Cross-Reactive Human IgM-Derived Monoclonal Antibodies that Bind to HIV-1 Envelope Glycoproteins
Abstract
:1. Introduction
2. Results
2.1. Construction of a large naive human antibody library by using the phagemid vector pZYD-N1
2.2. Selection of HIV-1 Env-specific cross-reactive human IgM-derived antibodies
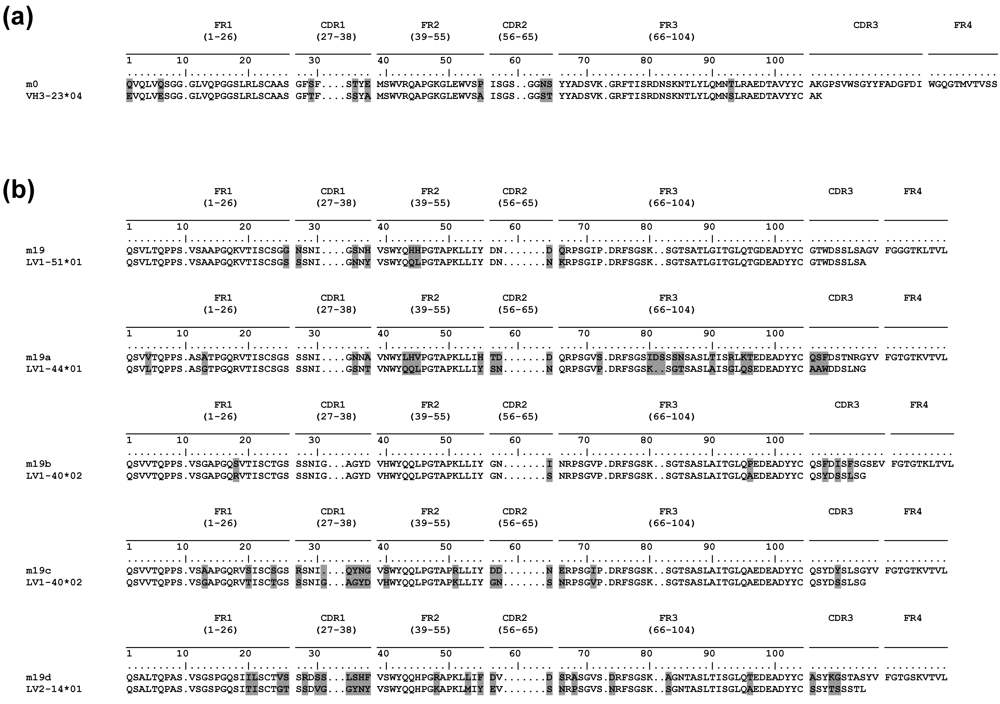
 |
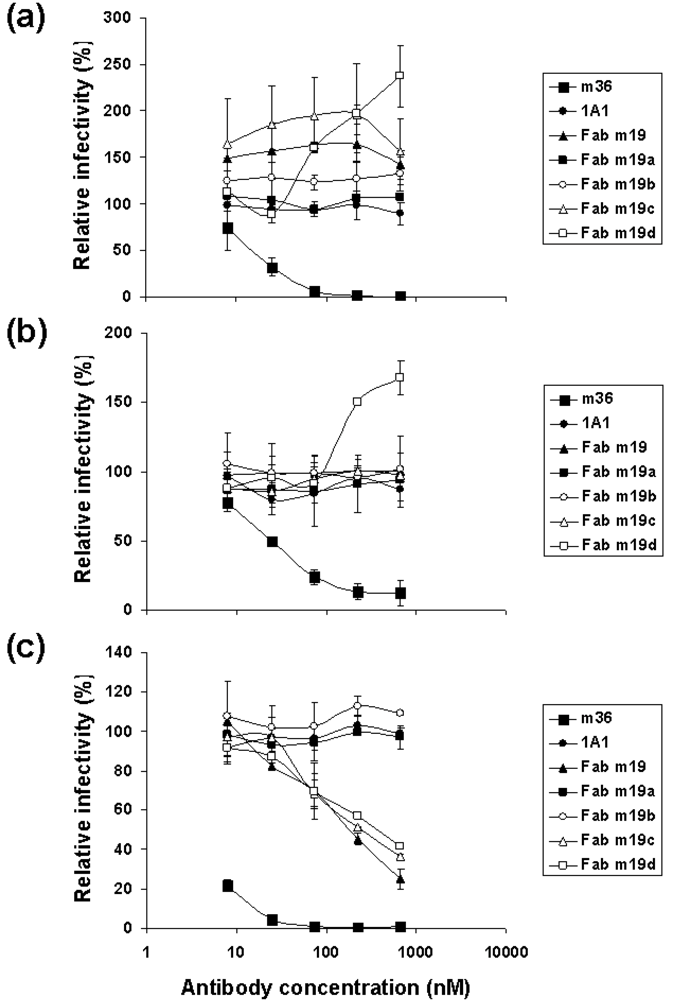
2.3. The selected antibodies did not neutralize, neutralized weakly or enhanced HIV-1 infections

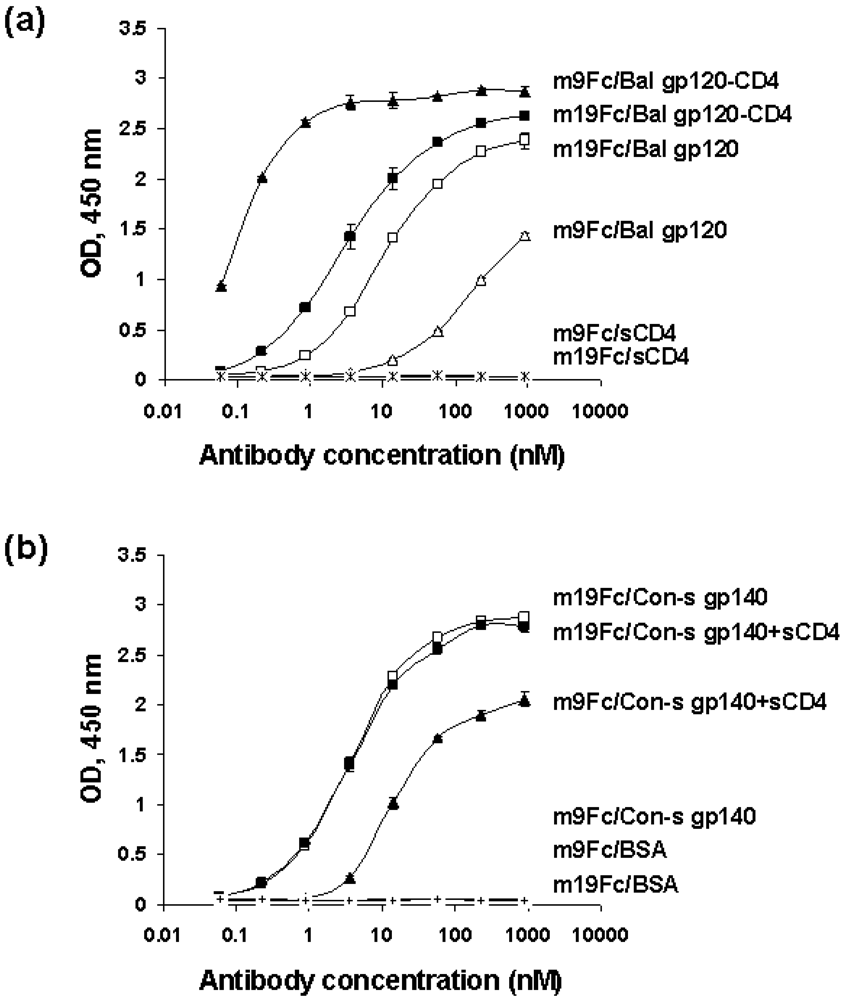
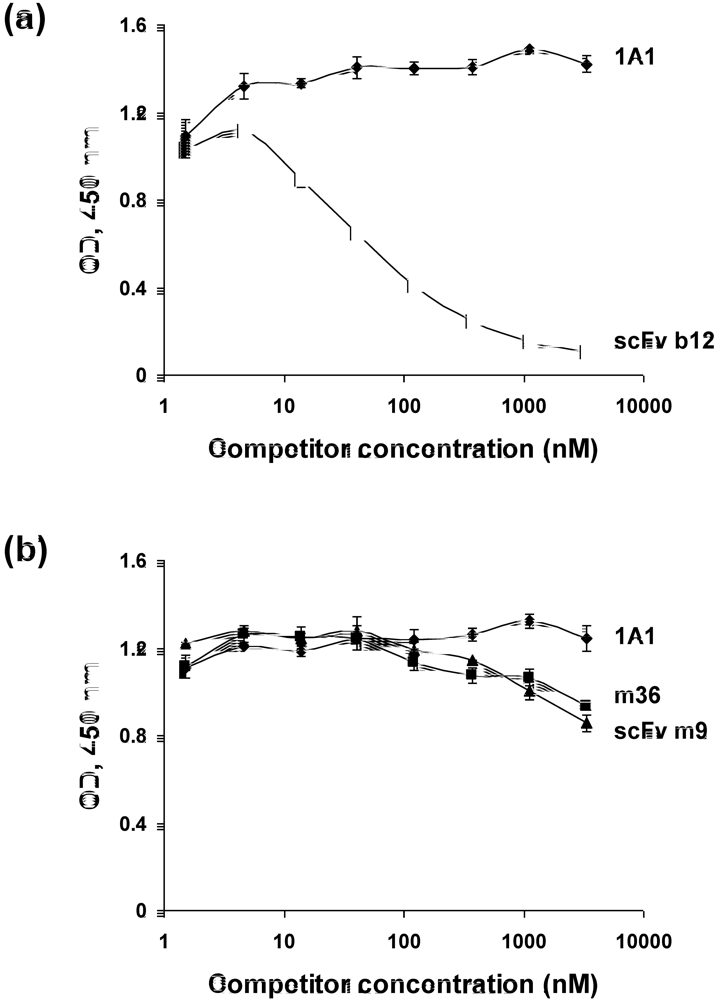
2.4. Characterization of the antibody epitopes
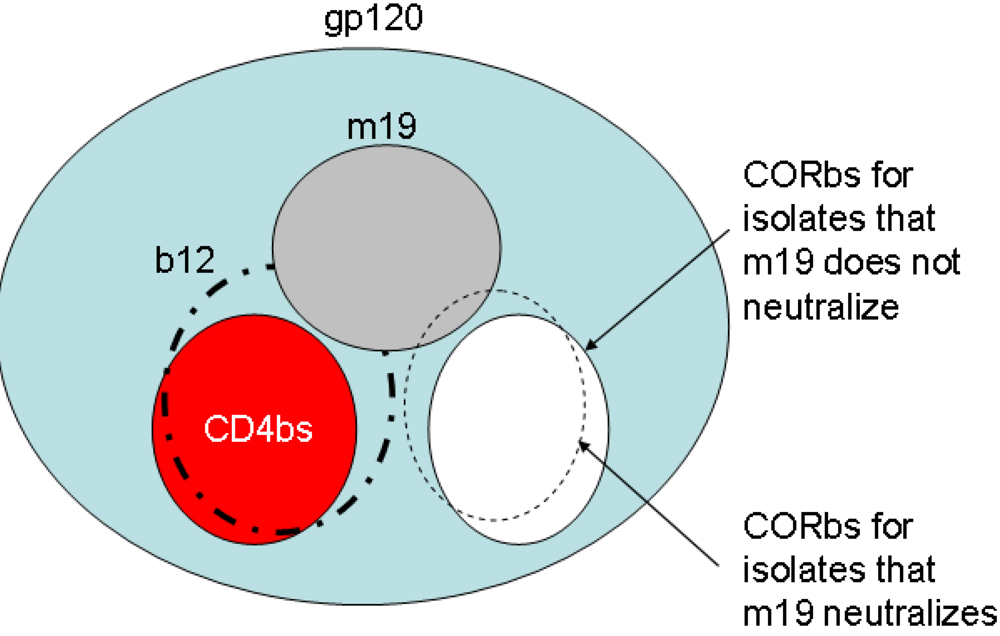
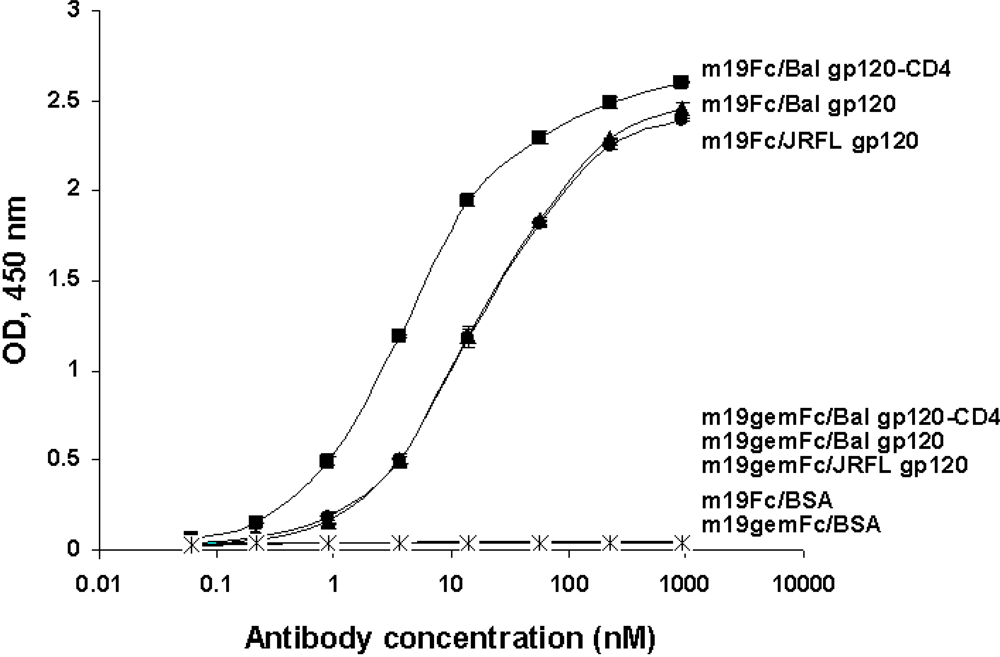
2.5. Somatic mutations are required for the antibody binding activity
3. Discussion
4. Materials and Methods
4.1. Cells, viruses, plasmids, gp120, gp140, and antibodies
4.2. Library construction
4.3. Selection of antibodies against HIV-1 antigens
4.4. Construction of the scFv and Fc-fusion proteins of selected antibodies
4.5. Expression and purification of antibodies
4.6. ELISA
4.7. Pseudovirus neutralization assay
5. Conclusions
Acknowledgments
References
- Roben, P.; Moore, J.P.; Thali, M.; Sodroski, J.; Barbas, C.F.; Burton, D.R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 1994, 68, 4821–4828. [Google Scholar] [PubMed]
- Moulard, M.; Phogat, S.K.; Shu, Y.; Labrijn, A.F.; Xiao, X.; Binley, J.M.; Zhang, M.Y.; Sidorov, I.A.; Broder, C.C.; Robinson, J.; Parren, P.W.; Burton, D.R.; Dimitrov, D.S. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 6913–6918. [Google Scholar] [CrossRef]
- Trkola, A.; Purtscher, M.; Muster, T.; Ballaun, C.; Buchacher, A.; Sullivan, N.; Srinivasan, K.; Sodroski, J.; Moore, J.P.; Katinger, H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996, 70, 1100–1108. [Google Scholar] [PubMed]
- Stiegler, G.; Kunert, R.; Purtscher, M.; Wolbank, S.; Voglauer, R.; Steindl, F.; Katinger, H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 2001, 17, 1757–1765. [Google Scholar] [PubMed]
- Zwick, M.B.; Labrijn, A.F.; Wang, M.; Spenlehauer, C.; Saphire, E.O.; Binley, J.M.; Moore, J.P.; Stiegler, G.; Katinger, H.; Burton, D.R.; Parren, P.W. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001, 75, 10892–10905. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dimitrov, D.S. Human monoclonal antibodies and engineered antibody domains as HIV-1 entry inhibitors. Curr. Opin. HIV. AIDS 2009, 4, 112–117. [Google Scholar] [CrossRef]
- Zhang, P.F.; Cham, F.; Dong, M.; Choudhary, A.; Bouma, P.; Zhang, Z.; Shao, Y.; Feng, Y.R.; Wang, L.; Mathy, N.; Voss, G.; Broder, C.C.; Quinnan, G.V. Extensively cross-reactive anti-HIV-1 neutralizing antibodies induced by gp140 immunization. Proc. Natl. Acad. Sci. USA 2007, 104, 10193–10198. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Peterson, M.L.; Follmann, D.; Hudgens, M.G.; Francis, D.P.; Gurwith, M.; Heyward, W.L.; Jobes, D.V.; Popovic, V.; Self, S.G.; Sinangil, F.; Burke, D.; Berman, P.W. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 2005, 191, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Mooij, P.; van der, K.M.; Bogers, W.M.; Ten Haaft, P.J.; Van Der, M.P.; Almond, N.; Stott, J.; Deschamps, M.; Labbe, D.; Momin, P.; Voss, G.; Von, H.P.; Bruck, C.; Heeney, J.L. A clinically relevant HIV-1 subunit vaccine protects rhesus macaques from in vivo passaged simian-human immunodeficiency virus infection. AIDS 1998, 12, F15–F22. [Google Scholar] [CrossRef] [PubMed]
- Voss, G.; Manson, K.; Montefiori, D.; Watkins, D.I.; Heeney, J.; Wyand, M.; Cohen, J.; Bruck, C. Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J. Virol. 2003, 77, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Montefiori, D.C. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 2006, 5, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002, 2, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Geleziunas, R.; Bianchi, E.; Lennard, S.; Hrin, R.; Zhang, H.; Lu, M.; An, Z.; Ingallinella, P.; Finotto, M.; Mattu, M.; Finnefrock, A.C.; Bramhill, D.; Cook, J.; Eckert, D.M.; Hampton, R.; Patel, M.; Jarantow, S.; Joyce, J.; Ciliberto, G.; Cortese, R.; Lu, P.; Strohl, W.; Schleif, W.; McElhaugh, M.; Lane, S.; Lloyd, C.; Lowe, D.; Osbourn, J.; Vaughan, T.; Emini, E.; Barbato, G.; Kim, P.S.; Hazuda, D.J.; Shiver, J.W.; Pessi, A. A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc. Natl. Acad. Sci. USA 2005, 102, 14759–14764. [Google Scholar] [CrossRef]
- Toran, J.L.; Kremer, L.; Sanchez-Pulido, L.; de, A.; del, R.G.; Llorente, M.; Valencia, A.; de Mon, M.A.; Martinez, A. Molecular analysis of HIV-1 gp120 antibody response using isotype IgM and IgG phage display libraries from a long-term non-progressor HIV-1-infected individual. Eur. J. Immunol. 1999, 29, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, N.C.; Bates, A.C.; Sattentau, Q.J. A functional human IgM response to HIV-1 Env after immunization with NYVAC HIV C. AIDS 2007, 21, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Bomsel, M.; Heyman, M.; Hocini, H.; Lagaye, S.; Belec, L.; Dupont, C.; Desgranges, C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 1998, 9, 277–287. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D.S. Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc. Natl. Acad. Sci. USA 2008, 105, 17121–17126. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Z.; Feng, Y.; Xiao, X.; Dimitrov, D.S. Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J. Mol. Biol. 2008, 382, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.X.; Sutherland, L.L.; Xia, S.M.; Brock, M.E.; Scearce, R.M.; Vanleeuwen, S.; Alam, S.M.; McAdams, M.; Weaver, E.A.; Camacho, Z.; Ma, B.J.; Li, Y.; Decker, J.M.; Nabel, G.J.; Montefiori, D.C.; Hahn, B.H.; Korber, B.T.; Gao, F.; Haynes, B.F. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 2006, 353, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Fouts, T.R.; Tuskan, R.; Godfrey, K.; Reitz, M.; Hone, D.; Lewis, G.K.; DeVico, A.L. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J. Virol. 2000, 74, 11427–11436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Shu, Y.; Rudolph, D.; Prabakaran, P.; Labrijn, A.F.; Zwick, M.B.; Lal, R.B.; Dimitrov, D.S. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J. Mol. Biol. 2004, 335, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Saphire, E.O.; Parren, P.W.; Pantophlet, R.; Zwick, M.B.; Morris, G.M.; Rudd, P.M.; Dwek, R.A.; Stanfield, R.L.; Burton, D.R.; Wilson, I.A. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 2001, 293, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; Benenson, M.; Gurunathan, S.; Tartaglia, J.; McNeil, J.G.; Francis, D.P.; Stablein, D.; Birx, D.L.; Chunsuttiwat, S.; Khamboonruang, C.; Thongcharoen, P.; Robb, M.L.; Michael, N.L.; Kunasol, P.; Kim, J.H. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.; Kwong, P.D.; Desjardins, E.; Sweet, R.W.; Robinson, J.; Hendrickson, W.A.; Sodroski, J.G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 1998, 393, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.W.; Lu, S.; Srivastava, I.; Cherpelis, S.; Gettie, A.; Blanchard, J.; Wang, S.; Mboudjeka, I.; Leung, L.; Lian, Y.; Fong, A.; Buckner, C.; Ly, A.; Hilt, S.; Ulmer, J.; Wild, C.T.; Mascola, J.R.; Stamatatos, L. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 2001, 75, 5526–5540. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Han, D.P.; Cao, C.; Cho, M.W. Immunogenicity and ability of variable loop-deleted human immunodeficiency virus type 1 envelope glycoproteins to elicit neutralizing antibodies. Virology 2003, 305, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wyatt, R.; Richmond, J.F.; Mustafa, F.; Wang, S.; Weng, J.; Montefiori, D.C.; Sodroski, J.; Robinson, H.L. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res. Hum. Retroviruses 1998, 14, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Chakrabarti, B.K.; Xu, L.; Welcher, B.; Kong, W.P.; Leung, K.; Panet, A.; Mascola, J.R.; Nabel, G.J. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol. 2004, 78, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Bolmstedt, A.; Sjolander, S.; Hansen, J.E.; Akerblom, L.; Hemming, A.; Hu, S.L.; Morein, B.; Olofsson, S. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 glycoprotein gp160 on induction of a virus-neutralizing humoral response. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1996, 12, 213–220. [Google Scholar] [PubMed]
- Quinones-Kochs, M.I.; Buonocore, L.; Rose, J.K. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 2002, 76, 4199–4211. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Quan, F.S.; Huang, C.; Guo, L.; Ye, L.; Yang, C.; Compans, R.W. Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology 2005, 331, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Sugiura, W.; Montefiori, D.C.; Broder, C.C.; Lee, S.A.; Wild, C.; Lifson, J.; Moss, B. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 2001, 75, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wyatt, R.; Sodroski, J. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 2001, 75, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Fouts, T.; Godfrey, K.; Bobb, K.; Montefiori, D.; Hanson, C.V.; Kalyanaraman, V.S.; DeVico, A.; Pal, R. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc. Natl. Acad. Sci. USA 2002, 99, 11842–11847. [Google Scholar] [CrossRef]
- Xiao, X.; Phogat, S.; Shu, Y.; Phogat, A.; Chow, Y.H.; Wei, O.L.; Goldstein, H.; Broder, C.C.; Dimitrov, D.S. Purified complexes of HIV-1 envelope glycoproteins with CD4 and CCR5(CXCR4): production, characterization and immunogenicity. Vaccine 2003, 21, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.H.; Kwong, P.D.; Gupta, R.; Rizzuto, C.D.; Casper, D.J.; Wyatt, R.; Wang, L.; Hendrickson, W.A.; Doyle, M.L.; Sodroski, J. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 2002, 76, 9888–9899. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Scearce, R.M.; Alam, S.M.; Hora, B.; Xia, S.; Hohm, J.E.; Parks, R.J.; Ogburn, D.F.; Tomaras, G.D.; Park, E.; Lomas, W.E.; Maino, V.C.; Fiscus, S.A.; Cohen, M.S.; Moody, M.A.; Hahn, B.H.; Korber, B.T.; Liao, H.; Haynes, B.F. Broadly reactive monoclonal antibodies to multiple HIV-1 subtype and SIVcpz Envelope glycoproteins . Virology (in press). [CrossRef]
- Sheppard, N.C.; Davies, S.L.; Jeffs, S.A.; Vieira, S.M.; Sattentau, Q.J. Production and characterization of high-affinity human monoclonal antibodies to human immunodeficiency virus type 1 envelope glycoproteins in a mouse model expressing human immunoglobulins. Clin. Vaccine Immunol. 2007, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Z.; Zhang, M.; Macagno, A.; Prabakaran, P.; Owens, J.; Longo, N.S.; Markowitz, M.; Lanzavecchia, A.; Haynes, B.F.; Dimitrov, D.S. All known cross reactive HIV-1 neutralizing antibodies are highly divergent from germline and their elicitation may require prolonge periods of time. AIDS Res. Hum. Retroviruses 2008, 24, 11–12. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, W.; Feng, Y.; Zhu, Z.; Prabakaran, P.; Wang, Y.; Zhang, M.Y.; Longo, N.S.; Dimitrov, D.S. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: Implications for evasion of immune responses and design of vaccine immunogens. Biochem. Biophys. Res. Commun. 2009, 390, 404–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Chen, W.; Feng, Y.; Dimitrov, D.S. Maturation pathways of cross-reactive HIV-1 neutralizing antibodies. Viruses 2009, 1, 802–817. [Google Scholar] [CrossRef]
- Berberian, L.; Goodglick, L.; Kipps, T.J.; Braun, J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 1993, 261, 1588–1591. [Google Scholar] [PubMed]
- Karray, S.; Zouali, M. Identification of the B cell superantigen-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. USA 1997, 94, 1356–1360. [Google Scholar] [CrossRef]
- Karray, S.; Juompan, L.; Maroun, R.C.; Isenberg, D.; Silverman, G.J.; Zouali, M. Structural basis of the gp120 superantigen-binding site on human immunoglobulins. J. Immunol. 1998, 161, 6681–6688. [Google Scholar] [PubMed]
- Caton, A.J.; Koprowski, H. Influenza virus hemagglutinin-specific antibodies isolated from a combinatorial expression library are closely related to the immune response of the donor. Proc. Natl. Acad. Sci. USA 1990, 87, 6450–6454. [Google Scholar] [CrossRef]
- Gherardi, E.; Milstein, C. Original and artificial antibodies. Nature 1992, 357, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Chapal, N.; Chardes, T.; Bresson, D.; Pugniere, M.; Mani, J.C.; Pau, B.; Bouanani, M.; Peraldi-Roux, S. Thyroid peroxidase autoantibodies obtained from random single chain FV libraries contain the same heavy/light chain combinations as occur in vivo. Endocrinology 2001, 142, 4740–4750. [Google Scholar] [CrossRef] [PubMed]
- Jaume, J.C.; Costante, G.; Portolano, S.; McLachlan, S.M.; Rapoport, B. Recombinant thyroid peroxidase-specific autoantibodies I. How diverse is the pool of heavy and light chains in immunoglobulin gene libraries constructed from thyroid tissue-infiltrating plasma cells. Endocrinology 1994, 135, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Costante, G.; Portolano, S.; Nishikawa, T.; Jaume, J.C.; Chazenbalk, G.D.; Rapoport, B.; McLachlan, S.M. Recombinant thyroid peroxidase-specific autoantibodies II. Role of individual heavy and light chains in determining epitope recognition. Endocrinology 1994, 135, 25–30. [Google Scholar] [CrossRef] [PubMed]
- de Wildt, R.M.; Hoet, R.M.; van Venrooij, W.J.; Tomlinson, I.M.; Winter, G. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J. Mol. Biol. 1999, 285, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Dimitrov, D.S. Construction of a large naive human phage-displayed Fab library through one-step cloning. Methods Mol. Biol. 2009, 525, 129–142. [Google Scholar] [PubMed]
- Zhu, Z.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Bishop, K.A.; Choudhry, V.; Mungall, B.A.; Feng, Y.R.; Choudhary, A.; Zhang, M.Y.; Feng, Y.; Wang, L.F.; Xiao, X.; Eaton, B.T.; Broder, C.C.; Dimitrov, D.S. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 2006, 80, 891–899. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Chen, W.; Zhu, Z.; Liao, H.; Quinnan, G.V., Jr.; Broder, C.C.; Haynes, B.F.; Dimitrov, D.S. Cross-Reactive Human IgM-Derived Monoclonal Antibodies that Bind to HIV-1 Envelope Glycoproteins. Viruses 2010, 2, 547-565. https://doi.org/10.3390/v2020547
Chen W, Zhu Z, Liao H, Quinnan GV Jr., Broder CC, Haynes BF, Dimitrov DS. Cross-Reactive Human IgM-Derived Monoclonal Antibodies that Bind to HIV-1 Envelope Glycoproteins. Viruses. 2010; 2(2):547-565. https://doi.org/10.3390/v2020547
Chicago/Turabian StyleChen, Weizao, Zhongyu Zhu, Huaxin Liao, Gerald V. Quinnan, Jr., Christopher C. Broder, Barton F. Haynes, and Dimiter S. Dimitrov. 2010. "Cross-Reactive Human IgM-Derived Monoclonal Antibodies that Bind to HIV-1 Envelope Glycoproteins" Viruses 2, no. 2: 547-565. https://doi.org/10.3390/v2020547




