Molecular Aspects of HTLV-1 Entry: Functional Domains of the HTLV-1 Surface Subunit (SU) and Their Relationships to the Entry Receptors
Abstract
:1. Introduction
2. The Host Cell Actors: The HTLV-1 Receptors
2.1. Identification of the Roles of GLUT1, NRP-1 and HSPGs in HTLV-1 Entry
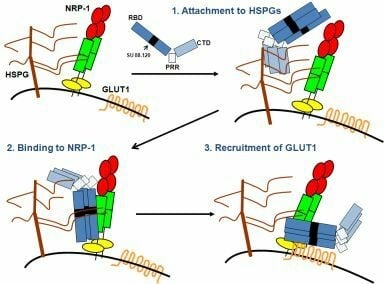
2.2. Cooperation between the HTLV-1 Receptors
2.3. A Multireceptor Model for HTLV-1 Entry
3. The Viral Actor: The HTLV-1 SU Protein
3.1. Function of Retroviral SU Proteins
3.2. General Organization of the HTLV-1 SU
3.3. Functional Residues of the HTLV-1 SU
3.4. Relationships between the Neutralizing Domains of the SU and the Residues Involved in Receptor Binding
3.5. Conformational Changes in the HTLV-1 SU
4. Comparison between HTLV-1 and the other PTLVs
4.1. Difference between HTLV-1 and HTLV-2
4.2. Receptor Usage of Other PTLV
5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Ilinskaya, A.; Heidecker, G.; Jones, K. Interaction between the HTLV-1 envelope and cellular proteins: impact on virus infection and restriction. Future Med. Chem. 1651, 2, 1651–1668. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Lepelletier, Y.; Jones, K.S.; Pique, C.; Hermine, O. Current concepts regarding the HTLV-1 receptor complex. Retrovirology 2010, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Agrawal, L.; VanHorn-Ali, Z.; Alkhatib, G. Infection of CD4+ T lymphocytes by the human T cell leukemia virus type 1 is mediated by the glucose transporter GLUT-1: Evidence using antibodies specific to the receptor's large extracellular domain. Virology 2006, 349, 184–196. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef]
- He, Z.; Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90, 739–751. [Google Scholar] [CrossRef]
- Bagri, A.; Tessier-Lavigne, M.; Watts, R.J. Neuropilins in tumor biology. Clin. Cancer Res. 2009, 15, 1860–1864. [Google Scholar] [CrossRef]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Romeo, P.H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef]
- Pinon, J.D.; Klasse, P.J.; Jassal, S.R.; Welson, S.; Weber, J.; Brighty, D.W.; Sattentau, Q.J. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 2003, 77, 9922–9930. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Fugo, K.; Petrow-Sadowski, C.; Huang, Y.; Bertolette, D.C.; Lisinski, I.; Cushman, S.W.; Jacobson, S.; Ruscetti, F.W. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells. J. Virol. 2006, 80, 8291–8302. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Alkhatib, B.; Cornetta, K.; Alkhatib, G. Alternate receptor usage of neuropilin-1 and glucose transporter protein 1 by the human T cell leukemia virus type 1. Virology 2009, 396, 203–212. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Vander Kooi, C.W.; Jusino, M.A.; Perman, B.; Neau, D.B.; Bellamy, H.D.; Leahy, D.J. Structural basis for ligand and heparin binding to neuropilin B domains. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Heveker, N.; Ruscetti, F.W.; Perret, G.; Jones, K.S.; et al. HTLV-1 uses HSPG and neuropilin 1 for entry by molecular mimicry of VEGF165. Blood 2009, 6, 6. [Google Scholar] [CrossRef]
- Shintani, Y.; Takashima, S.; Asano, Y.; Kato, H.; Liao, Y.; Yamazaki, S.; Tsukamoto, O.; Seguchi, O.; Yamamoto, H.; Fukushima, T.; et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006, 25, 3045–3055. [Google Scholar] [CrossRef]
- Takenouchi, N.; Jones, K.S.; Lisinski, I.; Fugo, K.; Yao, K.; Cushman, S.W.; Ruscetti, F.W.; Jacobson, S. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1. J. Virol. 2007, 81, 1506–1510. [Google Scholar] [CrossRef]
- Moulard, M.; Decroly, E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim. Biophys. Acta 2000, 1469, 121–132. [Google Scholar] [CrossRef]
- Waheed, A.A.; Freed, E.O. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009, 143, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Melikyan, G.B. Common principles and intermediates of viral protein-mediated fusion: The HIV-1 paradigm. Retrovirology 2008, 5, 111. [Google Scholar] [CrossRef]
- Permanyer, M.; Ballana, E.; Este, J.A. Endocytosis of HIV: Anything goes. Trends Microbiol. 2010, 18, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Narayan, S.; Dornadula, G.; Miller, M.D.; Young, J.A. Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating. J. Virol. 2004, 78, 10433–10441. [Google Scholar] [CrossRef]
- Ross, S.R.; Schofield, J.J.; Farr, C.J.; Bucan, M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 12386–12390. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Ekstrom, M.; Garoff, H. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 2004, 23, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Kopelman, R.; Li, Z.; Kayman, S.C.; Sanders, D.A. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 1997, 71, 8073–8077. [Google Scholar] [CrossRef]
- Ray, N.; Doms, R.W. HIV-1 coreceptors and their inhibitors. Curr. Top. Microbiol. Immunol. 2006, 303, 97–120. [Google Scholar]
- Markovic, I.; Stantchev, T.S.; Fields, K.H.; Tiffany, L.J.; Tomic, M.; Weiss, C.D.; Broder, C.C.; Strebel, K.; Clouse, K.A. Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood 2004, 103, 1586–1594. [Google Scholar] [CrossRef]
- Weissenhorn, W.; Hinz, A.; Gaudin, Y. Virus membrane fusion. FEBS Lett. 2007, 581, 2150–2155. [Google Scholar] [CrossRef]
- Pique, C.; Pham, D.; Tursz, T.; Dokhelar, M.C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J. Virol. 1992, 66, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Lavillette, D.; Maurice, M.; Roche, C.; Russell, S.J.; Sitbon, M.; Cosset, F.L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 1998, 72, 9955–9965. [Google Scholar] [CrossRef] [PubMed]
- Fass, D.; Davey, R.A.; Hamson, C.A.; Kim, P.S.; Cunningham, J.M.; Berger, J.M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science 1997, 277, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Battini, J.L.; Danos, O.; Heard, J.M. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 1995, 69, 713–719. [Google Scholar] [CrossRef]
- Kim, F.J.; Seiliez, I.; Denesvre, C.; Lavillette, D.; Cosset, F.L.; Sitbon, M. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 2000, 275, 23417–23420. [Google Scholar] [CrossRef]
- Kim, F.J.; Manel, N.; Garrido, E.N.; Valle, C.; Sitbon, M.; Battini, J.L. HTLV-1 and -2 envelope SU subdomains and critical determinants in receptor binding. Retrovirology 2004, 1, 41. [Google Scholar] [CrossRef]
- Argaw, T.; Figueroa, M.; Salomon, D.R.; Wilson, C.A. Identification of residues outside of the receptor binding domain that influence the infectivity and tropism of porcine endogenous retrovirus. J. Virol. 2008, 82, 7483–7491. [Google Scholar] [CrossRef]
- Rey, M.A.; Prasad, R.; Tailor, C.S. The C domain in the surface envelope glycoprotein of subgroup C feline leukemia virus is a second receptor-binding domain. Virology 2008, 370, 273–284. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Kronqvist, M.; Wallin, M.; Ekstrom, M.; Derse, D.; Garoff, H. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 2008, 82, 7135–7143. [Google Scholar] [CrossRef]
- Delamarre, L.; Pique, C.; Pham, D.; Tursz, T.; Dokhelar, M.C. Identification of functional regions in the human T-cell leukemia virus type I SU glycoprotein. J. Virol. 1994, 68, 3544–3549. [Google Scholar] [CrossRef]
- Lamb, D.; Mirsaliotis, A.; Kelly, S.M.; Brighty, D.W. Basic residues are critical to the activity of peptide inhibitors of human T cell leukemia virus type 1 entry. J. Biol. Chem. 2009, 284, 6575–6584. [Google Scholar] [CrossRef] [PubMed]
- Mirsaliotis, A.; Nurkiyanova, K.; Lamb, D.; Woof, J.M.; Brighty, D.W. Conformation-specific antibodies targeting the trimer-of-hairpins motif of the human T-cell leukemia virus type 1 transmembrane glycoprotein recognize the viral envelope but fail to neutralize viral entry. J. Virol. 2007, 81, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Palker, T.J.; Riggs, E.R.; Spragion, D.E.; Muir, A.J.; Scearce, R.M.; Randall, R.R.; McAdams, M.W.; McKnight, A.; Clapham, P.R.; Weiss, R.A.; et al. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J. Virol. 1992, 66, 5879–5889. [Google Scholar] [CrossRef]
- Delamarre, L.; Rosenberg, A.R.; Pique, C.; Pham, D.; Dokhelar, M.C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 1997, 71, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Souche, S.; Vernant, J.C.; Smadja, D.; Vahlne, A.; Horal, P. Identification of novel neutralization-inducing regions of the human T cell lymphotropic virus type I envelope glycoproteins with human HTLV-I-seropositive sera. AIDS Res. Hum. Retrovir. 1994, 10, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baba, E.; Nakamura, M.; Tanaka, Y.; Kuroki, M.; Itoyama, Y.; Nakano, S.; Niho, Y. Multiple neutralizing B-cell epitopes of human T-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. A basis for the design of an HTLV-1 peptide vaccine. J. Immunol. 1993, 151, 1013–1024. [Google Scholar] [CrossRef]
- Kuroki, M.; Nakamura, M.; Itoyama, Y.; Tanaka, Y.; Shiraki, H.; Baba, E.; Esaki, T.; Tatsumoto, T.; Nagafuchi, S.; Nakano, S.; et al. Identification of new epitopes recognized by human monoclonal antibodies with neutralizing and antibody-dependent cellular cytotoxicity activities specific for human T cell leukemia virus type 1. J. Immunol. 1992, 149, 940–948. [Google Scholar] [CrossRef]
- Le Blanc, I.; Grange, M.P.; Delamarre, L.; Rosenberg, A.R.; Blot, V.; Pique, C.; Dokhelar, M.C. HTLV-1 structural proteins. Virus Res. 2001, 78, 5–16. [Google Scholar] [CrossRef]
- Johnston, E.R.; Albritton, L.M.; Radke, K. Envelope proteins containing single amino acid substitutions support a structural model of the receptor-binding domain of bovine leukemia virus surface protein. J. Virol. 2002, 76, 10861–10872. [Google Scholar] [CrossRef]
- Bruck, C.; Mathot, S.; Portetelle, D.; Berte, C.; Franssen, J.D.; Herion, P.; Burny, A. Monoclonal antibodies define eight independent antigenic regions on the bovine leukemia virus (BLV) envelope glycoprotein gp51. Virology 1982, 122, 342–352. [Google Scholar] [CrossRef]
- Sommerfelt, M.A.; Weiss, R.A. Receptor interference groups of 20 retroviruses plating on human cells. Virology 1990, 176, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Salzwedel, K.; Berger, E.A. Cooperative subunit interactions within the oligomeric envelope glycoprotein of HIV-1: functional complementation of specific defects in gp120 and gp41. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 12794–12799. [Google Scholar] [CrossRef] [PubMed]
- Mahieux, R.; Gessain, A. The human HTLV-3 and HTLV-4 retroviruses: New members of the HTLV family. Pathol. Biol. (Paris) 2009, 57, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, F.; Lafay, B.; Switzer, W.M.; Locatelli, S.; Mpoudi-Ngole, E.; Loul, S.; Heneine, W.; Delaporte, E.; Peeters, M. Identification and molecular characterization of new STLV-1 and STLV-3 strains in wild-caught nonhuman primates in Cameroon. Virology 2008, 371, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30. [Google Scholar] [CrossRef]
- Shimotohno, K.; Takahashi, Y.; Shimizu, N.; Gojobori, T.; Golde, D.W.; Chen, I.S.; Miwa, M.; Sugimura, T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 3101–3105. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Delamarre, L.; Preira, A.; Dokhelar, M.C. Analysis of functional conservation in the surface and transmembrane glycoprotein subunits of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 1998, 72, 7609–7614. [Google Scholar] [CrossRef]
- Feuer, G.; Green, P.L. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 2005, 24, 5996–6004. [Google Scholar] [CrossRef]
- Xie, L.; Green, P.L. Envelope is a major viral determinant of the distinct in vitro cellular transformation tropism of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 2005, 79, 14536–14545. [Google Scholar] [CrossRef]
- Milpied, P.; Renand, A.; Bruneau, J.; Mendes-da-Cruz, D.A.; Jacquelin, S.; Asnafi, V.; Rubio, M.T.; MacIntyre, E.; Lepelletier, Y.; Hermine, O. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1466–1471. [Google Scholar] [CrossRef]
- Nath, M.D.; Ruscetti, F.W.; Petrow-Sadowski, C.; Jones, K.S. Regulation of the cell-surface expression of an HTLV-I binding protein in human T cells during immune activation. Blood 2003, 101, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kinet, S.; Battini, J.L.; Kim, F.J.; Taylor, N.; Sitbon, M. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood 2003, 101, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Huang, Y.K.; Chevalier, S.A.; Afonso, P.V.; Petrow-Sadowski, C.; Bertolette, D.C.; Gessain, A.; Ruscetti, F.W.; Mahieux, R. The receptor complex associated with human T-cell lymphotropic virus type 3 (HTLV-3) Env-mediated binding and entry is distinct from, but overlaps with, the receptor complexes of HTLV-1 and HTLV-2. J. Virol. 2009, 83, 5244–5255. [Google Scholar] [CrossRef] [PubMed]
- Nerrienet, E.; Meertens, L.; Kfutwah, A.; Foupouapouognigni, Y.; Gessain, A. Molecular epidemiology of simian T-lymphotropic virus (STLV) in wild-caught monkeys and apes from Cameroon: a new STLV-1, related to human T-lymphotropic virus subtype F, in a Cercocebus agilis. J. Gen. Virol. 2001, 82, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.J.; Lavanya, M.; Gessain, A.; Gallego, S.; Battini, J.L.; Sitbon, M.; Courgnaud, V. Intrahost variations in the envelope receptor-binding domain (RBD) of HTLV-1 and STLV-1 primary isolates. Retrovirology 2006, 3, 29. [Google Scholar] [CrossRef]
- Vandamme, A.M.; Salemi, M.; Van Brussel, M.; Liu, H.F.; Van Laethem, K.; Van Ranst, M.; Michels, L.; Desmyter, J.; Goubau, P. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. J. Virol. 1998, 72, 4327–4340. [Google Scholar] [CrossRef]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Afonso, P.; Froment, A.; Gessain, A.; Mahieux, R. Human T-cell lymphotropic virus type 3: complete nucleotide sequence and characterization of the human tax3 protein. J. Virol. 2006, 80, 9876–9888. [Google Scholar] [CrossRef]




© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jones, K.S.; Lambert, S.; Bouttier, M.; Bénit, L.; Ruscetti, F.W.; Hermine, O.; Pique, C. Molecular Aspects of HTLV-1 Entry: Functional Domains of the HTLV-1 Surface Subunit (SU) and Their Relationships to the Entry Receptors. Viruses 2011, 3, 794-810. https://doi.org/10.3390/v3060794
Jones KS, Lambert S, Bouttier M, Bénit L, Ruscetti FW, Hermine O, Pique C. Molecular Aspects of HTLV-1 Entry: Functional Domains of the HTLV-1 Surface Subunit (SU) and Their Relationships to the Entry Receptors. Viruses. 2011; 3(6):794-810. https://doi.org/10.3390/v3060794
Chicago/Turabian StyleJones, Kathryn S., Sophie Lambert, Manuella Bouttier, Laurence Bénit, Frank W. Ruscetti, Olivier Hermine, and Claudine Pique. 2011. "Molecular Aspects of HTLV-1 Entry: Functional Domains of the HTLV-1 Surface Subunit (SU) and Their Relationships to the Entry Receptors" Viruses 3, no. 6: 794-810. https://doi.org/10.3390/v3060794