Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Cultures, Virus and Animals
2.3. Cytotoxic Assay
2.4. Antiviral Assay
2.5. Virus Titration
2.6. Time-of-Addition Assay
2.7. Viral Adsorption, Entry and Inactivation Assay
2.8. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.9. In-Cell Western Assay
2.10. Detection of mTOR-Dependent Autophagy by Western Blotting
2.11. In Vivo Assay of PGG Anti-RABV Effects
2.12. Statistical Analysis
3. Results
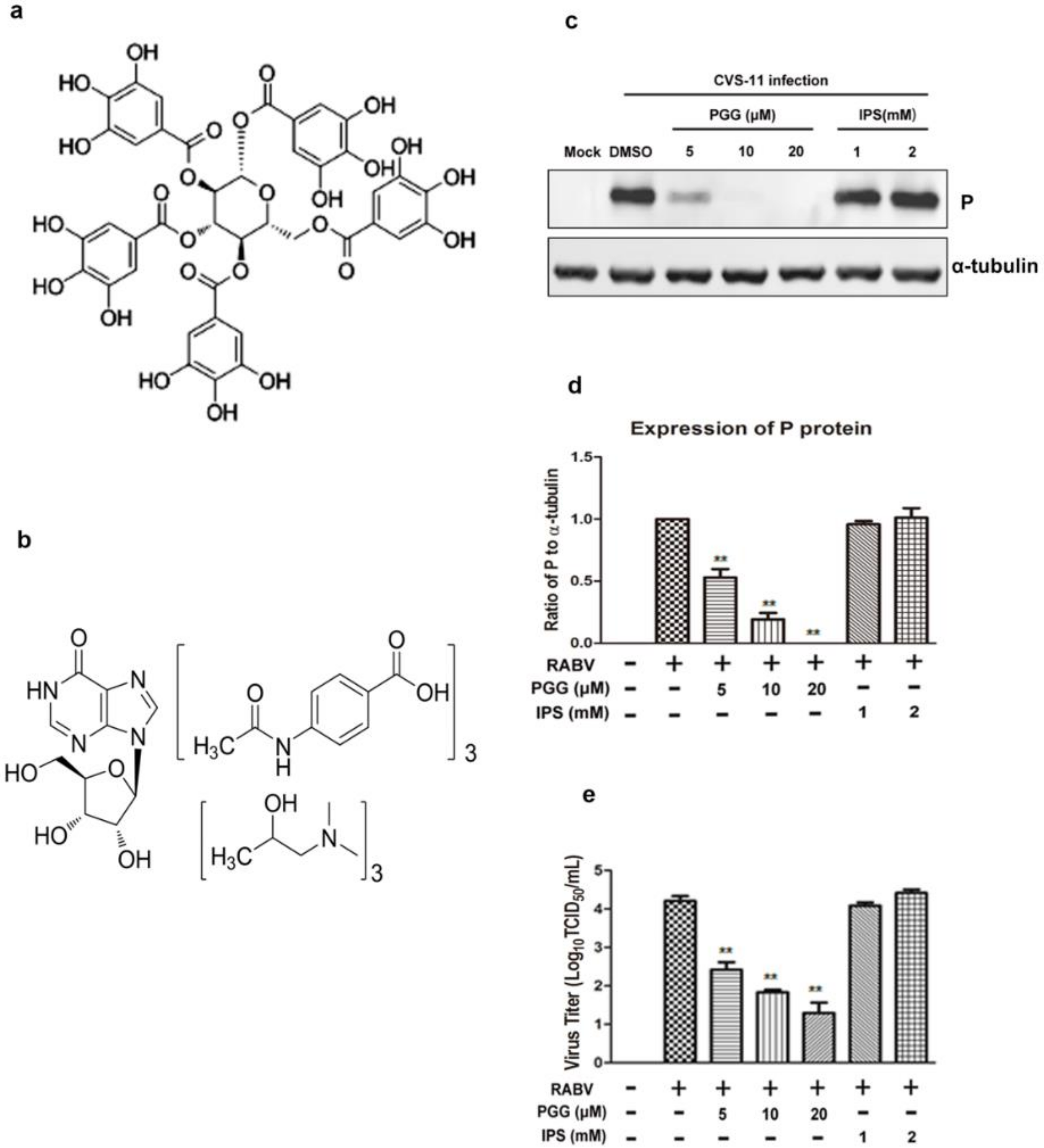
3.1. Cytotoxicity and Inactivation of RABV by PGG
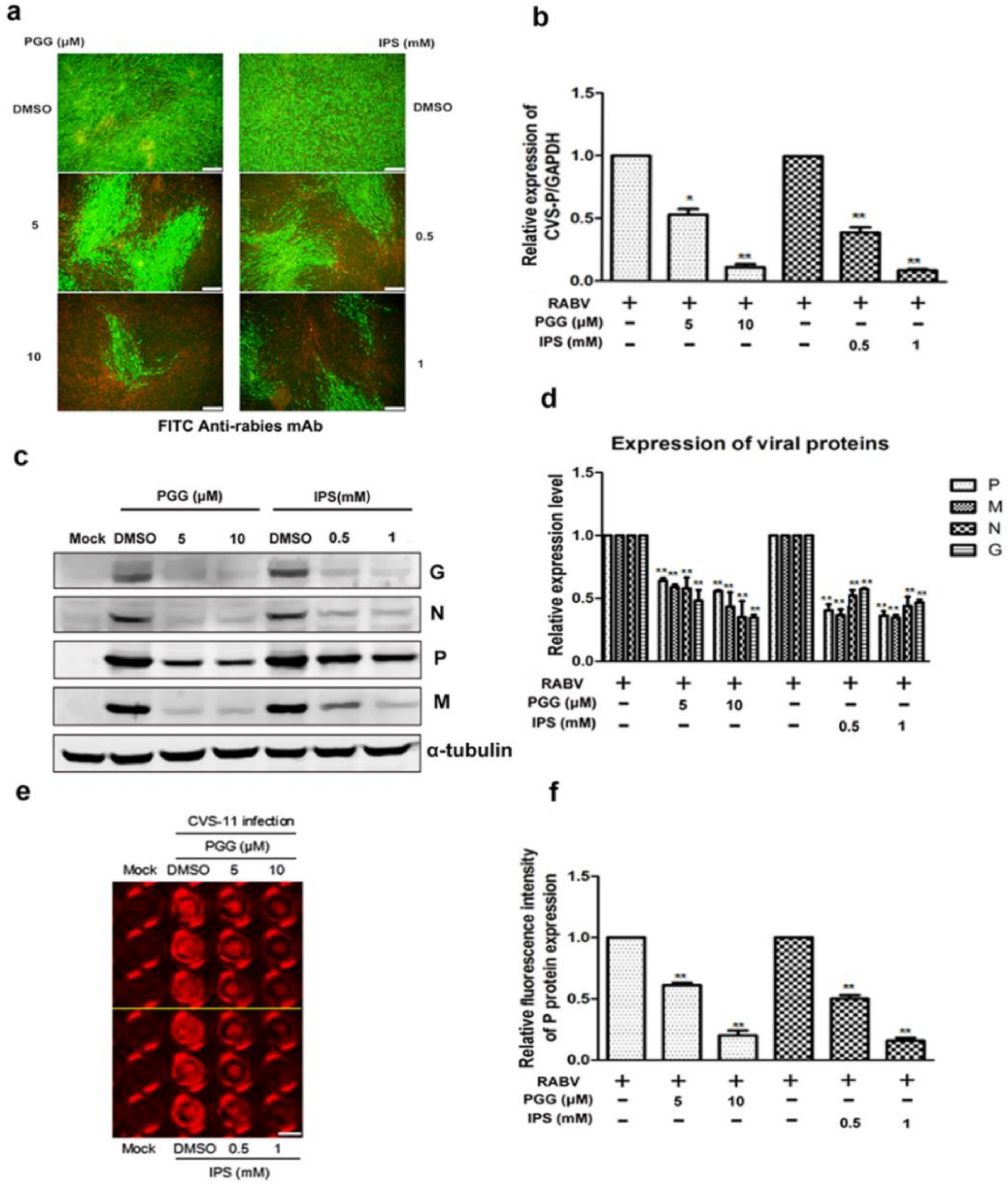
3.2. Antiviral Effects of PGG In Vitro
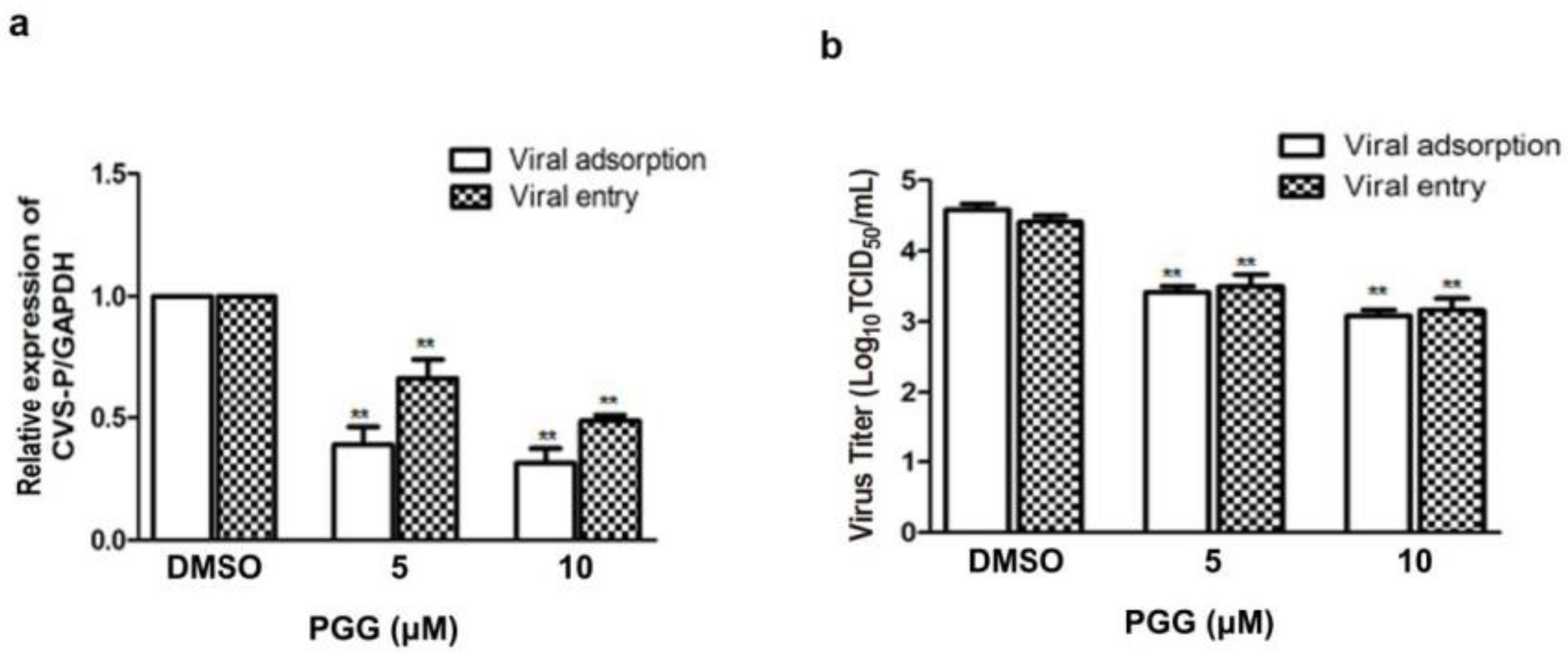
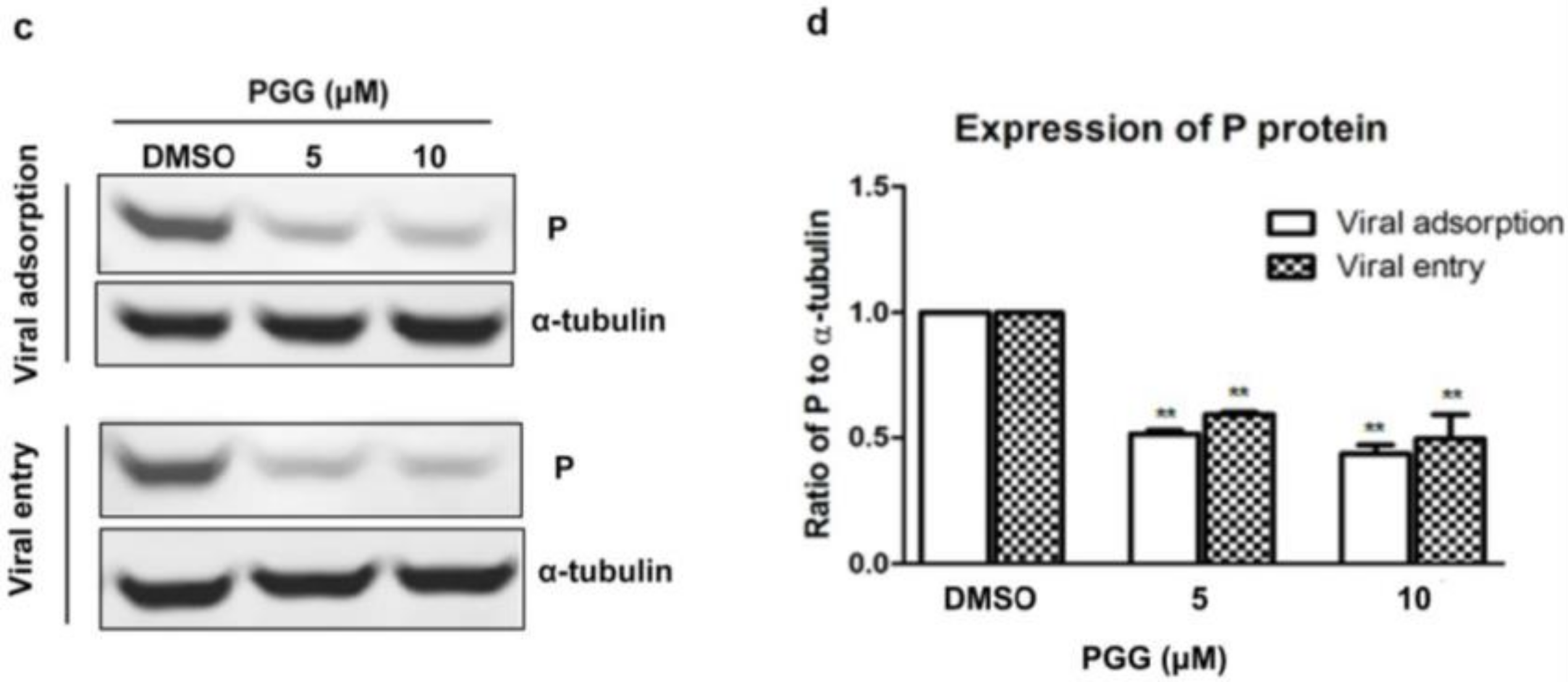
3.3. PGG Inhibits the Adsorption and Entry of RABV
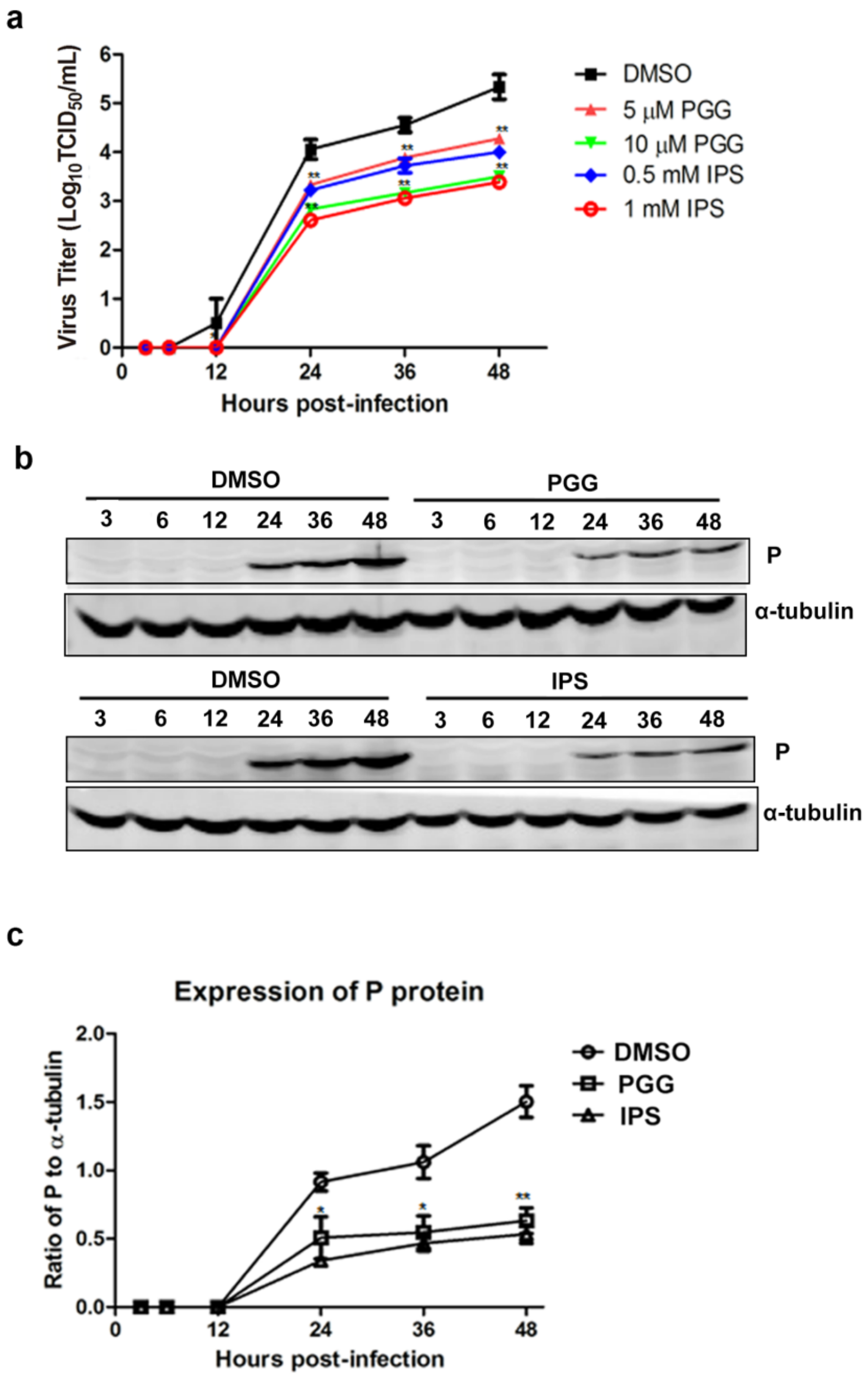
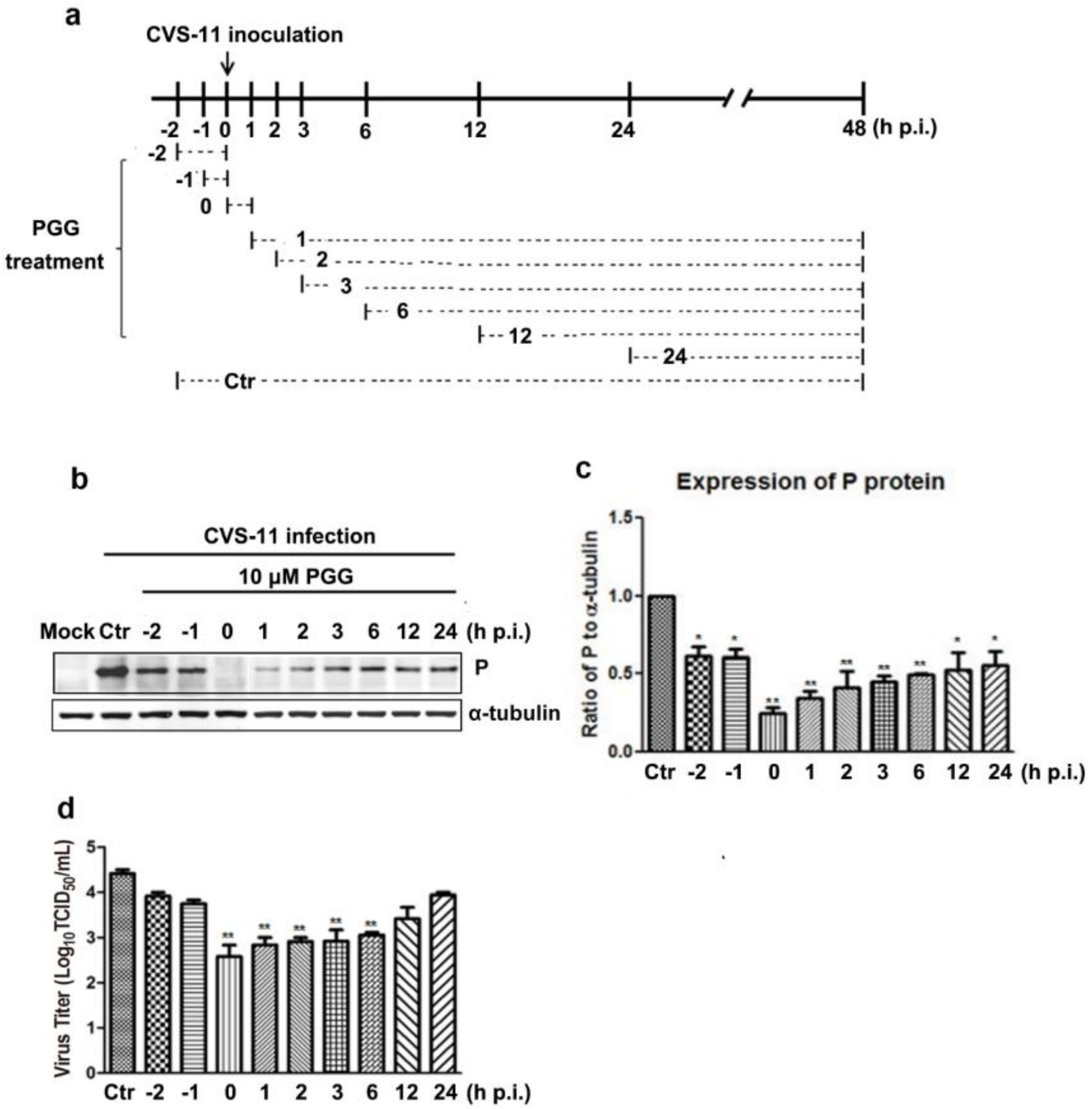
3.4. Time Course of PGG-Mediated Inhibition of RABV Replication
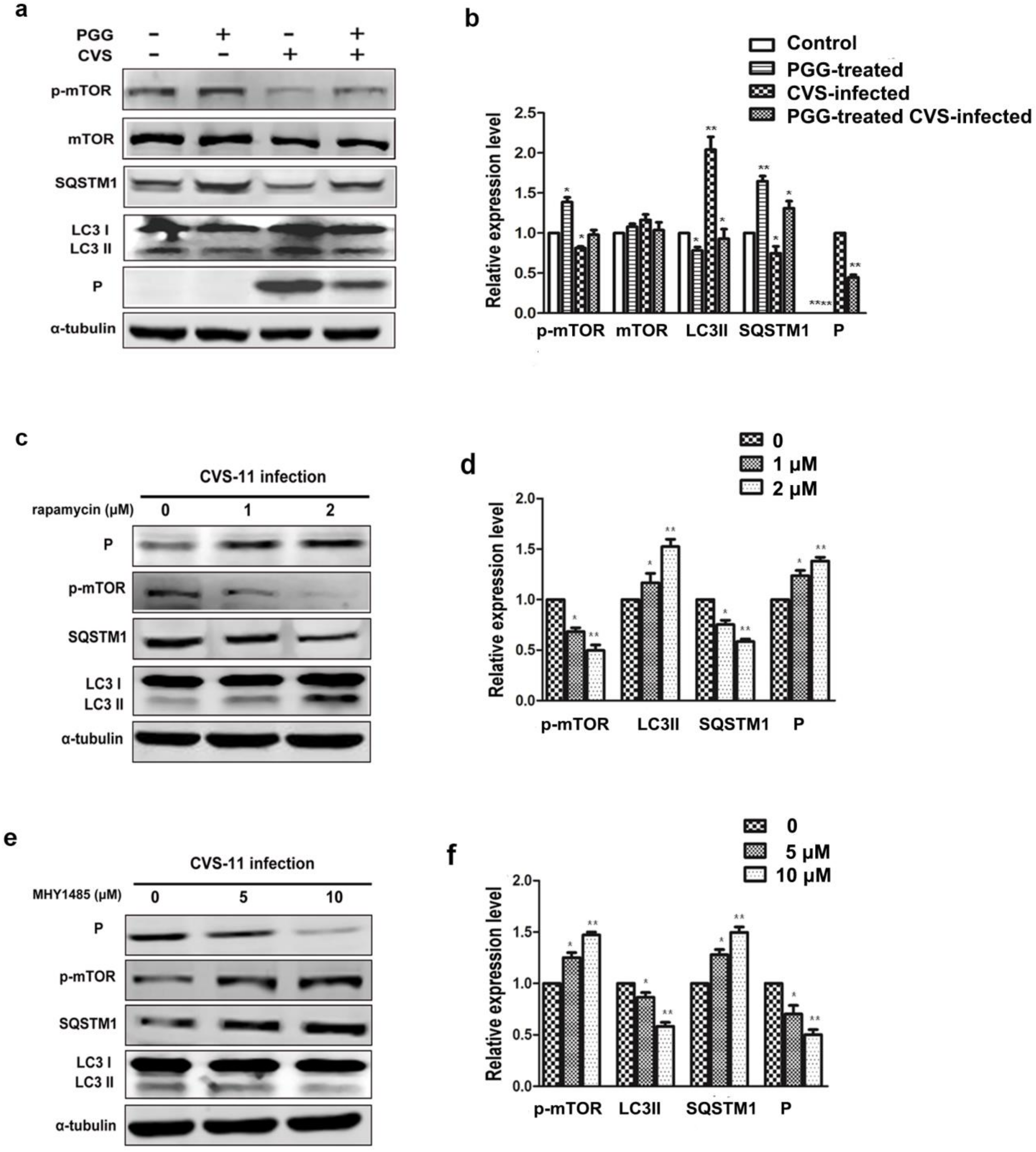
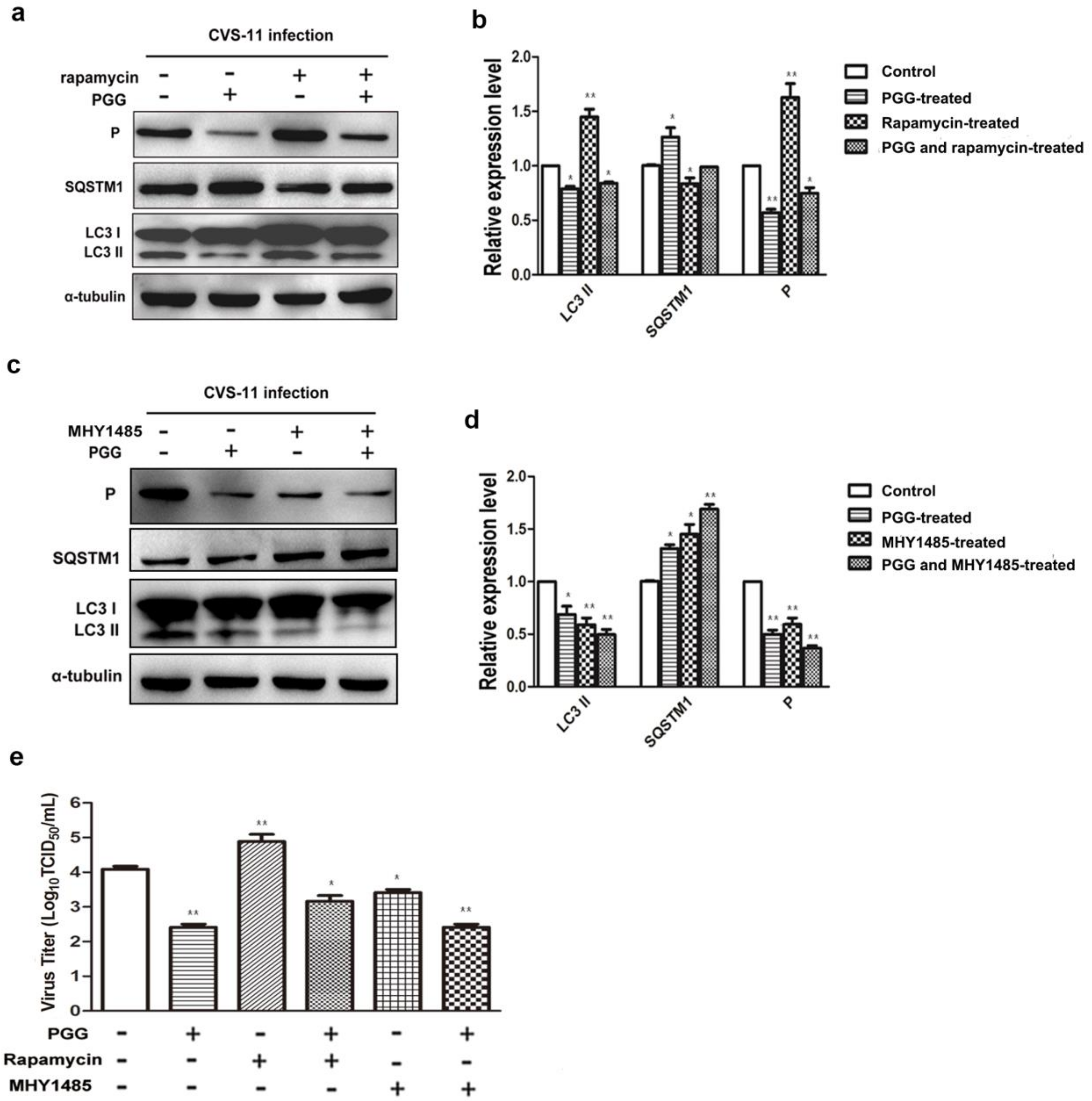
3.5. PGG Inhibits RABV Replication Via Suppressing mTOR-Dependent Autophagy
3.6. Antiviral Effects of PGG In Vivo
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, R.; Singh, K.P.; Cherian, S.; Saminathan, M.; Kapoor, S.; Manjunatha Reddy, G.B.; Panda, S.; Dhama, K. Rabies—Epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: A comprehensive review. Vet. Q. 2017, 37, 212–251. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, R.E., Jr.; Tieves, K.S.; Hoffman, G.M.; Ghanayem, N.S.; Amlie-Lefond, C.M.; Schwabe, M.J.; Chusid, M.J.; Rupprecht, C.E. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 2005, 352, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Wilde, H.; Hemachudha, T. The “Milwaukee protocol” for treatment of human rabies is no longer valid. Pediatr. Infect. Dis. J. 2015, 34, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Jackson, A.C. Critical Appraisal of the Milwaukee Protocol for Rabies: This Failed Approach Should Be Abandoned. Can. J. Neurol. Sci. 2016, 43, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Raux, H.; Flamand, A.; Blondel, D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 2000, 74, 10212–10216. [Google Scholar] [CrossRef] [PubMed]
- Wiltzer, L.; Okada, K.; Yamaoka, S.; Larrous, F.; Kuusisto, H.V.; Sugiyama, M.; Blondel, D.; Bourhy, H.; Jans, D.A.; Ito, N.; et al. Interaction of rabies virus P-protein with STAT proteins is critical to lethal rabies disease. J. Infect. Dis. 2014, 209, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Real, E.; Rain, J.C.; Battaglia, V.; Jallet, C.; Perrin, P.; Tordo, N.; Chrisment, P.; D’Alayer, J.; Legrain, P.; Jacob, Y. Antiviral drug discovery strategy using combinatorial libraries of structurally constrained peptides. J. Virol. 2004, 78, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yang, X.; Yamashita, S.; Kumazoe, M.; Huang, Y.; Nakahara, K.; Won, Y.S.; Murata, M.; Lin, I.C.; Tachibana, H. 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose increases a population of T regulatory cells and inhibits IgE production in ovalbumin-sensitized mice. Int. Immunopharmacol. 2015, 26, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Xiang, Y.F.; Chen, J.N.; Lu, C.H.; Hao, J.; Du, Q.; Lai, C.C.; Qu, C.; Li, S.; Ju, H.Q.; et al. Pentagalloylglucose downregulates cofilin1 and inhibits HSV-1 infection. Antiviral Res. 2011, 89, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Nagase, H.; Ose, Y.; Kito, H.; Sato, T.; Kawai, M.; Mizuno, M. Inhibitory action of peony root extract on the mutagenicity of benzo[a]pyrene. Mutat. Res. 1990, 244, 129–134. [Google Scholar] [CrossRef]
- Kang, D.G.; Moon, M.K.; Choi, D.H.; Lee, J.K.; Kwon, T.O.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG) via a nitric oxide-cGMP pathway. Eur. J. Pharmacol. 2005, 524, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Zhang, J.; Pugliese, A.; Kim, S.H.; Lu, J. Anti-cancer gallotannin penta-O-galloyl-β-d-glucose is a nanomolar inhibitor of select mammalian DNA polymerases. Biochem. Pharmacol. 2010, 80, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lu, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem. Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, H.K.; Jung, M.K.; Mar, W. In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose against hepatitis B virus. Biol. Pharm. Bull. 2006, 29, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, Z.P.; Ju, H.Q.; Komatsu, M.; Ji, Y.H.; Liu, G.; Guo, C.W.; Zhang, Y.J.; Yang, C.R.; Wang, Y.F.; et al. Autophagy is involved in anti-viral activity of pentagalloylglucose (PGG) against Herpes simplex virus type 1 infection in vitro. Biochem. Biophys. Res. Commun. 2011, 405, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Ma, K.; Chen, M.; Zou, M.; Wu, Y.; Li, F.; Wang, Y. Pentagalloylglucose Blocks the Nuclear Transport and the Process of Nucleocapsid Egress to Inhibit HSV-1 Infection. Jpn. J. Infect. Dis. 2016, 69, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; Kim, C.Y.; Lee, J.S.; Kim, T.G.; Kim, S.H.; Lee, C.K.; Lee, B.B.; Shin, C.G.; Huh, H.; Kim, J. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002, 68, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.J.; Yun, Y.J.; Lyu, M.A.; Woo, S.Y.; Woo, E.R.; Kim, S.J.; Lee, H.J.; Park, H.K.; Kook, Y.H. Respiratory syncytial virus infection induces matrix metalloproteinase-9 expression in epithelial cells. Arch. Virol. 2002, 147, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Li, Z.; Luo, H.; Zhang, W.; Chen, L.; Xu, X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiong, S.; Xiang, Y.F.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.J.; Wang, Y.F.; Kitazato, K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, M.; Guo, C.; Komatsu, M.; Xu, J.; Wang, Y.; Kitazato, K. Autophagy is involved in regulating influenza A virus RNA and protein synthesis associated with both modulation of Hsp90 induction and mTOR/p70S6K signaling pathway. Int. J. Biochem. Cell Biol. 2016, 72, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiang, Y.; Guo, C.; Pei, Y.; Wang, Y.; Kitazato, K. Cofilin-1 is involved in regulation of actin reorganization during influenza A virus assembly and budding. Biochem. Biophys. Res. Commun. 2014, 453, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Gu, J.; Deng, T.; Yuan, Z.; Hu, B.; Xu, Y.; Yan, Y.; Zan, J.; Liao, M.; et al. BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy 2017, 13, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhu, S.; Hu, L.; Ye, P.; Wang, Y.; Tian, Q.; Mei, M.; Chen, H.; Guo, X. Wild-type rabies virus induces autophagy in human and mouse neuroblastoma cell lines. Autophagy 2016, 12, 1704–1720. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Jauregui, P.; Gonzalez-Vega, D.; Cruz-Lavin, E.; Hernandez-Baumgarten, E. In vitro effect of isoprinosine on rabies virus. Am. J. Vet. Res. 1980, 41, 1475–1478. [Google Scholar] [PubMed]
- Glasgow, L.A.; Galasso, G.J. Isoprinosine: Lack of antiviral activity in experimental model infections. J. Infect. Dis. 1972, 126, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Hanh, T.T.H.; Thanh, N.V.; Thao, D.T.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Cytotoxic Steroids from the Vietnamese Soft Coral Sinularia leptoclados. Chem. Pharm. Bull. 2017, 65, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mukherjee, S.; Pawar, S.; Chowdhary, A. Evaluation of In vitro Antiviral Activity of Datura metel Linn. Against Rabies Virus. Pharmacognosy Res. 2016, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [PubMed]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Wang, X.; Wei, J.C.; Li, B.B.; Shao, D.H.; Li, Y.M.; Liu, K.; Shi, Y.Y.; Zhou, B.; Qiu, Y.F.; et al. Antiviral activity of doxycycline against vesicular stomatitis virus in vitro. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Q.; Tang, J.; Feng, W.H. Lipid rafts both in cellular membrane and viral envelope are critical for PRRSV efficient infection. Virology 2015, 484, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tian, D.; Zhou, M.; Xiao, W.; Zhang, Y.; Li, M.; Sui, B.; Wang, W.; Guan, H.; Chen, H.; et al. lambda-Carrageenan P32 Is a Potent Inhibitor of Rabies Virus Infection. PLoS ONE 2015, 10, e0140586. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C., Jr.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Sun, J.; Guo, H.; Tu, C. Genomic expression profiling of peripheral blood leukocytes of pigs infected with highly virulent classical swine fever virus strain Shimen. J. Gen. Virol. 2009, 90, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, Y.; Shi, Z.; Yan, Y.; Guo, H.; He, F.; Tu, C. Proteomic alteration of PK-15 cells after infection by classical swine fever virus. J. Proteome Res. 2008, 7, 5263–5269. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Jin, H.; Cao, Z.; Feng, N.; Zhao, Y.; Zheng, X.; Wang, J.; Li, Q.; Zhao, G.; et al. Interferon-inducible GTPase: A novel viral response protein involved in rabies virus infection. Arch. Virol. 2016, 161, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.H.; Leal, P.C.; Yunes, R.A.; Nunes, R.J.; Barardi, C.R.; Pinto, A.R.; Simoes, C.M.; Zanetti, C.R. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 2006, 116, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Hur, H.J.; Lee, H.J.; Lee, C.Y. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005, 53, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lee, H.J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.O.; Kim, S.H.; Lu, J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.; Menna, J.H.; Sung, C.C.; Yeh, Y.C. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tillekeratne, L.M.; Sherette, A.; Fulmer, J.A.; Hupe, L.; Hupe, D.; Gabbara, S.; Peliska, J.A.; Hudson, R.A. Differential inhibition of polymerase and strand-transfer activities of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2002, 12, 525–528. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Hay, A.J. In vitro anti-influenza virus activity of a plant preparation from Geranium sanguineum L. Antiviral Res. 1998, 37, 121–130. [Google Scholar] [CrossRef]
- Campbell, G.R.; Bruckman, R.S.; Herns, S.D.; Joshi, S.; Durden, D.; Spector, S.A. Induction of autophagy by PI3K/MTOR and PI3K/MTOR/BRD4 inhibitors suppresses HIV-1 replication. J. Biol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Yang, J.; Liu, J.; Zhang, Z.; Song, X.; Yao, Z.; Ma, C.; Li, W.; Zeng, R.; et al. RSV replication is promoted by autophagy-mediated inhibition of apoptosis. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chai, Y.; Wang, L.; Zhang, J.; Lee, H.J.; Kim, S.H.; Lu, J. Pentagalloylglucose induces autophagy and caspase-independent programmed deaths in human PC-3 and mouse TRAMP-C2 prostate cancer cells. Mol. Cancer Ther. 2009, 8, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Jiang, C.; Luo, X.; Guo, X.; Zhao, C.; Fan, L.; Meng, Y.; Lu, J.; Song, X.; et al. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-β-d-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310. [Google Scholar] [CrossRef] [PubMed]

| Compounds | CC50 (95% CL) | IC50 (95% CL) | SI (CC50/IC50) |
|---|---|---|---|
| PGG (μM) | 22.48 (20.327–24.921) | 3.90 (3.145–4.837) | 5.76 |
| IPS (mM) | 1.946 (1.766–2.145) | 0.3327 (0.3000–0.3691) | 5.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Z.; Gong, W.; Zhang, Y.; Feng, Y.; Liu, Y.; Tu, C. Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy. Viruses 2018, 10, 201. https://doi.org/10.3390/v10040201
Tu Z, Gong W, Zhang Y, Feng Y, Liu Y, Tu C. Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy. Viruses. 2018; 10(4):201. https://doi.org/10.3390/v10040201
Chicago/Turabian StyleTu, Zhongzhong, Wenjie Gong, Yan Zhang, Ye Feng, Yan Liu, and Changchun Tu. 2018. "Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy" Viruses 10, no. 4: 201. https://doi.org/10.3390/v10040201





