Study of the Interactions Between Bacteriophage phiIPLA-RODI and Four Chemical Disinfectants for the Elimination of Staphylococcus aureus Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Phages Used and Growth Conditions
2.2. Phage Susceptibility to Different Disinfectants
2.3. Minimal Inhibitory Concentration (MIC) Determination and Checkerboard Assays
2.4. BIMs
2.5. Phage Adsorption Rate
2.6. Biofilm Formation and Treatment Assays
2.7. Statistical Analysis
3. Results
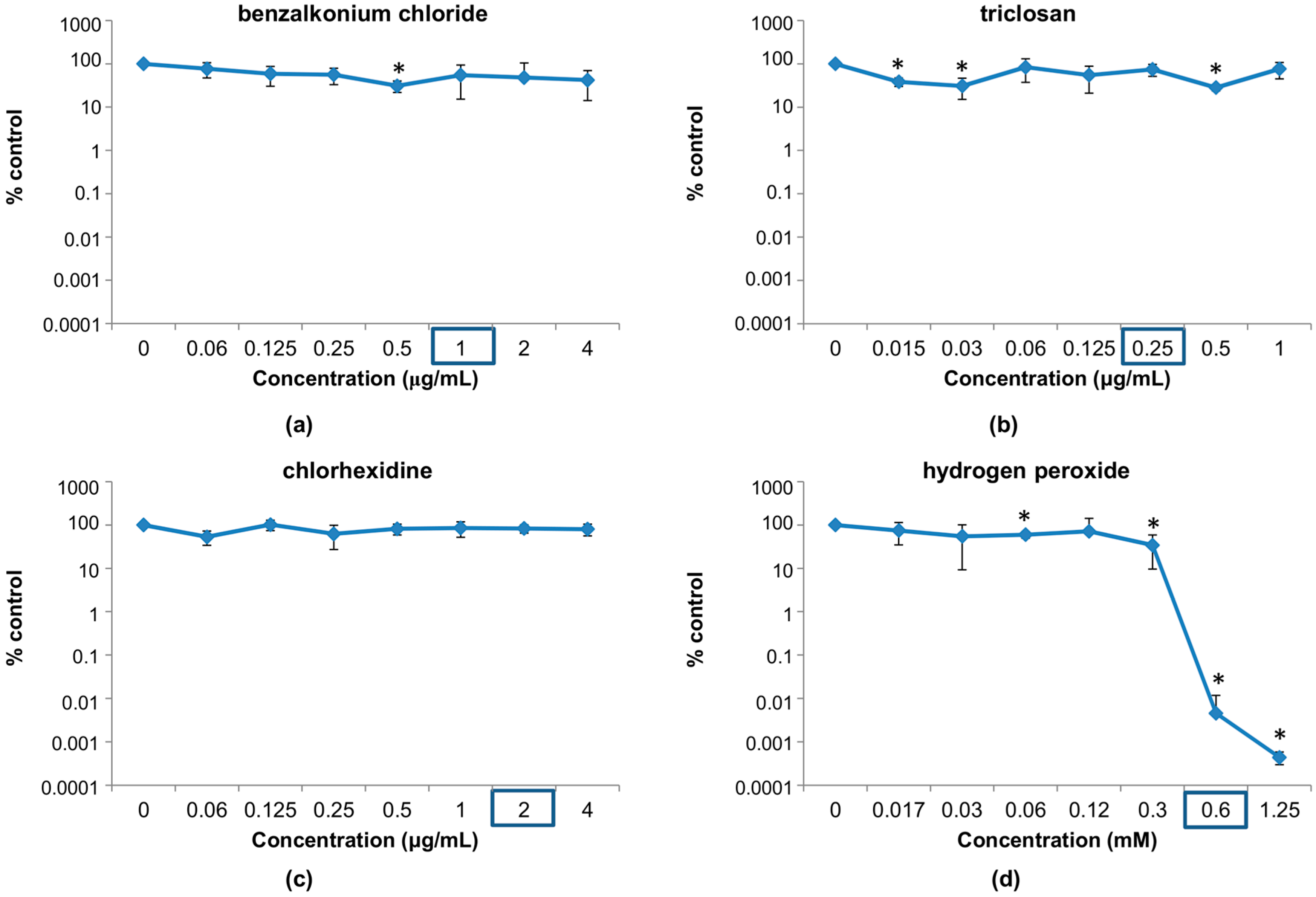
3.1. Susceptibility of Phage phiIPLA-RODI to Different Disinfectants
3.1.1. Susceptibility at Concentrations Used in Household and Industrial Products
3.1.2. Susceptibility at Disinfectant Concentrations Near the MIC of the Host Bacterium
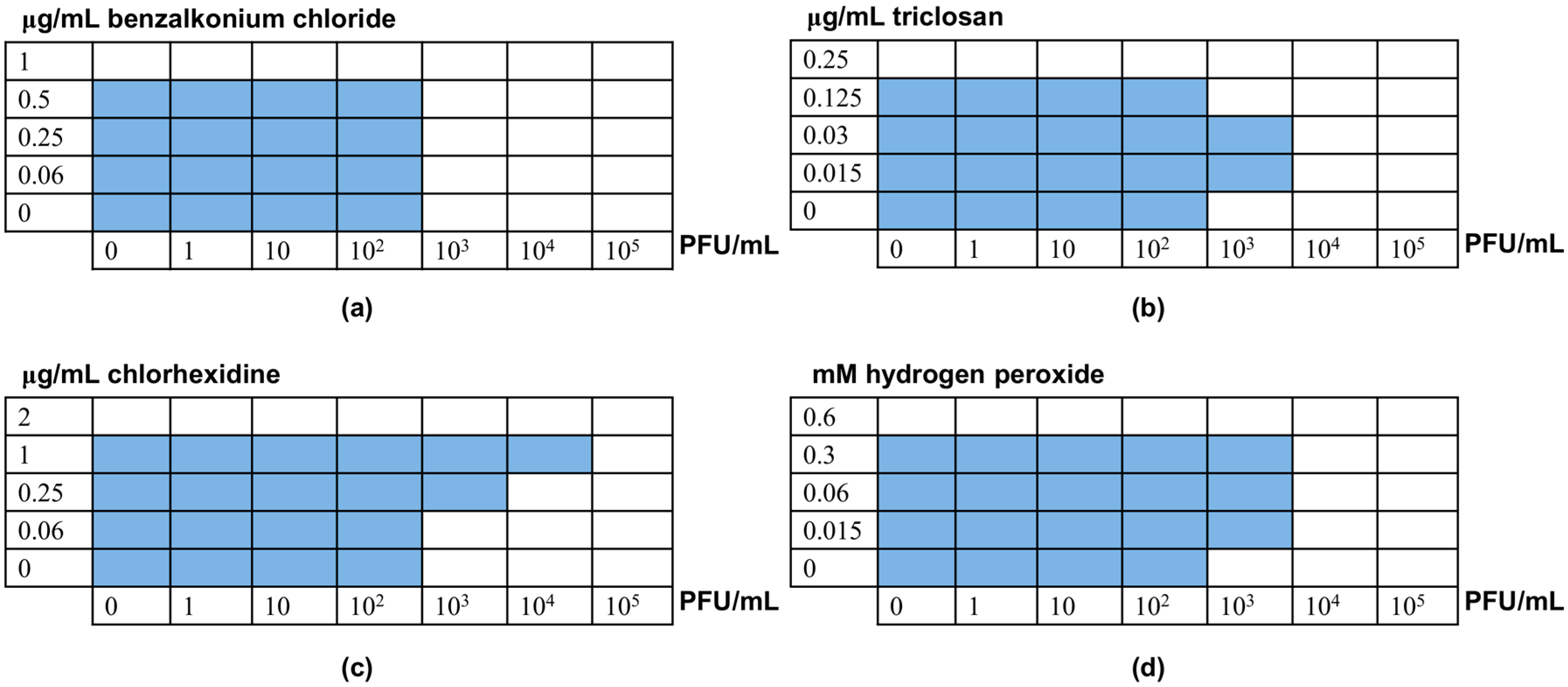
3.2. Interactions between Phage phiIPLA-RODI and Disinfectants
3.3. Elimination of S. aureus Biofilms by Combinations between Phage phiIPLA-RODI and Disinfectants
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. CR Acad. Sci. Paris 1917, 165, 373–375. [Google Scholar]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ramon, E.; Hoyo, J.; Tzanov, T. Innovative approaches for controlling clinically relevant biofilms: Current trends and future prospects. Curr. Top. Med. Chem. 2017, 17, 1889–1914. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Escobedo, S.; Gutiérrez, D.; Portilla, S.; Martínez, B.; García, P.; Rodríguez, A. Bacteriophages in the dairy environment: From enemies to allies. Antibiotics 2017, 6, E27. [Google Scholar] [CrossRef] [PubMed]
- Campagna, C.; Villion, M.; Labrie, S.J.; Duchaine, C.; Moineau, S. Inactivation of dairy bacteriophages by commercial sanitizers and disinfectants. Int. J. Food Microbiol. 2014, 171, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Murphy, J.; Mahony, J.; Lugli, G.A.; Ventura, M.; Noben, J.P.; Franz, C.M.; Neve, H.; Nauta, A.; van Sinderen, D. Biocidal inactivation of Lactococcus lactis bacteriophages: Efficacy and targets of commonly used sanitizers. Front. Microbiol. 2017, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2017, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Allué-Guardia, A.; Martínez-Castillo, A.; Muniesa, M. Persistence of infectious Shiga toxin-encoding bacteriophages after disinfection treatments. Appl. Environ. Microbiol. 2014, 80, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chaitiemwong, N.; Hazeleger, W.C.; Beumer, R.R. Inactivation of Listeria monocytogenes by disinfectants and bacteriophages in suspension and stainless steel carrier tests. J. Food Prot. 2014, 77, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Vandenheuvel, D.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Two Phages, phiIPLA-RODI and phiIPLA-C1C, lyse mono- and dual-species staphylococcal biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Fernández, L.; Campelo, A.B.; Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. The behavior of Staphylococcus aureus dual-species biofilms treated with bacteriophage phiIPLA-RODI depends on the accompanying microorganism. Appl. Environ. Microbiol. 2017, 83, e02821-16. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 7th ed.; Approved Standard M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Approved Standard M100-S17; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Dougherty, P.F.; Yotter, D.W.; Matthews, T.R. Microdilution transfer plate technique for determining in vitro synergy of antimicrobial agents. Antimicrob. Agents Chemother. 1977, 11, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B.; Singer, S.; Hitchings, G.H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J. Biol. Chem. 1954, 208, 477–488. [Google Scholar] [PubMed]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef] [PubMed]

| Disinfectant | Chemical Class | Mode of Action |
|---|---|---|
Benzalkonium chloride | Quaternarium ammonium compound (QAC) | Membrane damage |
Chlorhexidine | Biguanide | Membrane damage |
Hydrogen peroxide | Oxidizing agent | Oxidative damage |
Triclosan | Phenolic compound | Membrane disruption, FabI inhibition |
| Disinfectant | Concentration | % Survival Compared to Untreated Control |
|---|---|---|
| Benzalkonium chloride | 0.002% (20 μg/mL) | <0.02 |
| 0.02% (200 μg/mL) | <0.02 | |
| 5% (50 mg/mL) | <0.02 | |
| Chlorhexidine | 0.02% (200 μg/mL) | 3.67 ± 2.07 |
| 0.2% (2 mg/mL) | <0.02 | |
| Hydrogen peroxide | 0.3% (80 mM) | <0.02 |
| 3% (0.8 M) | <0.02 | |
| Triclosan | 0.003% (30 μg/mL) | 22.00 ± 7.21 |
| 0.03% (300 μg/mL) | 2.63 ± 0.94 |
| Chlorhexidine Concentration | BIM Frequency | Phage Adsorption Rate |
|---|---|---|
| 0 μg/mL | 3.79 × 10−7 | 87.22 ± 3.16 |
| 0.25 μg/mL | 3.55 × 10−7 | 84.08 ± 14.17 |
| 0.5 μg/mL | 3.09 × 10−7 | 89.00 ± 5.36 |
| 1 μg/mL | 2.34 × 10−7 | Not determined |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agún, S.; Fernández, L.; González-Menéndez, E.; Martínez, B.; Rodríguez, A.; García, P. Study of the Interactions Between Bacteriophage phiIPLA-RODI and Four Chemical Disinfectants for the Elimination of Staphylococcus aureus Contamination. Viruses 2018, 10, 103. https://doi.org/10.3390/v10030103
Agún S, Fernández L, González-Menéndez E, Martínez B, Rodríguez A, García P. Study of the Interactions Between Bacteriophage phiIPLA-RODI and Four Chemical Disinfectants for the Elimination of Staphylococcus aureus Contamination. Viruses. 2018; 10(3):103. https://doi.org/10.3390/v10030103
Chicago/Turabian StyleAgún, Seila, Lucía Fernández, Eva González-Menéndez, Beatriz Martínez, Ana Rodríguez, and Pilar García. 2018. "Study of the Interactions Between Bacteriophage phiIPLA-RODI and Four Chemical Disinfectants for the Elimination of Staphylococcus aureus Contamination" Viruses 10, no. 3: 103. https://doi.org/10.3390/v10030103






