Biological Invasions of Geminiviruses: Case Study of TYLCV and Bemisia tabaci in Reunion Island
Abstract
:1. Introduction
2. Discussion
2.1. Emergence of Geminivirus Diseases in Agricultural Settings
2.1.1. Maize Streak Disease in Africa
2.1.2. The African Cassava Mosaic Disease Pandemic
2.2. The Worldwide Emergence of a Whitefly Pest and Plant Virus Vector
2.2.1. The Bemisia Tabaci Species Complex
2.2.2. Shifts in Whitefly Populations
2.2.3. Whitefly Population Differentiation Based on Endosymbionts
2.2.4. Involvement of Endosymbionts in the Survival of Begomoviruses in their Insect Vector
2.3. The Worldwide Spread of TYLCV
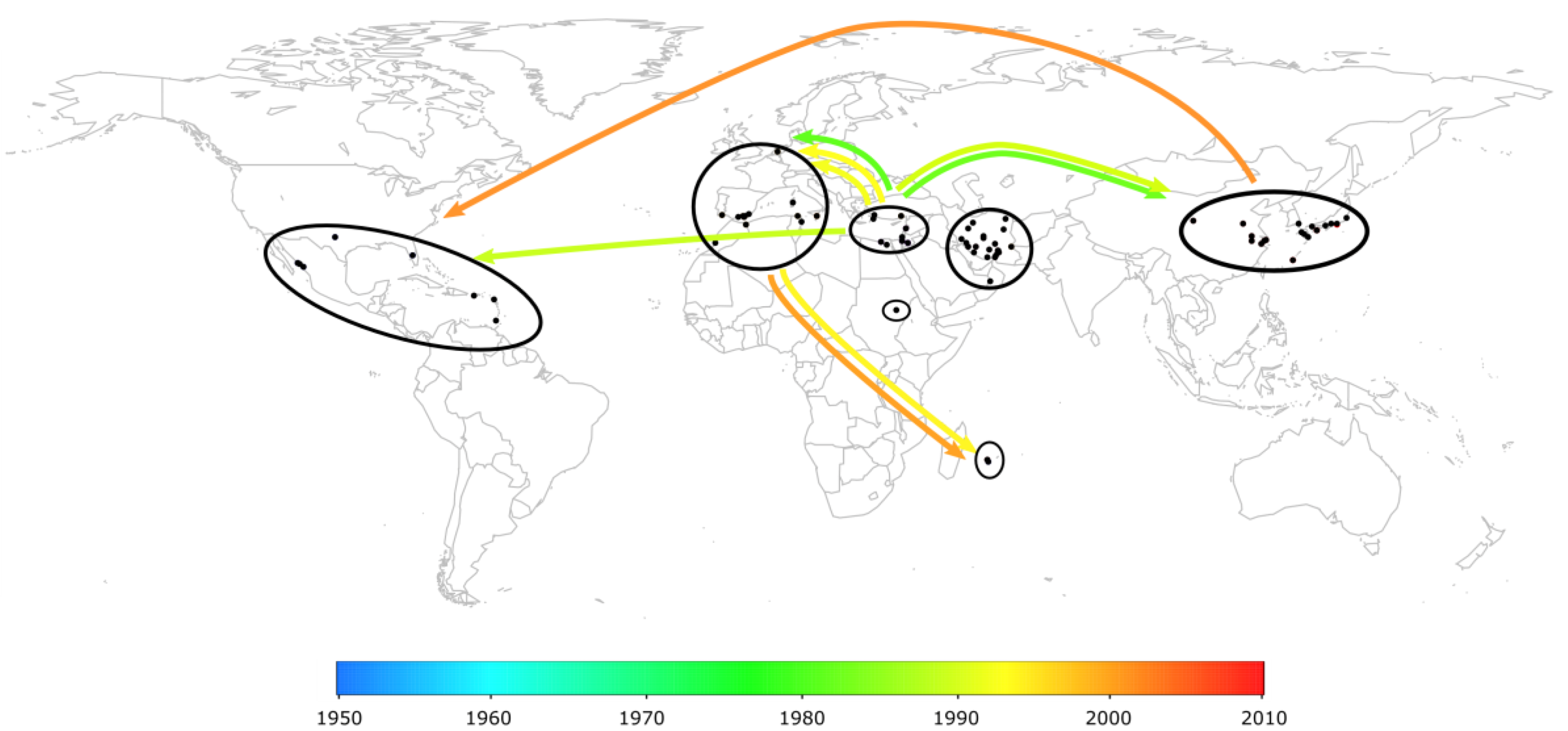
2.4. Case Study in Reunion Island
2.4.1. Invasion of the World Invasive MEAM1 Species of Bemisia Tabaci

2.4.2. Co-Existence of Invasive and Indigenous Species and Hybrids of Bemisia Tabaci
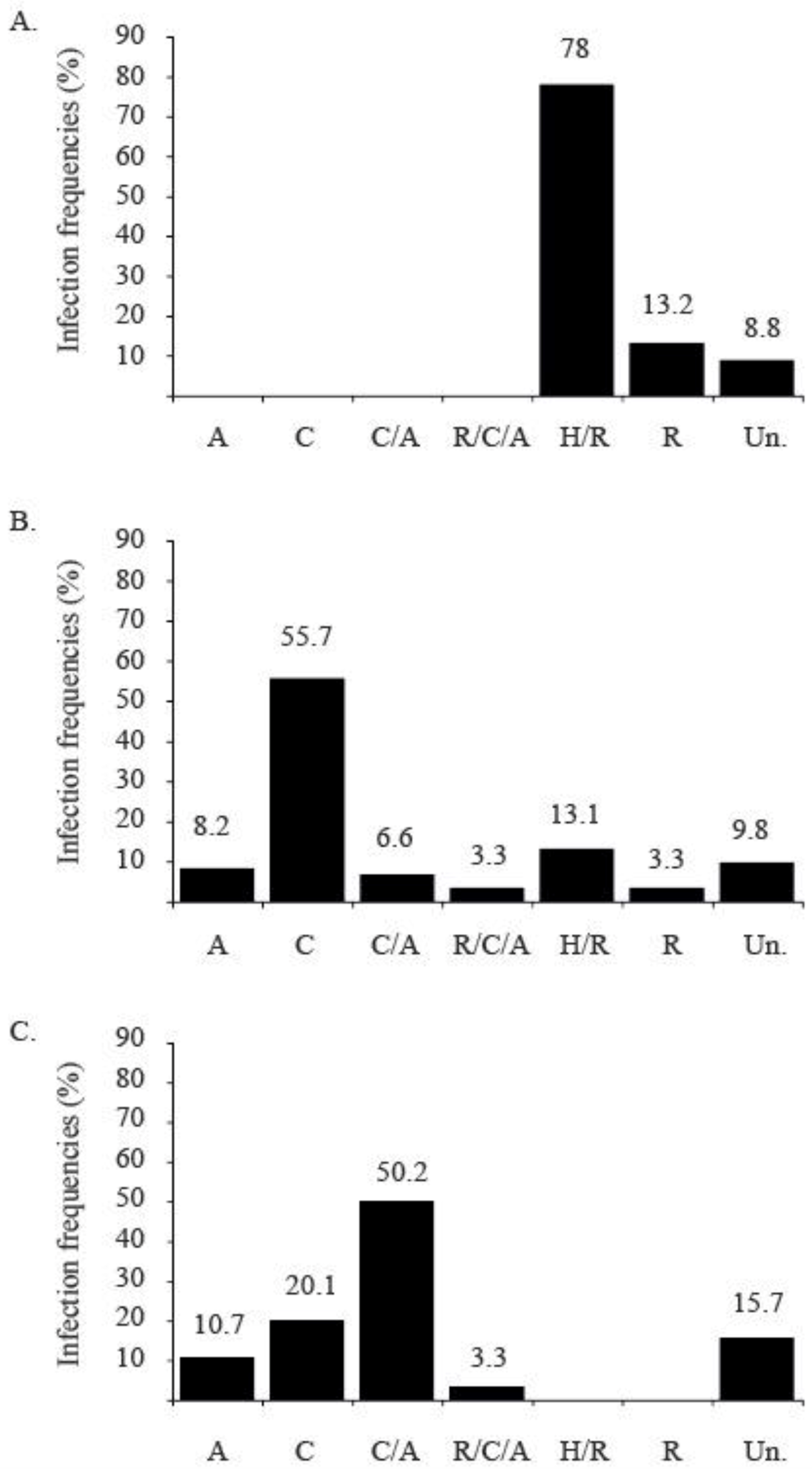
2.4.3. Successive Introduction of TYLCV Strains
3. Major Conclusions and Perspectives
Conflict of Interest
References
- Fargette, D.; Konaté, G.; Fauquet, C.; Muller, E.; Peterschmitt, M.; Thresh, J.M. Molecular ecology and emergence of tropical plant viruses. Ann. Rev. Phytopathol. 2006, 44, 35–260. [Google Scholar]
- Sax, D.F.; Brown, J.H. The paradox of invasion. Global Ecol. Biogeogr. 2000, 9, 363–371. [Google Scholar] [CrossRef]
- Gillespie, R.G.; Roderick, G.K. Arthropods on islands: Colonization, speciation, and conservation. Ann. Rev. Entomol. 2002, 47, 595–632. [Google Scholar] [CrossRef]
- Gillespie, R.G.; Claridge, E.M.; Roderick, G.K. Biodiversity dynamics in isolated island communities: Interaction between natural and human-mediated processes. Mol. Ecol. 2008, 17, 45–57. [Google Scholar]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Harlan, J.R. Agricultural origins: Centers and noncenters. Science 1971, 174, 468–474. [Google Scholar]
- Balter, M. Seeking agriculture's ancient roots. Science 2007, 316, 1830–1835. [Google Scholar] [CrossRef]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; Tsomondo, T. Economic and environmental threats of alien plant, animal, and microbe invasions. Agr. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H.; Ericson, L. The current and future dynamics of disease in plant communities. Ann. Rev. Phytopathol. 2006, 44, 19–39. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Salam, M.U.; Maling, T.J.; Diggle, A.J.; Thackray, D.J. Principles or predicting plant virus disease epidemics. Ann. Rev. Phytopathol. 2010, 48, 179–203. [Google Scholar] [CrossRef]
- Thrall, P.H.; Burdon, J.J. The spatial scale of pathogen dispersal: Consequences for disease dynamics and persistence. Evol. Ecol. Res. 1999, 1, 681–701. [Google Scholar]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Martin, D.; Shepherd, D. The epidemiology, economic impact and control of maize streak disease. Food Secur. 2009, 1, 305–315. [Google Scholar] [CrossRef]
- Shepherd, D.N.; Martin, D.P.; van der Walt, E.; Dent, K.; Varsani, A.; Rybicki, E.P. Maize streak virus: An old and complex ‘emerging’ pathogen. Mol. Plant Pathol. 2010, 11, 1–12. [Google Scholar]
- Lefeuvre, P.; Martin, D.P.; Hoareau, M.; Naze, F.; Delatte, H.; Thierry, M.; Varsani, A.; Becker, N.; Reynaud, B.; Lett, J.M. Begomovirus 'melting pot' in the south-west Indian Ocean islands: Molecular diversity and evolution through recombination. J. Gen. Virol. 2007, 88, 3458–3468. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Lett, J.M.; Varsani, A.; Martin, D.P. Widely conserved recombination patterns among single-stranded DNA viruses. J. Virol. 2009, 83, 2697–2707. [Google Scholar] [CrossRef]
- Harkins, G.W.; Martin, D.P.; Duffy, S.; Monjane, A.L.; Shepherd, D.N.; Windram, O.P.; Owor, B.E.; Donaldson, L.; van Antwerpen, T.; Sayed, R.A.; et al. Dating the origins of the maize-adapted strain of maize streak virus, MSV-A. J. Gen. Virol. 2009, 90, 3066–3074. [Google Scholar]
- Varsani, A.; Shepherd, D.N.; Monjane, A.L.; Owor, B.E.; Erdmann, J.B.; Rybicki, E.P.; Peterschmitt, M.; Briddon, R.W.; Markham, P.G.; Oluwafemi, S.; et al. Recombination, decreased host specificity and increased mobility may have driven the emergence of maize streak virus as an agricultural pathogen. J. Gen. Virol. 2008, 89, 2063–2074. [Google Scholar]
- Monjane, A.L.; Harkins, G.W.; Martin, D.P.; Lemey, P.; Lefeuvre, P.; Shepherd, D.N.; Oluwafemi, S.; Simuyandi, M.; Zinga, I.; Komba, E.K.; et al. Reconstructing the history of maize streak virus strain a dispersal to reveal diversification hot spots and its origin in Southern Africa. J. Virol. 2011, 85, 9623–9636. [Google Scholar]
- Fauquet, C.M.; Briddon, R.W.; Brown, J.K.; Moriones, E.; Stanley, J.; Zerbini, M.; Zhou, X. Geminivirus strain demarcation and nomenclature. Arch. Virol. 2008, 153, 783–821. [Google Scholar]
- Patil, B.L.; Fauquet, C.M. Cassava mosaic geminiviruses: Actual knowledge and perspectives. Mol. Plant Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef]
- Legg, J.P.; Thresh, J.M. Cassava mosaic virus disease in East Africa: A dynamic disease in a changing environment. Virus Res. 2000, 71, 135–149. [Google Scholar] [CrossRef]
- Legg, J.P.; Fauquet, C.M. Cassava mosaic geminiviruses in Africa. Plant Mol. Biol. 2004, 56, 585–599. [Google Scholar] [CrossRef]
- Legg, J.P.; Ogwal, S. Changes in the incidence of African cassava mosaic virus disease and the abundance of its whitefly vector along south-north transects in Uganda. J. Appl. Entomol. 1998, 122, 169–178. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Calvert, L.; Munoz, C.; Otim-Nape, G.W.; Robinson, D.J.; Harrison, B.D. Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 1997, 78, 2101–2111. [Google Scholar]
- Pita, J.S.; Fondong, V.N.; Sangaré, A.; Otim-Nape, G.W.; Ogwal, S.; Fauquet, C.M. Recombination, pseudorecombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J. Gen. Virol. 2001, 82, 655–665. [Google Scholar]
- Legg, J.P.; French, R.; Rogan, D.; Okao-Okuja, G.; Brown, J.K. A distinct Bemisia tabaci (Gennadius) (Hemiptera: Sternorrhyncha: Aleyrodidae) genotype cluster is associated with the epidemic of severe cassava mosaic virus disease in Uganda. Mol. Ecol. 2002, 11, 1219–1229. [Google Scholar] [CrossRef]
- Vanitharani, R.; Chellappan, P.; Pita, J.S.; Fauquet, C.M. Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J. Virol. 2004, 78, 9487–9498. [Google Scholar] [CrossRef]
- Berry, S.; Rey, M.E.C. Molecular evidence for diverse populations of cassava-infecting begomoviruses in southern Africa. Arch. Virol. 2001, 146, 1795–1802. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Lefeuvre, P.; Hoareau, M.; Traoré, V.S.E.; Barro, N.; Reynaud, B.; Traoré, A.S.; Konaté, G.; Traoré, O.; Lett, J.-M. Occurrence of East African cassava mosaic virus-Uganda (EACMV-UG) in Burkina Faso. Plant Pathol. 2009, 58, 783–783. [Google Scholar]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Ann. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- De Barro, J.; Liu, S.; Boykin, L.; Dinsdale, A. Bemisia tabaci: A statement of species status. Ann. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; Barro, P.D. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Liu, S.S.; Colvin, J.; De Barro, P.J. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there? J. Integr. Agr. 2012, 11, 176–186. [Google Scholar]
- Hu, J.; De Barro, P.; Zhao, H.; Wang, J.; Nardi, F.; Liu, S.S. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS One 2011, 6, e16061. [Google Scholar]
- Boykin, L.M.; Shatters, R.G.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemipter: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Available online: www.issg.org/database/welcome (accessed on 10 October 2012).
- Brown, J.K. Phylogenetic Biology of the Bemisia tabaci Sibling Species Group. In Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Amsterdam, The Netherlands, 2010; pp. 31–67. [Google Scholar]
- Varma, A.; Malathi, V.G. Emerging geminivirus problems: A serious threat to crop production. Ann. Appl. Biol. 2003, 142, 145–164. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195. [Google Scholar] [CrossRef]
- Cheek, S.; Macdonald, O. Extended summaries sci pesticides group symposium management of Bemisia tabaci. Pestic. Sci. 1994, 42, 135–142. [Google Scholar] [CrossRef]
- Dalton, R. Whitefly infestations: The Christmas Invasion. Nature 2006, 443, 898–900. [Google Scholar] [CrossRef]
- Moya, A.; Guirao, P.; Cifuentes, D.; Beitia, F.; Cenis, J.L. Genetic diversity of Iberian populations of Bemisia tabaci (Hemiptera: Aleyrodidae) based on random amplified polymorphic DNA-polymerase chain reaction. Mol. Ecol. 2001, 10, 891–897. [Google Scholar] [CrossRef]
- Luo, C.; Jones, C.M.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. 2005, 58, 216–225. [Google Scholar] [CrossRef]
- Horowitz, A.; Denholm, I.; Gorman, K.; Cenis, J.L.; Kontsedalov, S.; Ishaaya, I. Biotype Q of Bemisia tabaci identified in Israel. Phytoparasitica 2003, 31, 94–98. [Google Scholar] [CrossRef]
- Simon, B.; Cenis, J.L.; De La Rua, P. Distribution patterns of the Q and B biotypes of Bemisia tabaci in the Mediterranean Basin based on microsatellite variation. Entomol. Exp. Appl. 2007, 124, 327–336. [Google Scholar] [CrossRef]
- Gueguen, G.; Vavre, F.; Gnankine, O.; Peterschmitt, M.; Charif, D.; Chiel, E.; Gottlieb, Y.; Ghanim, M.; Zchori-Fein, E.; Fleury, F. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 2010, 19, 4365–4376. [Google Scholar] [CrossRef]
- Gnankiné, O.; Mouton, L.; Henri, H.; Terraz, G.; Houndeté, T.; Martin, T.; Vavre, F.; Fleury, F. Distribution of Bemisia tabaci (Homoptera: Aleyrodidae) biotypes and their associated symbiotic bacteria on host plants in West Africa. Insect Conserv. Diver. 2012. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhang, Y.J.; Zhang, W.J.; Wu, Q.J.; Xu, B.Y.; Chu, D. Analysis of genetic diversity among different geographical populations and determination of biotypes of Bemisia tabaci in China. J. Appl. Entomol. 2005, 129, 121–128. [Google Scholar] [CrossRef]
- Ueda, S.; Brown, J.K. First report of the Q biotype of Bemisia tabaci in Japan by mitochondrial cytochrome oxidase I sequence analysis. Phytoparasitica 2006, 34, 405–411. [Google Scholar] [CrossRef]
- Brown, J. The Bemisia tabaci Complex: Genetic and Phenotypic Variability Drives Begomovirus Spread and Virus Diversification. Available online: http://www.apsnet.org/online/feature/btabaci/ (accessed on 10 October 2012).
- Liu, S.-S.; De Barro, P.J.; Xu, J.; Luan, J.-B.; Zang, L.-S.; Ruan, Y.-M.; Wan, F.-H. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Chu, D.; Wan, F.H.; Zhang, Y.J.; Brown, J.K. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ. Entomol. 2010, 39, 1028–1036. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Abu-Moch, F.; Lebedev, G.; Czosnek, H.; Horowitz, A.R.; Ghanim, M. Bemisia tabaci biotype dynamics and resistance to insecticides in Israel during the years 2008–2010. J. Integr. Agr. 2012, 11, 312–320. [Google Scholar]
- Crowder, D.W.; Horowitz, A.R.; Breslauer, H.; Rippa, M.; Kontsedalov, S.; Ghanim, M.; Carriere, Y. Niche partitioning and stochastic processes shape community structure following whitefly invasions. Basic Appl. Ecol. 2011, 12, 685–694. [Google Scholar] [CrossRef]
- Pascual, S. Mechanisms in competition, under laboratory conditions, between Spanish biotypes B and Q of Bemisia tabaci (Gennadius). Span. J. Agric. Res. 2006, 4, 351–354. [Google Scholar]
- Muniz, M. Host suitability of two biotypes of Bemisia tabaci on some common weeds. Entomol. Exp. Appl. 2000, 95, 63–70. [Google Scholar]
- Muniz, M.; Nombela, G. Differential variation in development of the B- and Q-biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on sweet pepper at constant temperatures. Environ. Entomol. 2001, 30, 720–727. [Google Scholar] [CrossRef]
- Elbaz, M.; Lahav, N.; Morin, S. Evidence for pre-zygotic reproductive barrier between the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 2010, 100, 581–590. [Google Scholar] [CrossRef]
- Sun, D.B.; Xu, J.; Luan, J.B.; Liu, S.S. Reproductive incompatibility between the B and Q biotypes of the whitefly Bemisia tabaci in China: Genetic and behavioural evidence. B. Entomol. Res. 2010, 101, 211–220. [Google Scholar]
- Wang, X.W.; Luan, J.B.; Li, J.M.; Su, Y.L.; Xia, J.; Liu, S.S. Transcriptome analysis and comparison reveal divergence between two invasive whitefly cryptic species. BMC Genom. 2011, 12, 458. [Google Scholar] [CrossRef]
- Seal, S.; Patel, M.V.; Collins, C.; Colvin, J.; Bailey, D. Next generation transcriptome sequencing and quantitative real-time PCR technologies for characterisation of the bemisia tabaci Asia 1 mtCOI Phylogenetic Clade. J. Integr. Agr. 2012, 11, 281–292. [Google Scholar]
- Guillemaud, T.; Ciosi, M.; Lombaert, É.; Estoup, A. Biological invasions in agricultural settings: Insights from evolutionary biology and population genetics. C. R. Biol. 2011, 334, 237–246. [Google Scholar] [CrossRef]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Chiel, E.; Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Katzir, N.; Inbar, M.; Ghanim, M. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull. Entomol. Res. 2007, 97, 407. [Google Scholar] [CrossRef]
- Everett, K.D.; Thao, M.; Horn, M.; Dyszynski, G.E.; Baumann, P. Novel chlamydiae in whiteflies and scale insects: Endosymbionts 'Candidatus Fritschea bemisiae' strain Falk and 'Candidatus Fritschea eriococci' strain Elm. Int. J. Syst. Evol. Micr. 2005, 55, 1581–1587. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, A.R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microb. 2006, 72, 3646–3652. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Brown, J.K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2002, 95, 711–718. [Google Scholar] [CrossRef]
- Chiel, E.; Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Katzir, N.; Inbar, M.; Ghanim, M. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull. Entomol. Res. 2007, 97, 407–413. [Google Scholar] [CrossRef]
- Skaljac, M.; Zanic, K.; Ban, S.G.; Kontsedalov, S.; Ghanim, M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 2010, 10, 142. [Google Scholar] [CrossRef]
- Thierry, M.; Becker, N.; Hajri, A.; Reynaud, B.; Lett, J.M.; Delatte, H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011, 20, 2172–2187. [Google Scholar] [CrossRef]
- Engelstadter, J.; Hurst, G.D.D. The ecology and evolution of microbes that manipulate host reproduction. Ann. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Ann. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; Hunter, M.S. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011, 332, 254–256. [Google Scholar]
- Zchori-Fein, E.; Gottlieb, Y.; Kelly, S.E.; Brown, J.K.; Wilson, J.M.; Karr, T.L.; Hunter, M.S. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. P. Natl. Acad. Sci. USA 2001, 98, 12555–12560. [Google Scholar]
- Zchori-Fein, E.; Perlman, S.J.; Kelly, S.E.; Katzir, N.; Hunter, M.S. Characterization of a 'Bacteroidetes' symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of 'Candidatus Cardinium hertigii'. Int. J. Syst. Evol. Microbiol. 2004, 54, 961–968. [Google Scholar] [CrossRef]
- Hunter, M.S.; Perlman, S.J.; Kelly, S.E. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. B. 2003, 270, 2185–2190. [Google Scholar] [CrossRef]
- Scarborough, C.L.; Ferrari, J.; Godfray, H.C.J. Aphid protected from pathogen by endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. R. Soc. B 2008, 275, 353–361. [Google Scholar] [CrossRef]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. P. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar]
- Ghanim, M.; Kontsedalov, S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag. Sci. 2009, 65, 939–942. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef]
- Hinrich, J.; Schulenburg, G.V.D.; Hurst, G.D.D.; Tetzlaff, D.; Booth, G.E.; Zakharov, I.A.; Majerus, M.E.N. History of infection with different male-killing bacteria in the two-spot ladybird beetle Adalia bipunctata revealed through mitochondrial DNA sequence analysis. Genetics 2002, 160, 1075–1086. [Google Scholar]
- Turelli, M.; Hoffmann, A.A. Rapid spread of an inherited incompatibility factor in California drosophila. Nature 1991, 353, 440–442. [Google Scholar] [CrossRef]
- Caspi-Fluger, A.; Inbar, M.; Mozes-Daube, N.; Mouton, L.; Hunter, M.S.; Zchori-Fein, E. Rickettsia 'in' and 'out': Two different localization patterns of a bacterial symbiont in the same insect species. PLoS One 2011, 6, e21096. [Google Scholar]
- Caspi-Fluger, A.; Inbar, M.; Mozes-Daube, N.; Katzir, N.; Portnoy, V.; Belausov, E.; Hunter, M.S.; Zchori-Fein, E. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc Biol Sci 2012, 279, 1791–1796. [Google Scholar]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Science 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Czosnek, H.; Ghanim, M. Back to basics: Are begomoviruses whitefly pathogens? J. Integr. Agr. 2012, 11, 225–234. [Google Scholar]
- Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F.; Ghanim, M. The transmission efficiency of To mato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 2010, 84, 9310–9317. [Google Scholar]
- Rana, V.S.; Singh, S.T.; Priya, N.G.; Kumar, J.; Rajagopal, R. Arsenophonus GroEL interacts with CLCuV and is localized in midgut and salivary gland of whitefly B. tabaci. PLoS One 2012, 7, e42168. [Google Scholar]
- Morin, S.; Ghanim, M.; Zeidan, M.; Czosnek, H.; Verbeek, M.; van den Heuvel, J. A GroEL homologue from endosymbiotic bacteria of the whitefly Bemisia tabaci is implicated in the circulative transmission of Tomato yellow leaf curl virus. Virology 1999, 256, 75. [Google Scholar] [CrossRef]
- Morin, S.; Ghanim, M.; Sobol, I.; Czosnek, H. The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 2000, 276, 404–416. [Google Scholar] [CrossRef]
- Azzam, O.; Frazer, J.; de la Rosa, D.; Beaver, J.S.; Ahlquist, P.; Maxwell, D.P. Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 1994, 204, 289–296. [Google Scholar] [CrossRef]
- Briddon, R.W.; Pinner, M.S.; Stanley, J.; Markham, P.G. Geminivirus coat protein gene replacement alters insect specificity. Virology 1990, 177, 85–94. [Google Scholar] [CrossRef]
- Hofer, P.; Bedford, I.D.; Markham, P.G.; Jeske, H.; Frischmuth, T. Coat protein gene replacement results in whitefly transmission of an insect nontransmissible geminivirus isolate. Virology 1997, 236, 288–295. [Google Scholar]
- Noris, E.; Vaira, A.M.; Caciagli, P.; Masenga, V.; Gronenborn, B.; Accotto, G.P. Amino acids in the capsid protein of Tomato yellow leaf curl virus that are crucial for systemic infection, particle formation, and insect transmission. J. Virol. 1998, 72, 10050–10057. [Google Scholar]
- Delatte, H.; Martin, D.P.; Naze, F.; Goldbach, R.; Reynaud, B.; Peterschmitt, M.; Lett, J.-M. South West Indian Ocean islands tomato begomovirus populations represent a new major monopartite begomovirus group. J. Gen. Virol. 2005, 86, 1533–1542. [Google Scholar] [CrossRef]
- Xie, W.; Meng, Q.S.; Wu, Q.J.; Wang, S.L.; Yang, X.; Yang, N.N.; Li, R.M.; Jiao, X.G.; Pan, H.P.; Liu, B.M.; et al. Pyrosequencing the Bemisia tabaci transcriptome reveals a highly diverse bacterial community and a robust system for insecticide resistance. PLoS One 2012, 7, e35181. [Google Scholar]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging viral diseases of tomato crops. Mol. Plant Microbe Int. 2010, 23, 539–548. [Google Scholar]
- Moriones, E.; Navas-Castillo, J.; Díaz-Pendón, J.A. Emergence of Begomoviruses Diseases. In Recent Advances in Plant Virology; Caranta, C., Aranda, M.A., Tepfer, M., Lopez-Moya, J.J., Eds.; Caister Academic Press: Nortfolk, United Kingdom, 2011; pp. 301–320. [Google Scholar]
- Nakhla, M.K.; Maxwell, D.P.; Martinez, R.T.; Carvalho, M.G.; Gilbertson, R.L. Widespread occurrence of the eastern mediterranean strain of tomato yellow leaf curl geminivirus in tomatoes in the Dominican Republic. Plant Dis. 1994, 78, 926–926. [Google Scholar]
- Picó, B. Viral diseases causing the greatest economic losses to the tomato crop. II. The Tomato yellow leaf curl virus—A review. Sci. Hortic. 1996, 67, 151–196. [Google Scholar]
- Cohen, S.; Lapidot, M. Appearance and Expansion of TYLCV: A Historical Point of View. In Tomato yellow leaf curl disease; Czosnek, H., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 3–12. [Google Scholar]
- Polston, J.E.; Anderson, P.K. The emergence of whitefly-transmitted geminiviruses in tomato in the western hemisphere. Plant Dis. 1997, 81, 1358–1369. [Google Scholar] [CrossRef]
- Duffy, S.; Holmes, E.C. Multiple introductions of the Old World begomovirus Tomato yellow leaf curl virus into the New World. Appl. Environ. Microb. 2007, 73, 7114–7117. [Google Scholar] [CrossRef]
- Lett, J.M.; Péréfarres, F.; Hoareau, M.; Lefeuvre, P.; De Bruyn, A.; Dottin, M.; Prior, P.; Wicker, E.; Umaharan, P. Tomatoes showing yellow leaf curl symptoms in the island of Grenada exhibit an infection with Tomato yellow leaf curl virus either alone or in combination with Potato yellow mosaic virus. New Disease Reports 2011, 24, 19–19. [Google Scholar] [CrossRef] [Green Version]
- Kheyr-Pour, A.; Bendahmane, M.; Matzeit, V. Tomato yellow leaf curl virus from sardinia is a whitefly-transmitted monoparatite geminivirus. Nucleic Acids Res. 1991, 19, 6763–6769. [Google Scholar] [CrossRef]
- Navot, N.; Pichersky, E.; Zeidan, M.; Zamir, D.; Czosnek, H. Tomato yellow leaf curl virus: A whitefly-transmitted geminivirus with a single genomic component. Virology 1991, 185, 151–161. [Google Scholar] [CrossRef]
- Antignus, Y.; Cohen, S. Cloning of tomato yellow leaf curl virus (TYLCV) and the complete nucleotide sequence of a mild infectious clone. Phytopathology 1994, 84, 707–712. [Google Scholar] [CrossRef]
- Abhary, M.; Patil, B.; Fauquet, C. Molecular Biodiversity, Taxonomy, and Nomenclature of Tomato Yellow Leaf Curl-like Viruses. In Tomato yellow leaf curl disease; Czosnek, H., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 85–118. [Google Scholar]
- Fauquet, C.M.; Sawyer, S.; Idris, A.M.; Brown, J.K. Sequence analysis and classification of apparent recombinant begomoviruses infecting tomato in the Nile and Mediterranean Basins. Phytopathology 2005, 95, 549–555. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Harkins, G.; Lemey, P.; Gray, A.J.A.; Meredith, S.; Lakay, F.; Monjane, A.; Lett, J.-M.; Varsani, A.; Heydarnejad, J. The spread of Tomato eellow leaf curl virus from the Middle East to the world. PLoS Pathogens 2010, 6, e1001164–e1001164. [Google Scholar] [CrossRef] [Green Version]
- Lombaert, E.; Guillemaud, T.; Cornuet, J.-M.; Malausa, T.; Facon, B.; Estoup, A. Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE 2010, 5, e9743–e9743. [Google Scholar]
- Facon, B.; Genton, B.J.; Shykoff, J.; Jarne, P.; Estoup, A.; David, P. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol. Evol. 2006, 21, 130–135. [Google Scholar] [CrossRef]
- Bourriquet, G. Note concernant les maladies des plantes cultivées à La Réunion. Revue Agricole Réunion 1938, 43, 33–38. [Google Scholar]
- Luziau, R. Contribution à la prospection phytosanitaire de l'île de la Réunion. Phytoma 1953, 6, 13–19. [Google Scholar]
- Peterschmitt, M.; Granier, M.; Mekdoud, R.; Dalmon, A.; Gambin, O.; Vayssieres, J.F.; Reynaud, B. First report of Tomato Yellow Leaf Curl virus in Réunion Island. Plant Dis. 1999, 83, 303–303. [Google Scholar]
- Reynaud, B.; Wuster, G.; Delatte, H.; Soustrade, I.; Lett, J.-M.; Gambin, O.; Peterschmitt, M. Les maladies à bégomovirus chez la tomate dans les départements français d'Outre-Mer—Le Tomato yellow leaf curl virus (TYLCV) à la Réunion. Phytoma, la défense des végétaux 2003, 13–17. [Google Scholar]
- Delatte, H.; Lett, J.-M.; Lefeuvre, P.; Reynaud, B.; Peterschmitt, M. An Insular Environment before and after TYLCV Introduction. In Tomato yellow leaf curl disease; Czosnek, H., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 13–23. [Google Scholar]
- Delatte, H.; Holota, H.; Warren, B.H.; Becker, N.; Thierry, M.; Reynaud, B. Genetic diversity, geographical range and origin of Bemisia tabaci biotype Ms. Bull. Ent. Res. 2011, 101, 487–497. [Google Scholar] [CrossRef]
- Delatte, H.; David, P.; Granier, M.; Lett, J.M.; Goldbach, R.; Peterschmitt, M.; Reynaud, B. Microsatellites reveal the coexistence and genetic relationships between invasive and indigenous whitefly biotypes in an insular environment. Genet. Res. 2006, 87, 109–124. [Google Scholar] [CrossRef]
- Thierry, M.; Bile, A.; Grondin, M.; Reynaud, B.; Becker, N.; Delatte, H. Evidence from mitochondrial and endosymbiotic data of the Bemisia tabaci Q biotype introduction in La Réunion. 2012. submitted. [Google Scholar]
- Rimbaud, L.; Cottineau, J.-S.; Avril, J.-P.; Sorres, V.; Festin, C.; Minatchy, J.; Suzanne, W.; Leroux, K.; Tilma, P.; Maillary, L.; et al. La tomate face au TYLCV à la Réunion. Phytoma 2012, 650, 48–52. [Google Scholar]
- Delatte, H.; Holota, H.; Moury, B.; Reynaud, B.; Lett, J-M.; Peterschmitt, M. Evidence for a founder effect after introduction of Tomato yellow leaf curl virus-mild in an insular environment. J. Mol. Evol. 2007, 65, 112–118. [Google Scholar] [CrossRef]
- Delatte, H.; Holota, H.; Naze, F.; Peterschmitt, M.; Reynaud, B.; Lett, J.M. The presence of both recombinant and non recombinant strains of Tomato yellow leaf curl virus on tomato in Réunion Island. Plant Pathol. 2005, 54, 262. [Google Scholar] [CrossRef]
- Sánchez-Campos, S.; Navas-Castillo, J.; Camero, R.; Soria, C.; Díaz, J.A.; Moriones, E. Displacement of Tomato yellow leaf curl virus (TYLCV)-Sr by TYLCV-Is in Tomato epidemics in Spain. Phytopathology 1999, 89, 1038–1043. [Google Scholar] [CrossRef]
- Davino, S.; Napoli, C.; Davino, M.; Accotto, G.P. Spread of Tomato yellow leaf curl virus in Sicily: Partial displacement of another geminivirus originally present. Eur. J. Plant Pathol. 2006, 114, 293–299. [Google Scholar] [CrossRef]
- Monci, F.; Sanchez-Campos, S.; Navas-Castillo, J.; Moriones, E. A natural recombinant between the geminiviruses Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl virus exhibits a novel pathogenic phenotype and is becoming prevalent in Spanish populations. Virology 2002, 303, 317–326. [Google Scholar] [CrossRef]
- García-Andrés, S.; Accotto, G.P.; Navas-Castillo, J.; Moriones, E. Founder effect, plant host, and recombination shape the emergent population of begomoviruses that cause the tomato yellow leaf curl disease in the Mediterranean basin. Virology 2007, 359, 302–312. [Google Scholar] [CrossRef]
- Davino, S.; Napoli, C.; Dellacroce, C.; Miozzi, L.; Noris, E.; Davino, M.; Accotto, G.P. Two new natural begomovirus recombinants associated with the tomato yellow leaf curl disease co-exist with parental viruses in tomato epidemics in Italy. Virus Res. 2009, 143, 15–23. [Google Scholar] [CrossRef]
- Péréfarres, F.; Lefeuvre, P.; Hoareau, M.; Thierry, M.; Becker, N.; Reynaud, B.; Dintinger, J.; Lett, J.-M. Rapid displacement as a result of interaction between strains of TYLCV in Reunion Island. Acta Horticulturae 2011, 914, 197–201. [Google Scholar]
- Péréfarres, F.; Thébaud, G.; Lefeuvre, P.; Rimbaud, L.; Hoareau, M.; Chiroleu, F.; Reynaud, B.; Lett, J.-M. Long-term viral competition monitoring: A case of epidemiological rescue. 2012; in preparation. [Google Scholar]
- Castillo-Urquiza, G.P.; Beserra, J.E.A.; Bruckner, F.P.; Lima, A.T.M.; Varsani, A.; Alfenas-Zerbini, P.; Murilo Zerbini, F. Six novel begomoviruses infecting tomato and associated weeds in southeastern Brazil. Arch. Virol. 2008, 153, 1985–1989. [Google Scholar] [CrossRef]
- Fernandes, F.R.; de Albuquerque, L.C.; de Britto Giordano, L.; Boiteux, L.S.; de Avila, A.C.; Inoue-Nagata, A.K. Diversity and prevalence of Brazilian bipartite begomovirus species associated to tomatoes. Virus Genes 2008, 36, 251–258. [Google Scholar] [CrossRef]
- Martin, D.P.; Biagini, P.; Lefeuvre, P.; Golden, M.; Roumagnac, P.; Varsani, A. Recombination in eukaryotic single stranded DNA viruses. Viruses 2011, 3, 1699–1738. [Google Scholar] [CrossRef] [Green Version]
- Rey, M.E.C.; Ndunguru, J.; Berrie, L.C.; Paximadis, M.; Berry, S.; Cossa, N.; Nuaila, V.N.; Mabasa, K.G.; Abraham, N.; Rybicki, E.P.; et al. Diversity of dicotyledenous-infecting geminiviruses and their associated DNA molecules in Southern Africa, including the south-west Indian Ocean islands. Viruses 2012, 4, 1753–1791. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Sánchez-Campos, S.; Noris, E.; Louro, D.; Accotto, G.P.; Moriones, E. Natural recombination between Tomato yellow leaf curl virus—Is and Tomato leaf curl virus. J. Gen. Virol. 2000, 81, 2797–2801. [Google Scholar]
- Jones, R.A.C. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef]
- Polston, J.E.; McGovern, R.J.; Brown, L.G. Introduction of Tomato yellow leaf curl virus in Florida and implications for the spread of this and other geminiviruses of tomato. Plant Dis. 1999, 83, 984–988. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Péréfarres, F.; Thierry, M.; Becker, N.; Lefeuvre, P.; Reynaud, B.; Delatte, H.; Lett, J.-M. Biological Invasions of Geminiviruses: Case Study of TYLCV and Bemisia tabaci in Reunion Island. Viruses 2012, 4, 3665-3688. https://doi.org/10.3390/v4123665
Péréfarres F, Thierry M, Becker N, Lefeuvre P, Reynaud B, Delatte H, Lett J-M. Biological Invasions of Geminiviruses: Case Study of TYLCV and Bemisia tabaci in Reunion Island. Viruses. 2012; 4(12):3665-3688. https://doi.org/10.3390/v4123665
Chicago/Turabian StylePéréfarres, Frédéric, Magali Thierry, Nathalie Becker, Pierre Lefeuvre, Bernard Reynaud, Hélène Delatte, and Jean-Michel Lett. 2012. "Biological Invasions of Geminiviruses: Case Study of TYLCV and Bemisia tabaci in Reunion Island" Viruses 4, no. 12: 3665-3688. https://doi.org/10.3390/v4123665



