotide polymorphisms in cellular viral receptors and attachment factor-related genes and humoral immunity to rubella vaccination. PLoS ONE 2014, 9, e99997. [Google Scholar] [CrossRef] [PubMed]
- Perelygina, L.; Zheng, Q.; Metcalfe, M.; Icenogle, J. Persistent infection of human fetal endothelial cells with rubella virus. PLoS ONE 2013, 8, e73014. [Google Scholar] [CrossRef] [PubMed]
- Heggie, A.D. Pathogenesis of the rubella exanthem. Isolation of rubella virus from the skin. N. Engl. J. Med. 1971, 285, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.C.; Trompeter, R.S.; Risdon, R.A. Letter: Chronic rashes in congenital rubella: Isolation of virus from skin. Lancet 1975, 1, 1349. [Google Scholar] [CrossRef]
- Perelygina, L.; Plotkin, S.; Russo, P.; Hautala, T.; Bonilla, F.; Ochs, H.D.; Joshi, A.; Routes, J.; Patel, K.; Wehr, C.; et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J. Allergy Clin. Immunol. 2016, 138, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, N.; Sakata, M.; Saito, K.; Okamoto, K.; Mori, Y.; Hanada, K.; Takeda, M. Both sphingomyelin and cholesterol in the host cell membrane are essential for Rubella virus entry. J. Virol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Alvero, A.B.; Aldo, P.B.; Ma, Y.; Guller, S.; Romero, R.; Mor, G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan71. Placenta 2009, 30, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, Q.D.; Pham, N.T.K.; Takada, K.; Komine-Aizawa, S.; Hayakawa, S. Myelin Oligodendrocyte Glycoprotein-Independent Rubella Infection of Keratinocytes and Resistance of First-Trimester Trophoblast Cells to Rubella Virus In Vitro. Viruses 2018, 10, 23. https://doi.org/10.3390/v10010023
Trinh QD, Pham NTK, Takada K, Komine-Aizawa S, Hayakawa S. Myelin Oligodendrocyte Glycoprotein-Independent Rubella Infection of Keratinocytes and Resistance of First-Trimester Trophoblast Cells to Rubella Virus In Vitro. Viruses. 2018; 10(1):23. https://doi.org/10.3390/v10010023
Chicago/Turabian StyleTrinh, Quang Duy, Ngan Thi Kim Pham, Kazuhide Takada, Shihoko Komine-Aizawa, and Satoshi Hayakawa. 2018. "Myelin Oligodendrocyte Glycoprotein-Independent Rubella Infection of Keratinocytes and Resistance of First-Trimester Trophoblast Cells to Rubella Virus In Vitro" Viruses 10, no. 1: 23. https://doi.org/10.3390/v10010023




