Viral and Antibody Kinetics, and Mosquito Infectivity of an Imported Case of Zika Fever Due to Asian Genotype (American Strain) in Singapore
Abstract
:1. Introduction
2. The Study
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; WHO Zika Causality Working Group. Zika virus infection as a cause of congenital brain abnormalities and guillain-barre syndrome: Systematic review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; de Oliveira, W.K.; Pamplona de Góes Cavalcanti, L. Zika virus outbreak in Brazil. J. Infect. Dev. Ctries. 2016, 10, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.G.; Ksiazek, T.G.; Suhandiman; Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef]
- Ikejezie, J.; Shapiro, C.N.; Kim, J.; Chiu, M.; Almiron, M.; Ugarte, C.; Espinal, M.A.; Aldighieri, S. Zika virus transmission—Region of the Americas, May 15, 2015–December 15, 2016. Morb. Mortal. Wkly. Rep. 2017, 66, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of zika virus infection. J. Infect. Dis. 2017, 216, S868–S874. [Google Scholar] [CrossRef] [PubMed]
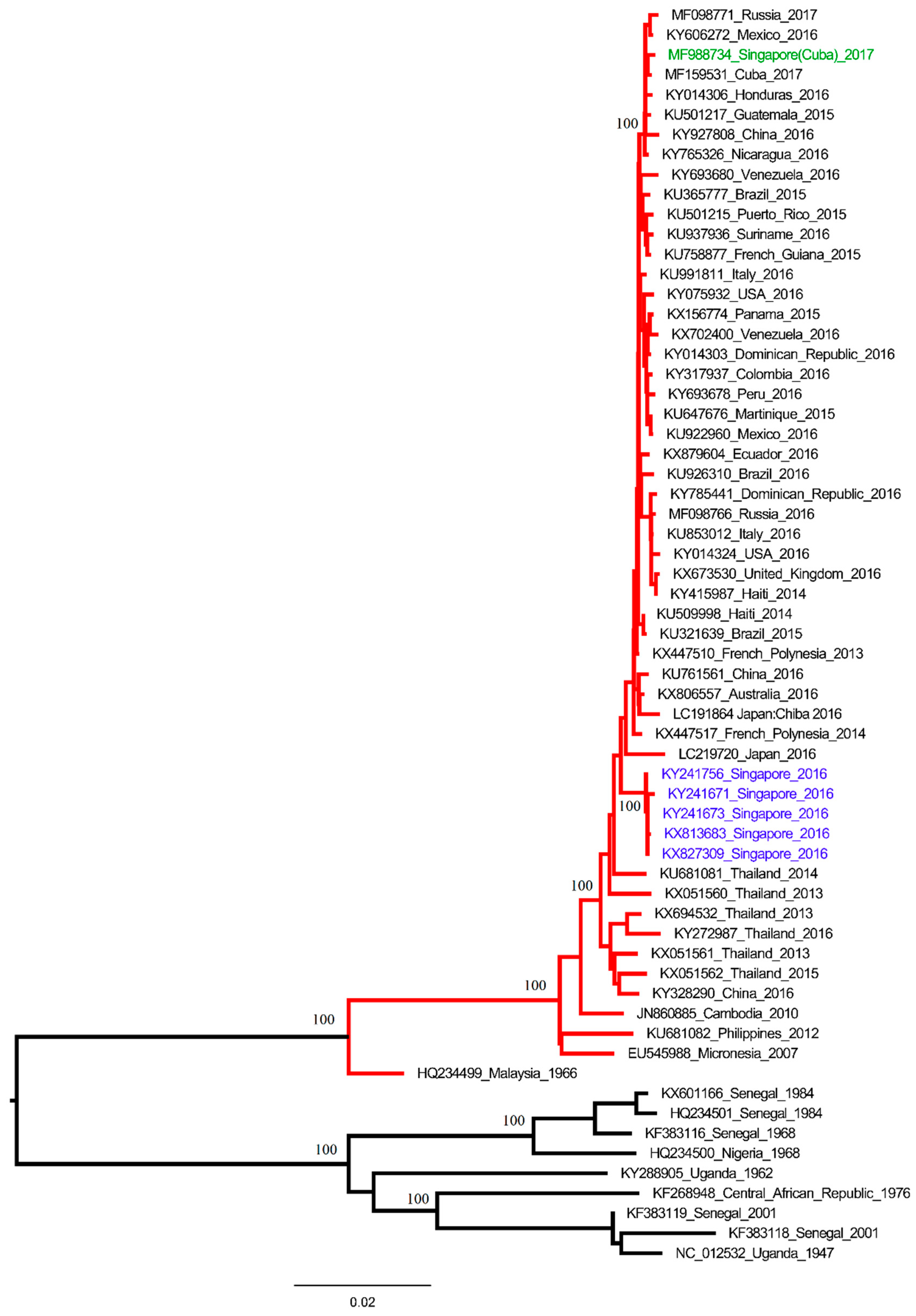
- Singapore Zika Study Group. Outbreak of zika virus infection in Singapore: An epidemiological, entomological, virological, and clinical analysis. Lancet Infect. Dis. 2017, 17, 813–821. [Google Scholar]
- Ministry of Health. Weekly Infectious Disease Bulletin. Available online: https://www.moh.gov.sg/content/dam/moh_web/Statistics/Infectious_Diseases_Bulletin/2017/December/2017_week_52.pdf. (assessed on 5 January 2018).
- Gardner, L.; Chen, N.; Sarkar, S. Vector status of Aedes species determines geographical risk of autochthonous zika virus establishment. PLoS Negl. Trop. Dis. 2017, 11, e0005487. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Reagan-Steiner, S.; Goodenough, D.; Russell, K.; Tanner, M.; Lewis, L.; Petersen, E.E.; Powers, A.M.; Kniss, K.; Meaney-Delman, D.; et al. Patterns in zika virus testing and infection, by report of symptoms and pregnancy status—United States, January 3–March 5, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Yap, G.; Sil, B.K.; Ng, L.C. Use of saliva for early dengue diagnosis. PLoS Negl. Trop. Dis. 2011, 5, e1046. [Google Scholar] [CrossRef] [PubMed]
- Low, S.L.; Lam, S.; Wong, W.Y.; Teo, D.; Ng, L.C.; Tan, L.K. Dengue seroprevalence of healthy adults in singapore: Serosurvey among blood donors, 2009. Am. J. Trop. Med. Hyg. 2015, 93, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, P.S.; Li, M.Z.; Yang, H.T.; Chong, C.S.; Lee, L.K.; Yuan, S.; Leo, Y.S.; Ng, L.C.; Lye, D.C. Membrane feeding of dengue patient’s blood as a substitute for direct skin feeding in studying aedes-dengue virus interaction. Parasites Vectors 2016, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Pompon, J.; Morales-Vargas, R.; Manuel, M.; Huat Tan, C.; Vial, T.; Hao Tan, J.; Sessions, O.M.; Vasconcelos, P.D.C.; Ng, L.C.; Missé, D. A zika virus from america is more efficiently transmitted than an asian virus by Aedes aegypti mosquitoes from Asia. Sci. Rep. 2017, 7, 1215. [Google Scholar] [CrossRef] [PubMed]
- Li, M.I.; Wong, P.S.; Ng, L.C.; Tan, C.H. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to zika virus. PLoS Negl. Trop. Dis. 2012, 6, e1792. [Google Scholar] [CrossRef] [PubMed]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Huang, J.H.; Leal, G.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.; Kitron, U.; et al. Variation in Aedes aegypti mosquito competence for zika virus transmission. Emerg. Infect. Dis. 2017, 23, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Bialosuknia, S.M.; Ehrbar, D.J.; Kramer, L.D. Vertical transmission of zika virus by Aedes aegypti and Ae. Albopictus mosquitoes. Emerg. Infect. Dis. 2017, 23, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Nguyen, D.; Debs, L.H.; Patel, A.P.; Liu, G.; Jhaveri, V.M.; S Kay, S.I.; Mittal, J.; Bandstra, E.S.; Younis, R.T.; et al. Zika virus: An emerging global health threat. Front. Cell. Infect. Microbiol. 2017, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; Turner, J.; Metelmann, S.; Hesson, J.C.; Blagrove, M.S.; Solomon, T.; Morse, A.P.; Baylis, M. Global risk model for vector-borne transmission of zika virus reveals the role of el niño 2015. Proc. Natl. Acad. Sci. USA 2017, 114, 119–124. [Google Scholar] [CrossRef] [PubMed]
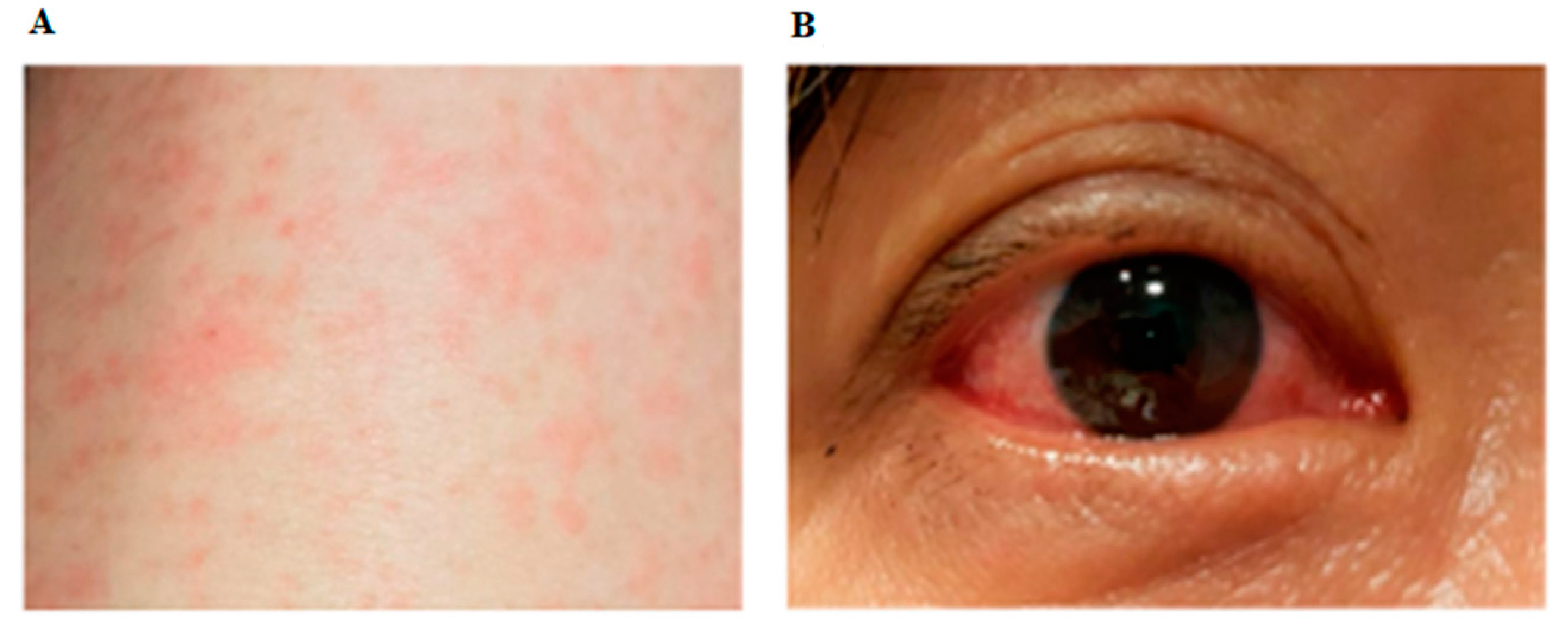
 represents the presence of symptoms and signs on any particular day. For Panel B laboratory tests, each circle represents a sample that was tested.
represents the presence of symptoms and signs on any particular day. For Panel B laboratory tests, each circle represents a sample that was tested.  represents negative, while
represents negative, while  represents positive results. Saliva was collected as previously described [14]. IgM and IgG serology was performed with Zika SD Biosensor rapid kit (SD Biosensor, Gyeonggi-do, Republic of Korea) according to the manufacturer’s instructions. RT-quantitative PCR (qPCR) was performed as previously reported [1]. RNA was extracted from sera, urine and saliva using QIAamp© Viral RNA Mini Kit (Qiagen, Hilden, Germany), and from whole blood using High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ instructions. Zika Virus (ZIKV) PRNT was performed as previously described [15], except that ZIKV was used instead of Dengue virus. PRNT: Plaque Reduction Neutralization Test; PCR: Polymerase Chain Reaction.
represents positive results. Saliva was collected as previously described [14]. IgM and IgG serology was performed with Zika SD Biosensor rapid kit (SD Biosensor, Gyeonggi-do, Republic of Korea) according to the manufacturer’s instructions. RT-quantitative PCR (qPCR) was performed as previously reported [1]. RNA was extracted from sera, urine and saliva using QIAamp© Viral RNA Mini Kit (Qiagen, Hilden, Germany), and from whole blood using High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ instructions. Zika Virus (ZIKV) PRNT was performed as previously described [15], except that ZIKV was used instead of Dengue virus. PRNT: Plaque Reduction Neutralization Test; PCR: Polymerase Chain Reaction.
 represents the presence of symptoms and signs on any particular day. For Panel B laboratory tests, each circle represents a sample that was tested.
represents the presence of symptoms and signs on any particular day. For Panel B laboratory tests, each circle represents a sample that was tested.  represents negative, while
represents negative, while  represents positive results. Saliva was collected as previously described [14]. IgM and IgG serology was performed with Zika SD Biosensor rapid kit (SD Biosensor, Gyeonggi-do, Republic of Korea) according to the manufacturer’s instructions. RT-quantitative PCR (qPCR) was performed as previously reported [1]. RNA was extracted from sera, urine and saliva using QIAamp© Viral RNA Mini Kit (Qiagen, Hilden, Germany), and from whole blood using High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ instructions. Zika Virus (ZIKV) PRNT was performed as previously described [15], except that ZIKV was used instead of Dengue virus. PRNT: Plaque Reduction Neutralization Test; PCR: Polymerase Chain Reaction.
represents positive results. Saliva was collected as previously described [14]. IgM and IgG serology was performed with Zika SD Biosensor rapid kit (SD Biosensor, Gyeonggi-do, Republic of Korea) according to the manufacturer’s instructions. RT-quantitative PCR (qPCR) was performed as previously reported [1]. RNA was extracted from sera, urine and saliva using QIAamp© Viral RNA Mini Kit (Qiagen, Hilden, Germany), and from whole blood using High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ instructions. Zika Virus (ZIKV) PRNT was performed as previously described [15], except that ZIKV was used instead of Dengue virus. PRNT: Plaque Reduction Neutralization Test; PCR: Polymerase Chain Reaction.
| Days Post Infection | Fever Days (Zika virus log10RNA Copy/mL)/Infection Rate (n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 (7.49) | Day 2 (6.03) | Day 3 (5.02) | Day 4 (Undetectable) | |||||||||
| MG | SG | Head | MG | SG | Head | MG | SG | Head | MG | SG | Head | |
| 5 | 10% * (10) | 0 (10) | 10% (10) | 0 (10) | 0 (10) | 20% (10) | ND | ND | ||||
| 7 | 0 (10) | 0 (10) | 10% (10) | 0 (10) | 0 (10) | 0 (10) | 0 (10) | 0 (10) | 10% (10) | 0 (10) | 0 (10) | 10% (10) |
| 14 | 0 (10) | 0 (10) | 10% (10) | 0 (10) | 0 (10) | 20% (10) | 0 (10) | 0 (10) | 10% (10) | 0 (10) | 0 (10) | 0 (10) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.H.; Tan, L.K.; Hapuarachchi, H.C.; Lai, Y.L.; Wong, P.S.J.; Yap, G.; Mak, K.W.; Wong, W.Y.; Leo, Y.S.; Wong, M.C.; et al. Viral and Antibody Kinetics, and Mosquito Infectivity of an Imported Case of Zika Fever Due to Asian Genotype (American Strain) in Singapore. Viruses 2018, 10, 44. https://doi.org/10.3390/v10010044
Tan CH, Tan LK, Hapuarachchi HC, Lai YL, Wong PSJ, Yap G, Mak KW, Wong WY, Leo YS, Wong MC, et al. Viral and Antibody Kinetics, and Mosquito Infectivity of an Imported Case of Zika Fever Due to Asian Genotype (American Strain) in Singapore. Viruses. 2018; 10(1):44. https://doi.org/10.3390/v10010044
Chicago/Turabian StyleTan, Cheong Huat, Li Kiang Tan, Hapuarachchige Chanditha Hapuarachchi, Yee Ling Lai, Pei Sze Jeslyn Wong, Grace Yap, Keng Wai Mak, Wing Yan Wong, Yee Sin Leo, Mei Chun Wong, and et al. 2018. "Viral and Antibody Kinetics, and Mosquito Infectivity of an Imported Case of Zika Fever Due to Asian Genotype (American Strain) in Singapore" Viruses 10, no. 1: 44. https://doi.org/10.3390/v10010044





