Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response
Abstract
:1. Introduction
2. Keratinocytes at the Initiation of HPV Infections

3. Role of DCs in HPV Infections
4. Down-Regulation of Toll-Like Receptors by HPV and the Use of TLR Agonists to Improve Immunity
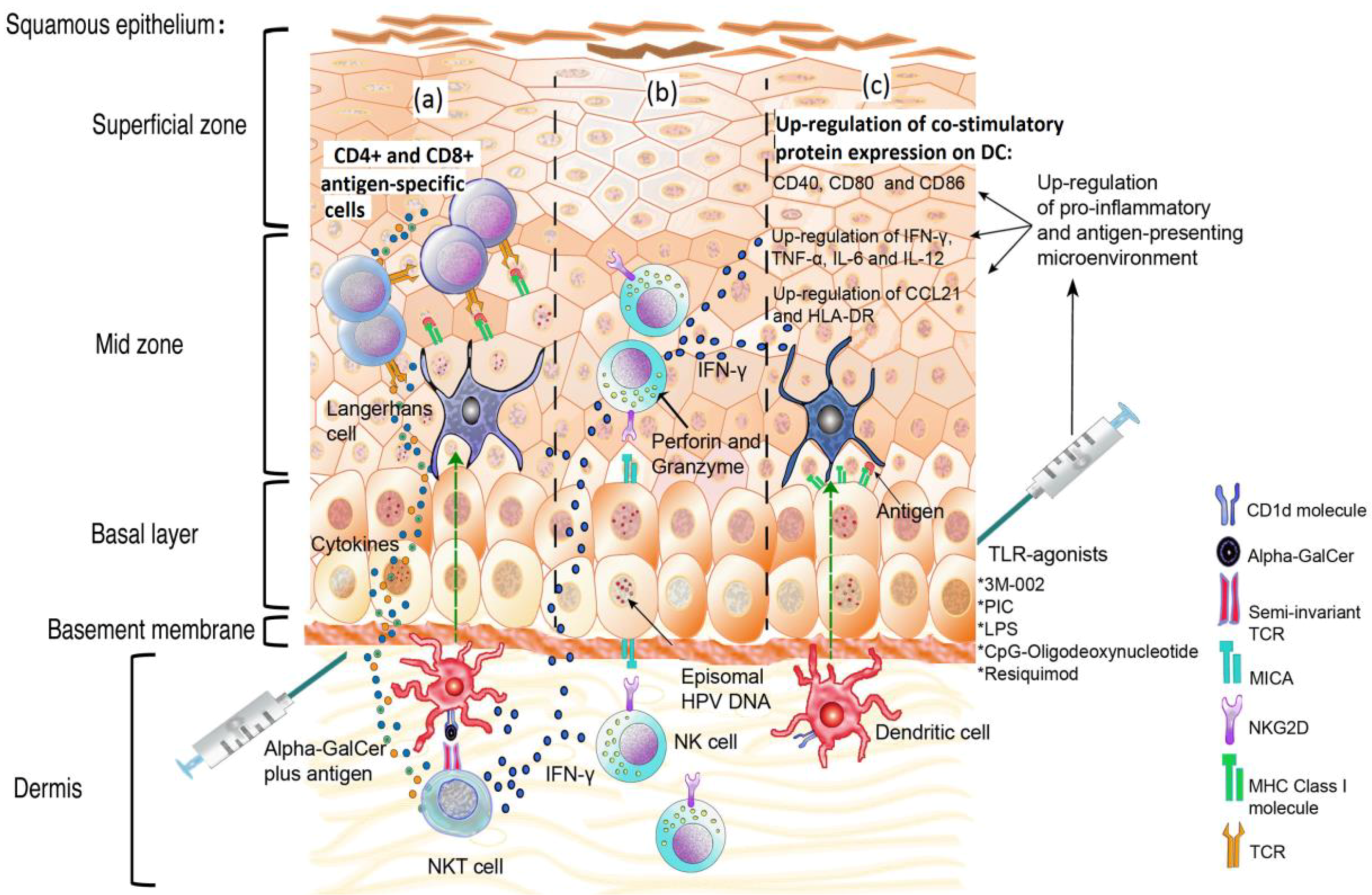
| Treatment | Effect | Model | Reference |
|---|---|---|---|
| CpG ODN (TLR-9 agonist) + E7 recombinant protein | Suppression of tumor formation. | Mouse | [52,53] |
| 3M002 (TLR-8 agonist), resiquimod or (TLR-8 and 7 agonist) + VLP-L1-L2 or VLP-L1-L2-E7 | Overexpression of chemokines and pro-inflammatory TH1 cytokines (MIP, IL-6, TNF-α, IL-12, IL-8). Stimulation of LC migration related to CCL21. Induction of specific CD8+ T cell response. | Human immune cells isolated from peripheral blood lymphocyte (PBL) | [54] |
| LPS (Lipopolysaccharide) TLR-4 agonist or polyinosinic acid-polycytidylic acid (PIC, TLR-3 agonist) + HPV11-E7 epitopes. | Up-regulation of CD40, CD80, CD86, CD83, HLA-DR, IL-12 and IFN-γ, in monocyte-derived dendritic cells (mdDC). Promotion of specific cytotoxic T lymphocyte response. | Human immune cells isolated from PBL | [55] |
| Live or inactivated Listeria monocytogenes or endotoxin. | Promote E7-specific T CD8+ cell immune response. | E7-Skin graft challenge. | [56] |
| Hydralazine and valproate | Decrease of soluble MICA and increase of susceptibility of target cells to NK attack. | NK cells isolated from PBL and tumor cells lines. | [57] |
| Short hairpin RNA (shRNA) plasmid targeting the IDO gene | Susceptibility to NK cell attack. | In vitro assays. | [58] |
| Gardasil HPV vaccine | Induction of protective antibodies. Increase NK cell population following immunization. Increase of the expression of NKG2D, NKp30, Nkp46 and ILT2 receptors. | Peripheral blood samples from vaccinated patients. | [26,59] |
| α-GalCer + DNA vaccine encoding the HPV16-E7 oncoprotein. | Increase of E7-specific CD8+ T cells and inhibition of tumor growth. | Mouse | [60] |
| β-GalCer | Inhibition of TC-1-tumor growth. | Mouse | [61] |
| B subunit of Shiga toxin coupled with ovalbumin or the E7 polypeptide + α-GalCer | Break tolerance generated against self Ag-elicited antiviral immunity | Mouse | [62] |
5. The Pro-Inflammatory Response: Deregulation of the Link between the Innate and the Acquired Immune Responses
6. Natural Killer Cells: An Important Barrier against Cells Expressing HPV Antigens
7. The Promising Role of NKT Cells in Controlling HPV Infection
8. Perspectives
Acknowledgments
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar]
- Clifford, G.M.; Smith, J.S.; Aguado, T.; Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br. J. Cancer 2003, 89, 101–105. [Google Scholar]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Munoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef]
- Doorbar, J. Papillomavirus life cycle organization and biomarker selection. Dis. Markers 2007, 23, 297–313. [Google Scholar] [CrossRef]
- Kasukawa, H.; Howley, P.M.; Benson, J.D. A fifteen-amino-acid peptide inhibits human papillomavirus E1-E2 interaction and human papillomavirus DNA replication in vitro. J. Virol. 1998, 72, 8166–8173. [Google Scholar]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar]
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 1995, 69, 2989–2997. [Google Scholar]
- Spartz, H.; Lehr, E.; Zhang, B.; Roman, A.; Brown, D.R. Progression from productive infection to integration and oncogenic transformation in human papillomavirus type 59-immortalized foreskin keratinocytes. Virology 2005, 336, 11–25. [Google Scholar]
- Jeon, S.; Lambert, P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 1654–1658. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomavirus infections − a major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar]
- Renoux, V.M.; Bisig, B.; Langers, I.; Dortu, E.; Clemenceau, B.; Thiry, M.; Deroanne, C.; Colige, A.; Boniver, J.; Delvenne, P.; Jacobs, N. Human papillomavirus entry into NK cells requires CD16 expression and triggers cytotoxic activity and cytokine secretion. Eur. J. Immunol. 2011, 41, 3240–3252. [Google Scholar] [CrossRef]
- Kanodia, S.; Fahey, L.M.; Kast, W.M. Mechanisms used by human papillomaviruses to escape the host immune response. Curr. Cancer Drug Targets 2007, 7, 79–89. [Google Scholar] [CrossRef]
- Sterling, J.C.; Skepper, J.N.; Stanley, M.A. Immunoelectron microscopical localization of human papillomavirus type 16 L1 and E4 proteins in cervical keratinocytes cultured in vivo. J. Investig. Dermatol. 1993, 100, 154–158. [Google Scholar]
- Stanley, M.A. Replication of human papillomaviruses in cell culture. Antivir. Res. 1994, 24, 1–15. [Google Scholar]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef]
- Gravitt, P.E. Evidence and impact of human papillomavirus latency. Open Virol. J. 2012, 6, 198–203. [Google Scholar] [CrossRef]
- Nestle, F.O.; di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar]
- Black, A.P.; Ardern-Jones, M.R.; Kasprowicz, V.; Bowness, P.; Jones, L.; Bailey, A.S.; Ogg, G.S. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 2007, 37, 1485–1493. [Google Scholar]
- Nasu, K.; Narahara, H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediat. Inflamm. 2010, 2010, 976024. [Google Scholar]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar]
- Sanghavi, S.K.; Reinhart, T.A. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: Implications for inflammation and immunodeficiency. J. Immunol. 2005, 175, 5314–5323. [Google Scholar]
- Yang, K.; Puel, A.; Zhang, S.; Eidenschenk, C.; Ku, C.L.; Casrouge, A.; Picard, C.; von Bernuth, H.; Senechal, B.; Plancoulaine, S.; et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 2005, 23, 465–478. [Google Scholar]
- Lebre, M.C.; van der Aar, A.M.; van Baarsen, L.; van Capel, T.M.; Schuitemaker, J.H.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef]
- Miller, L.S.; Modlin, R.L. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Investig. Dermatol. 2007, 127, 262–263. [Google Scholar]
- Stanley, M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef]
- Le Bon, A.; Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002, 14, 432–436. [Google Scholar] [CrossRef]
- Um, S.J.; Rhyu, J.W.; Kim, E.J.; Jeon, K.C.; Hwang, E.S.; Park, J.S. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett. 2002, 179, 205–212. [Google Scholar]
- Li, S.; Labrecque, S.; Gauzzi, M.C.; Cuddihy, A.R.; Wong, A.H.; Pellegrini, S.; Matlashewski, G.J.; Koromilas, A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene 1999, 18, 5727–5737. [Google Scholar]
- Karim, R.; Meyers, C.; Backendorf, C.; Ludigs, K.; Offringa, R.; van Ommen, G.J.; Melief, C.J.; van der Burg, S.H.; Boer, J.M. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One 2011, 6, e17848. [Google Scholar] [CrossRef]
- Eiben, G.L.; Velders, M.P.; Kast, W.M. The cell-mediated immune response to human papillomavirus-induced cervical cancer: Implications for immunotherapy. Adv. Cancer Res. 2002, 86, 113–148. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar]
- Valladeau, J.; Saeland, S. Cutaneous dendritic cells. Semin. Immunol. 2005, 17, 273–283. [Google Scholar] [CrossRef]
- Van der Aar, A.M.; Sylva-Steenland, R.M.; Bos, J.D.; Kapsenberg, M.L.; de Jong, E.C.; Teunissen, M.B. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J. Immunol. 2007, 178, 1986–1990. [Google Scholar]
- Klechevsky, E.; Banchereau, J. Human dendritic cells subsets as targets and vectors for therapy. Ann. N. Y. Acad. Sci. 2013, 1284, 24–30. [Google Scholar]
- Klechevsky, E.; Liu, M.; Morita, R.; Banchereau, R.; Thompson-Snipes, L.; Palucka, A.K.; Ueno, H.; Banchereau, J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum. Immunol. 2009, 70, 281–288. [Google Scholar]
- Huang, L.; Baban, B.; Johnson, B.A., 3rd; Mellor, A.L. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int. Rev. Immunol. 2010, 29, 133–155. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Hou, D.; Baban, B.; Lee, J.R.; Antonia, S.J.; Messina, J.L.; Chandler, P.; Koni, P.A.; Mellor, A.L. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Investig. 2004, 114, 280–290. [Google Scholar]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef]
- Mittal, D.; Kassianos, A.J.; Tran, L.S.; Bergot, A.S.; Gosmann, C.; Hofmann, J.; Blumenthal, A.; Leggatt, G.R.; Frazer, I.H. Indoleamine 2,3-Dioxygenase Activity Contributes to Local Immune Suppression in the Skin Expressing Human Papillomavirus Oncoprotein E7. J. Investig. Dermatol. 2013. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar]
- Yang, W.; Song, Y.; Lu, Y.L.; Sun, J.Z.; Wang, H.W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013, 139, 513–522. [Google Scholar]
- Hubert, P.; Caberg, J.H.; Gilles, C.; Bousarghin, L.; Franzen-Detrooz, E.; Boniver, J.; Delvenne, P. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre)neoplastic lesions. J. Pathol. 2005, 206, 346–355. [Google Scholar]
- Caberg, J.H.; Hubert, P.; Herman, L.; Herfs, M.; Roncarati, P.; Boniver, J.; Delvenne, P. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: Role of CCL20. Cancer Immunol. Immunother. 2009, 58, 39–47. [Google Scholar] [CrossRef]
- Caberg, J.H.; Hubert, P.M.; Begon, D.Y.; Herfs, M.F.; Roncarati, P.J.; Boniver, J.J.; Delvenne, P.O. Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes. Carcinogenesis 2008, 29, 1441–1447. [Google Scholar] [CrossRef]
- Sperling, T.; Oldak, M.; Walch-Ruckheim, B.; Wickenhauser, C.; Doorbar, J.; Pfister, H.; Malejczyk, M.; Majewski, S.; Keates, A.C.; Smola, S. Human papillomavirus type 8 interferes with a novel C/EBPbeta-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012, 8, e1002833. [Google Scholar] [CrossRef]
- Mota, F.F.; Rayment, N.B.; Kanan, J.H.; Singer, A.; Chain, B.M. Differential regulation of HLA-DQ expression by keratinocytes and Langerhans cells in normal and premalignant cervical epithelium. Tissue Antigens 1998, 52, 286–293. [Google Scholar] [CrossRef]
- Mota, F.F.; Rayment, N.B.; Chong, S.; Singer, A.; Chain, B.M. The antigen-presenting environment in normal and human papillomavirus (HPV)-related premalignant cervical epithelium. Clin. Exp. Immunol. 1999, 116, 33–40. [Google Scholar] [CrossRef]
- Leong, C.M.; Doorbar, J.; Nindl, I.; Yoon, H.S.; Hibma, M.H. Loss of epidermal Langerhans cells occurs in human papillomavirus alpha, gamma, and mu but not beta genus infections. J. Investig. Dermatol. 2010, 130, 472–480. [Google Scholar]
- Hasan, U.A.; Bates, E.; Takeshita, F.; Biliato, A.; Accardi, R.; Bouvard, V.; Mansour, M.; Vincent, I.; Gissmann, L.; Iftner, T.; et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007, 178, 3186–3197. [Google Scholar]
- Kim, T.Y.; Myoung, H.J.; Kim, J.H.; Moon, I.S.; Kim, T.G.; Ahn, W.S.; Sin, J.I. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: Involvement of CD4+ and CD8+ T cells in protection. Cancer Res. 2002, 62, 7234–7240. [Google Scholar]
- Kim, T.G.; Kim, C.H.; Won, E.H.; Bae, S.M.; Ahn, W.S.; Park, J.B.; Sin, J.I. CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model. Immunology 2004, 112, 117–125. [Google Scholar]
- Fahey, L.M.; Raff, A.B.; Da Silva, D.M.; Kast, W.M. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J. Immunol. 2009, 182, 2919–2928. [Google Scholar]
- Chen, X.Z.; Mao, X.H.; Zhu, K.J.; Jin, N.; Ye, J.; Cen, J.P.; Zhou, Q.; Cheng, H. Toll like receptor agonists augment HPV 11 E7-specific T cell responses by modulating monocyte-derived dendritic cells. Arch. Dermatol. Res. 2010, 302, 57–65. [Google Scholar] [CrossRef]
- Frazer, I.H.; de Kluyver, R.; Leggatt, G.R.; Guo, H.Y.; Dunn, L.; White, O.; Harris, C.; Liem, A.; Lambert, P. Tolerance or immunity to a tumor antigen expressed in somatic cells can be determined by systemic proinflammatory signals at the time of first antigen exposure. J. Immunol. 2001, 167, 6180–6187. [Google Scholar]
- Chavez-Blanco, A.; de la Cruz-Hernandez, E.; Dominguez, G.I.; Rodriguez-Cortez, O.; Alatorre, B.; Perez-Cardenas, E.; Chacon-Salinas, R.; Trejo-Becerril, C.; Taja-Chayeb, L.; Trujillo, J.E.; et al. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int. J. Oncol. 2011, 39, 1491–1499. [Google Scholar]
- Sato, N.; Saga, Y.; Mizukami, H.; Wang, D.; Takahashi, S.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; et al. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol. Rep. 2012, 28, 1574–1578. [Google Scholar]
- Colmenares, V.; Noyola, D.E.; Monsivais-Urenda, A.; Salgado-Bustamante, M.; Estrada-Capetillo, L.; Gonzalez-Amaro, R.; Baranda, L. Human papillomavirus immunization is associated with increased expression of different innate immune regulatory receptors. Clin. Vaccine Immunol. 2012, 19, 1005–1011. [Google Scholar]
- Kim, D.; Hung, C.F.; Wu, T.C.; Park, Y.M. DNA vaccine with alpha-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine 2010, 28, 7297–7305. [Google Scholar]
- Simova, J.; Indrova, M.; Bieblova, J.; Mikyskova, R.; Bubenik, J.; Reinis, M. Therapy for minimal residual tumor disease: Beta-galactosylceramide inhibits the growth of recurrent HPV16-associated neoplasms after surgery and chemotherapy. Int. J. Cancer 2010, 126, 2997–3004. [Google Scholar]
- Adotevi, O.; Vingert, B.; Freyburger, L.; Shrikant, P.; Lone, Y.C.; Quintin-Colonna, F.; Haicheur, N.; Amessou, M.; Herbelin, A.; Langlade-Demoyen, P.; et al. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J. Immunol. 2007, 179, 3371–3379. [Google Scholar]
- Azar, K.K.; Tani, M.; Yasuda, H.; Sakai, A.; Inoue, M.; Sasagawa, T. Increased secretion patterns of interleukin-10 and tumor necrosis factor-alpha in cervical squamous intraepithelial lesions. Hum. Pathol. 2004, 35, 1376–1384. [Google Scholar] [CrossRef]
- Peghini, B.C.; Abdalla, D.R.; Barcelos, A.C.; Teodoro, L.; Murta, E.F.; Michelin, M.A. Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Hum. Immunol. 2012, 73, 920–926. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yu, C.W.; Lai, L.C.; Tsao, C.H.; Ho, K.T.; Yang, S.C.; Lee, H.; Cheng, Y.W.; Wu, T.C.; Shiau, M.Y. Up-regulation of interleukin-17 expression by human papillomavirus type 16 E6 in nonsmall cell lung cancer. Cancer 2010, 116, 4800–4809. [Google Scholar] [CrossRef]
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Fan, L.C.; Yang, S.C.; Tsao, C.H.; Lee, H.; Cheng, Y.W.; Lai, L.C.; Chang, Y.H. Human papillomavirus up-regulates MMP-2 and MMP-9 expression and activity by inducing interleukin-8 in lung adenocarcinomas. PLoS One 2013, 8, e54423. [Google Scholar]
- Sutlu, T.; Alici, E. Natural killer cell-based immunotherapy in cancer: Current insights and future prospects. J. Intern. Med. 2009, 266, 154–181. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; Long, E.O. Line of attack: NK cell specificity and integration of signals. Curr. Opin. Immunol. 2008, 20, 344–352. [Google Scholar] [CrossRef]
- Garcia-Iglesias, T.; del Toro-Arreola, A.; Albarran-Somoza, B.; del Toro-Arreola, S.; Sanchez-Hernandez, P.E.; Ramirez-Duenas, M.G.; Balderas-Pena, L.M.; Bravo-Cuellar, A.; Ortiz-Lazareno, P.C.; Daneri-Navarro, A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 2009, 9, 186. [Google Scholar]
- Arreygue-Garcia, N.A.; Daneri-Navarro, A.; del Toro-Arreola, A.; Cid-Arregui, A.; Gonzalez-Ramella, O.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A.; Troyo-Sanroman, R.; Bravo-Cuellar, A.; Delgado-Rizo, V.; et al. Augmented serum level of major histocompatibility complex class I-related chain A (MICA) protein and reduced NKG2D expression on NK and T cells in patients with cervical cancer and precursor lesions. BMC Cancer 2008, 8, 16. [Google Scholar] [CrossRef]
- Jimenez-Perez, M.I.; Jave-Suarez, L.F.; Ortiz-Lazareno, P.C.; Bravo-Cuellar, A.; Gonzalez-Ramella, O.; Aguilar-Lemarroy, A.; Hernandez-Flores, G.; Pereira-Suarez, A.L.; Daneri-Navarro, A.; del Toro-Arreola, S. Cervical cancer cell lines expressing NKG2D-ligands are able to down-modulate the NKG2D receptor on NKL cells with functional implications. BMC Immunol. 2012, 13, 7. [Google Scholar] [CrossRef]
- Neuman, R.J.; Huettner, P.C.; Li, L.; Mardis, E.R.; Duffy, B.F.; Wilson, R.K.; Rader, J.S. Association between DQB1 and cervical cancer in patients with human papillomavirus and family controls. Obstet. Gynecol. 2000, 95, 134–140. [Google Scholar] [CrossRef]
- Wang, S.S.; Wheeler, C.M.; Hildesheim, A.; Schiffman, M.; Herrero, R.; Bratti, M.C.; Sherman, M.E.; Alfaro, M.; Hutchinson, M.L.; Morales, J.; et al. Human leukocyte antigen class I and II alleles and risk of cervical neoplasia: Results from a population-based study in Costa Rica. J. Infect. Dis. 2001, 184, 1310–1314. [Google Scholar]
- Albarran-Somoza, B.; Franco-Topete, R.; Delgado-Rizo, V.; Cerda-Camacho, F.; Acosta-Jimenez, L.; Lopez-Botet, M.; Daneri-Navarro, A. CEACAM1 in cervical cancer and precursor lesions: Association with human papillomavirus infection. J. Histochem. Cytochem. 2006, 54, 1393–1399. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Theate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef]
- Patel, O.; Cameron, G.; Pellicci, D.G.; Liu, Z.; Byun, H.S.; Beddoe, T.; McCluskey, J.; Franck, R.W.; Castano, A.R.; Harrak, Y.; et al. NKT TCR recognition of CD1d-alpha-C-galactosylceramide. J. Immunol. 2011, 187, 4705–4713. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Hammond, K.J.; Poulton, L.D.; Smyth, M.J.; Baxter, A.G. NKT cells: Facts, functions and fallacies. Immunol. Today 2000, 21, 573–583. [Google Scholar] [CrossRef]
- Juno, J.A.; Keynan, Y.; Fowke, K.R. Invariant NKT cells: Regulation and function during viral infection. PLoS Pathog. 2012, 8, e1002838. [Google Scholar] [CrossRef]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef]
- Cerundolo, V.; Barral, P.; Batista, F.D. Synthetic iNKT cell-agonists as vaccine adjuvants − finding the balance. Curr. Opin. Immunol. 2010, 22, 417–424. [Google Scholar] [CrossRef]
- Banchet-Cadeddu, A.; Henon, E.; Dauchez, M.; Renault, J.H.; Monneaux, F.; Haudrechy, A. The stimulating adventure of KRN 7000. Org. Biomol. Chem. 2011, 9, 3080–3104. [Google Scholar]
- Kinjo, Y.; Wu, D.; Kim, G.; Xing, G.W.; Poles, M.A.; Ho, D.D.; Tsuji, M.; Kawahara, K.; Wong, C.H.; Kronenberg, M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005, 434, 520–525. [Google Scholar]
- Wu, L.; van Kaer, L. Natural killer T cells in health and disease. Front. Biosci. (Schol. Ed.) 2011, 3, 236–251. [Google Scholar]
- Mattner, J.; Debord, K.L.; Ismail, N.; Goff, R.D.; Cantu, C., 3rd; Zhou, D.; Saint-Mezard, P.; Wang, V.; Gao, Y.; Yin, N.; et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005, 434, 525–529. [Google Scholar]
- Stewart, T.J.; Smyth, M.J.; Fernando, G.J.; Frazer, I.H.; Leggatt, G.R. Inhibition of early tumor growth requires J alpha 18-positive (natural killer T) cells. Cancer Res. 2003, 63, 3058–3060. [Google Scholar]
- Monnier-Benoit, S.; Mauny, F.; Riethmuller, D.; Guerrini, J.S.; Capilna, M.; Felix, S.; Seilles, E.; Mougin, C.; Pretet, J.L. Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol. Oncol. 2006, 102, 22–31. [Google Scholar]
- Molling, J.W.; de Gruijl, T.D.; Glim, J.; Moreno, M.; Rozendaal, L.; Meijer, C.J.; van den Eertwegh, A.J.; Scheper, R.J.; von Blomberg, M.E.; Bontkes, H.J. CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int. J. Cancer 2007, 121, 1749–1755. [Google Scholar]
- Miura, S.; Kawana, K.; Schust, D.J.; Fujii, T.; Yokoyama, T.; Iwasawa, Y.; Nagamatsu, T.; Adachi, K.; Tomio, A.; Tomio, K.; et al. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: A possible mechanism for immune evasion by HPV. J. Virol. 2010, 84, 11614–11623. [Google Scholar] [CrossRef]
- Liu, K.; Idoyaga, J.; Charalambous, A.; Fujii, S.; Bonito, A.; Mordoh, J.; Wainstok, R.; Bai, X.F.; Liu, Y.; Steinman, R.M. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J. Exp. Med. 2005, 202, 1507–1516. [Google Scholar] [CrossRef]
- Silk, J.D.; Hermans, I.F.; Gileadi, U.; Chong, T.W.; Shepherd, D.; Salio, M.; Mathew, B.; Schmidt, R.R.; Lunt, S.J.; Williams, K.J.; et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Investig. 2004, 114, 1800–1811. [Google Scholar]
- Ko, S.Y.; Ko, H.J.; Chang, W.S.; Park, S.H.; Kweon, M.N.; Kang, C.Y. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 2005, 175, 3309–3317. [Google Scholar]
- Choi, D.H.; Kim, K.S.; Yang, S.H.; Chung, D.H.; Song, B.; Sprent, J.; Cho, J.H.; Sung, Y.C. Dendritic cell internalization of alpha-galactosylceramide from CD8 T cells induces potent antitumor CD8 T-cell responses. Cancer Res. 2011, 71, 7442–7451. [Google Scholar]
- Mattarollo, S.R.; Rahimpour, A.; Choyce, A.; Godfrey, D.I.; Leggatt, G.R.; Frazer, I.H. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J. Immunol. 2010, 184, 1242–1250. [Google Scholar] [CrossRef]
- Mattarollo, S.R.; Yong, M.; Gosmann, C.; Choyce, A.; Chan, D.; Leggatt, G.R.; Frazer, I.H. NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins. J. Immunol. 2011, 187, 1601–1608. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624-2642. https://doi.org/10.3390/v5112624
Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses. 2013; 5(11):2624-2642. https://doi.org/10.3390/v5112624
Chicago/Turabian StyleAmador-Molina, Alfredo, José Fernando Hernández-Valencia, Edmundo Lamoyi, Adriana Contreras-Paredes, and Marcela Lizano. 2013. "Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response" Viruses 5, no. 11: 2624-2642. https://doi.org/10.3390/v5112624




