Gemcitabine and Nucleos(t)ide Synthesis Inhibitors Are Broad-Spectrum Antiviral Drugs that Activate Innate Immunity
Abstract
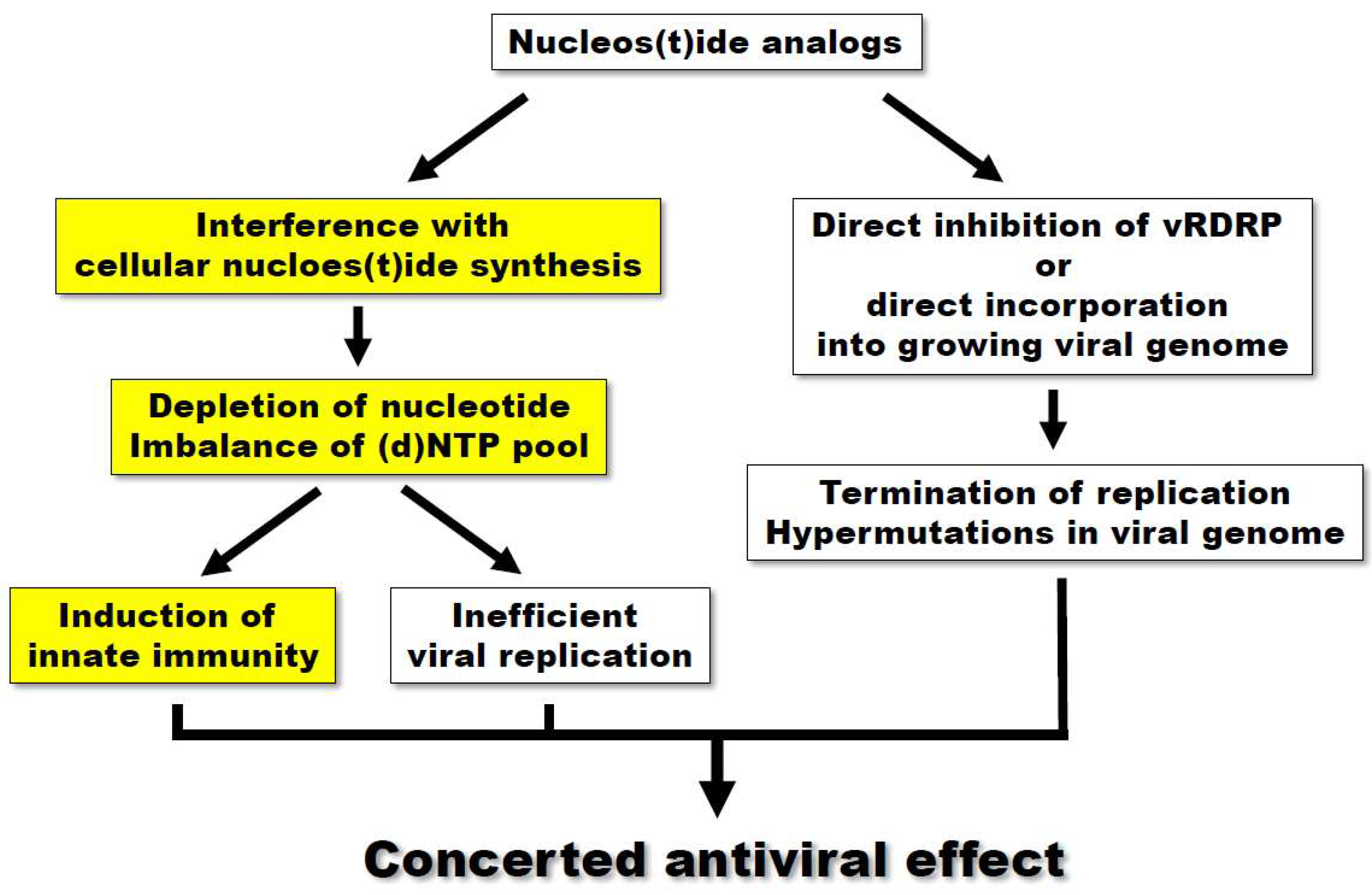
:1. Introduction
2. The Broad-Spectrum Antiviral Activity of Gemcitabine
3. Innate Immune Activation by Purine and Pyrimidine Biosynthesis Inhibitors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B. The purine path to chemotherapy. Science 1989, 244, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Alford, C.A.; Hirsch, M.S.; Schooley, R.T.; Luby, J.P.; Aoki, F.Y.; Hanley, D.; Nahmias, A.J.; Soong, S.J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Eng. J. Med. 1986, 314, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.O.; Lagakos, S.W.; Richman, D.D.; Cross, A.; Pettinelli, C.; Liou, S.H.; Brown, M.; Volberding, P.A.; Crumpacker, C.S.; Beall, G.; et al. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. The niaid aids clinical trials group. N. Eng. J. Med. 1992, 327, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Mangia, A.; Wyles, D.; Rodriguez-Torres, M.; Hassanein, T.; Gordon, S.C.; Schultz, M.; Davis, M.N.; Kayali, Z.; Reddy, K.R.; et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Eng. J. Med. 2013, 368, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Feld, J.J.; Li, Q.; Hu, Z.; Fried, M.W.; Liang, T.J. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis c virus cell culture models. Hepatology 2011, 53, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; Balzarini, J.; de Clercq, E.; Neyts, J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of imp dehydrogenase. J. Virol. 2005, 79, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Debing, Y.; Emerson, S.U.; Wang, Y.; Pan, Q.; Balzarini, J.; Dallmeier, K.; Neyts, J. Ribavirin inhibits in vitro hepatitis e virus replication through depletion of cellular gtp pools and is moderately synergistic with α interferon. Antimicrob. Agents Chemother. 2014, 58, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Karlsson, A.; Wang, L.; Bohman, C.; Horska, K.; Votruba, I.; Fridland, A.; van Aerschot, A.; Herdewijn, P.; de Clercq, E. Eicar (5-ethynyl-1-beta-d-ribofuranosylimidazole-4-carboxamide). A novel potent inhibitor of inosinate dehydrogenase activity and guanylate biosynthesis. J. Biol. Chem. 1993, 268, 24591–24598. [Google Scholar] [PubMed]
- Wang, Y.; Wang, W.; Xu, L.; Zhou, X.; Shokrollahi, E.; Felczak, K.; van der Laan, L.J.; Pankiewicz, K.W.; Sprengers, D.; Raat, N.J.; et al. Cross talk between nucleotide synthesis pathways with cellular immunity in constraining hepatitis e virus replication. Antimicrob. Agents Chemother. 2016, 60, 2834–2848. [Google Scholar] [CrossRef] [PubMed]
- Hertel, L.W.; Boder, G.B.; Kroin, J.S.; Rinzel, S.M.; Poore, G.A.; Todd, G.C.; Grindey, G.B. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res. 1990, 50, 4417–4422. [Google Scholar] [PubMed]
- Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Understanding ribonucleotide reductase inactivation by gemcitabine. Chemistry 2007, 13, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- Dyall, J.; Coleman, C.M.; Hart, B.J.; Venkataraman, T.; Holbrook, M.R.; Kindrachuk, J.; Johnson, R.F.; Olinger, G.G., Jr.; Jahrling, P.B.; Laidlaw, M.; et al. Repurposing of clinically developed drugs for treatment of middle east respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014, 58, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Kuivanen, S.; Bespalov, M.M.; Nandania, J.; Ianevski, A.; Velagapudi, V.; de Brabander, J.K.; Kainov, D.E.; Vapalahti, O. Obatoclax, saliphenylhalamide and gemcitabine inhibit zika virus infection in vitro and differentially affect cellular signaling, transcription and metabolism. Antivir. Res. 2017, 139, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, E.; Hu, C.; Cheng, H.; Chen, C.Y.; Huang, D.; Wang, R.; Zhao, Y.; Rong, L.; Vignuzzi, M.; et al. Cell-based high-throughput screening assay identifies 2′,2′-difluoro-2′-deoxycytidine gemcitabine as a potential antipoliovirus agent. ACS Infect. Dis. 2017, 3, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Denisova, O.V.; Kakkola, L.; Feng, L.; Stenman, J.; Nagaraj, A.; Lampe, J.; Yadav, B.; Aittokallio, T.; Kaukinen, P.; Ahola, T.; et al. Obatoclax, saliphenylhalamide, and gemcitabine inhibit influenza a virus infection. J. Biol. Chem. 2012, 287, 35324–35332. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Holtz, C.M.; Mullett, M.; Crankshaw, D.L.; Briggs, J.E.; O’Sullivan, M.G.; Patterson, S.E.; Mansky, L.M. Activity of a novel combined antiretroviral therapy of gemcitabine and decitabine in a mouse model for HIV-1. Antimicrob. Agents Chemother. 2012, 56, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, C.; Kim, D.E.; Song, J.H.; Choi, M.; Choi, K.; Kang, M.; Lee, K.; Kim, H.S.; Shin, J.S.; et al. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antivir. Res. 2015, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beran, R.K.; Sharma, R.; Corsa, A.C.; Tian, Y.; Golde, J.; Lundgaard, G.; Delaney, W.E.t.; Zhong, W.; Greenstein, A.E. Cellular growth kinetics distinguish a cyclophilin inhibitor from an hsp90 inhibitor as a selective inhibitor of hepatitis C virus. PLoS ONE 2012, 7, e30286. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, R.; Kantarjian, H.; Du, M.; Faucher, K.; Tarassoff, P.; Plunkett, W. Gemcitabine in leukemia: A phase I clinical, plasma, and cellular pharmacology study. J. Clin. Oncol. 1992, 10, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Exploiting drug repositioning for discovery of a novel HIV combination therapy. J. Virol. 2010, 84, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.R.; Heo, E.Y.; Lee, J.Y.; Kim, D.E.; Cho, S.; Chang, S.Y.; Yoon, B.I.; Seong, J.; Ko, H.J. Antiviral activity of gemcitabine against human rhinovirus in vitro and in vivo. Antivir. Res. 2017, 145, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.E.; Jang, K.S.; Kim, S.J.; Cho, S.; Kim, C. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget 2017, 8, 115315–115325. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Mok, K.Y.; Chan, A.S.; Cheung, N.N.; Wang, P.; Lui, Y.M.; Chan, J.F.; Chen, H.; Chan, K.H.; Kao, R.Y.; et al. Mycophenolic acid, an immunomodulator, has potent and broad-spectrum in vitro antiviral activity against pandemic, seasonal and avian influenza viruses affecting humans. J. Gen. Virol. 2016, 97, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Smee, D.F.; Sidwell, R.W.; Tseng, C. Identification of active antiviral compounds against a new york isolate of west nile virus. Antivir. Res. 2002, 55, 107–116. [Google Scholar] [CrossRef]
- Khan, M.; Dhanwani, R.; Patro, I.K.; Rao, P.V.; Parida, M.M. Cellular impdh enzyme activity is a potential target for the inhibition of chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir. Res. 2011, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takhampunya, R.; Ubol, S.; Houng, H.S.; Cameron, C.E.; Padmanabhan, R. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J. Gen. Virol. 2006, 87, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Colonno, R.; de Clercq, E.; Neyts, J. Ribavirin and mycophenolic acid markedly potentiate the anti-hepatitis B virus activity of entecavir. Antivir. Res. 2007, 73, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Debing, Y.; Chen, K.; van der Laan, L.J.; Neyts, J.; Janssen, H.L.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology 2014, 146, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; de Ruiter, P.E.; Metselaar, H.J.; Kwekkeboom, J.; de Jonge, J.; Tilanus, H.W.; Janssen, H.L.; van der Laan, L.J. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C virus infection in vitro and in vivo. Hepatology 2012, 55, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Panattoni, A.; D’Anna, F.; Triolo, E. Antiviral activity of tiazofurin and mycophenolic acid against grapevine leafroll-associated virus 3 in vitis vinifera explants. Antivir. Res. 2007, 73, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Ishibashi, M.; Okuno, T.; Kokado, Y.; Takahara, S.; Yamanishi, K.; Sonoda, T.; Takahashi, M. Effects of cyclosporine, azathioprine, mizoribine, and prednisolone on replication of human cytomegalovirus. Transplant. Proc. 1990, 22, 1682–1685. [Google Scholar] [PubMed]
- Furuta, Y.; Takahashi, K.; Fukuda, Y.; Kuno, M.; Kamiyama, T.; Kozaki, K.; Nomura, N.; Egawa, H.; Minami, S.; Watanabe, Y.; et al. In vitro and in vivo activities of anti-influenza virus compound t-705. Antimicrob. Agents Chemother. 2002, 46, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Qing, M.; Zou, G.; Wang, Q.Y.; Xu, H.Y.; Dong, H.; Yuan, Z.; Shi, P.Y. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob. Agents Chemother. 2010, 54, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Schlapfer, E.; Fischer, M.; Ott, P.; Speck, R.F. Anti-HIV-1 activity of leflunomide: A comparison with mycophenolic acid and hydroxyurea. AIDS 2003, 17, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Farasati, N.A.; Shapiro, R.; Vats, A.; Randhawa, P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 2005, 79, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Hourani, M.; Dauzonne, D.; Jorda, P.; Cousin, G.; Lupan, A.; Helynck, O.; Caignard, G.; Janvier, G.; Andre-Leroux, G.; Khiar, S.; et al. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog. 2013, 9, e1003678. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.N.; Lai, K.K.; Dai, J.; Kok, K.H.; Chen, H.; Chan, K.H.; Yuen, K.Y.; Kao, R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017, 98, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, A.; Franti, M.; Pusateri Keaney, E.; Kuhen, K.; Seepersaud, M.; Radetich, B.; Shao, J.; Honda, A.; Dewhurst, J.; Balabanis, K.; et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV). Proc. Natl. Acad. Sci. USA 2011, 108, 6739–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Bushell, S.; Qing, M.; Xu, H.Y.; Bonavia, A.; Nunes, S.; Zhou, J.; Poh, M.K.; Florez de Sessions, P.; Niyomrattanakit, P.; et al. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J. Virol. 2011, 85, 6548–6556. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Hurst, B.L.; Day, C.W. D282, a non-nucleoside inhibitor of influenza virus infection that interferes with de novo pyrimidine biosynthesis. Antivir. Chem. Chemother. 2012, 22, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Leaman, D.W.; Pisharody, S.; Flickinger, T.W.; Commane, M.A.; Schlessinger, J.; Kerr, I.M.; Levy, D.E.; Stark, G.R. Roles of jaks in activation of stats and stimulation of c-fos gene expression by epidermal growth factor. Mol. Cell. Biol. 1996, 16, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Snider, H.M.; Beatty, P.R.; Harris, E. Critical roles for both stat1-dependent and stat1-independent pathways in the control of primary dengue virus infection in mice. J. Immunol. 2005, 175, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-stimulated genes: Roles in viral pathogenesis. Curr. Opin. Virol. 2014, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
| Virus | Group | Family | Inhibition of | Cell Line/Animal Model | Antiviral Activity (EC50 or IC50) | Cell Toxicity (CC50) | Ref. |
|---|---|---|---|---|---|---|---|
| MERS-CoV | (+)ssRNA | Coronaviridae | Viral replication | Vero E6 cells | 1.2 μM | >10 μM | [13] |
| SARS-CoV | (+)ssRNA | Coronaviridae | Viral replication | Vero E6 cells | 4.9 μM | >10 μM | [13] |
| ZIKA | (+)ssRNA | Flaviviridae | Viral RNA and protein synthesis virus-mediated cell death | RPE cells | 0.01 μM | >10 μM | [14] |
| HCV | (+)ssRNA | Flaviviridae | Viral replication | Huh-7 cells | 12 nM | >44 μM | [19] |
| Poliovirus | (+)ssRNA | Picornaviridae | Viral replication RNA polymerase | HeLa cells | 0.3 μM | >100 μM | [15] |
| Influenza A virus (IAV) | (−)ssRNA | Orthomyxoviridae | Viral RNA transcription and replication virus entry | RPE cells | 0.068 μM | >10 μM | [16] |
| HIV | ssRNA-RT | Retroviridae | Viral replication | U373-MAGI-CXCR4CEM cells | 16.3 nM | [17] | |
| MuLV | ssRNA-RT | Retroviridae | Viral replication | U373-MAGI-CXCR4CEM ls/Murine AIDS mouse model | 1.6 nM | [17] | |
| CVB3 | (+)ssRNA | Picornaviridae | Viral proliferation Viral replication Early step of virus infection | Vero cells HeLa cells | 0.4 μM 2 μM | >50 μM >50 μM | [18] |
| EV71 | (+)ssRNA | Picornaviridae | Viral proliferation Viral replication | Vero cells | 1 μM | >50 μM | [18] |
| HRV | (+)ssRNA | Picornaviridae | Viral RNA synthesis | HeLa cells/Mouse | 1–5 μM | >50 μM | [18,22] |
| Sindbis virus (SINV) | (+)ssRNA | Togaviridae | Virus production | Vero cells | >2 logs (at 3 μM) | [16] | |
| HSV-1 | dsDNA | Herpesviridae | Virus production | RPE cells | >4 logs (at 3 μM) | [16] |
| Purine | Pyrimidine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Target | Antiviral Ref. | Innate Immunity Ref. | Chemical Structure (Pubchem) | Compound | Target | Antiviral Ref. | Innate Immunity Ref. | Chemical Structure (Pubchem) |
| Mycophenolic acid | IMPDH | [24,25,26,27,28,29] | [29,30] |  | Gemcitabine | Cytosine→Cytidine Uracil→Uridine | Table 1 | [23] |  |
| Tiazofurin | IMPDH | [31] |  | Brequinar | DHODH | [34] | [10] |  | |
| Mizoribine | IMPDH, GMP synthetase | [32] |  | Leflunomide Teriflunomide | DHODH | [35,36] |  | ||
| Ribavirin | IMPDH | [6,26,27,28] | [6] |  | 6-Azauridine | ODCase | [10,25,26] | [10] |  |
| Favipiravir (T-705) | ? | [33] |  | DD264 | DHODH | [37] | [37] | ||
| FA-613 | DHODH | [38] | [38] | ||||||
| 1346, 1347, 1348 | IMPDH | [10] | [10] | A3 | DHODH | [39] | |||
| 6b/14b | DHODH/CAD | [40] | |||||||
| NITD-982 | DHODH | [41] | |||||||
| D282 | DHODH | [42] | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.J.; Kim, C.; Cho, S. Gemcitabine and Nucleos(t)ide Synthesis Inhibitors Are Broad-Spectrum Antiviral Drugs that Activate Innate Immunity. Viruses 2018, 10, 211. https://doi.org/10.3390/v10040211
Shin HJ, Kim C, Cho S. Gemcitabine and Nucleos(t)ide Synthesis Inhibitors Are Broad-Spectrum Antiviral Drugs that Activate Innate Immunity. Viruses. 2018; 10(4):211. https://doi.org/10.3390/v10040211
Chicago/Turabian StyleShin, Hye Jin, Chonsaeng Kim, and Sungchan Cho. 2018. "Gemcitabine and Nucleos(t)ide Synthesis Inhibitors Are Broad-Spectrum Antiviral Drugs that Activate Innate Immunity" Viruses 10, no. 4: 211. https://doi.org/10.3390/v10040211





