A Semipersistent Plant Virus Differentially Manipulates Feeding Behaviors of Different Sexes and Biotypes of Its Whitefly Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Laboratory Whitefly Populations
2.3. Establishment of Non-Viruliferous and Viruliferous B. Tabaci Populations
2.4. Electrical Penetration Graph Recording
2.5. Data Analysis
3. Results
3.1. Effects of CCYV on Non-Phloem Feeding Behaviors of B. tabaci B and Q Biotypes
3.2. Effects of CCYV on Phloem Feeding Behaviors of B. tabaci B and Q Biotypes
3.3. Effects of CCYV on Feeding Behaviors of the Different Sexes of B. tabaci B and Q Biotypes
3.3.1. Feeding Behaviors of Male Whiteflies
3.3.2. Feeding Behaviors of the Female Whiteflies
3.3.3. Comparison of Feeding Behaviors of the Male and Female Whiteflies between Biotypes
3.4. Interaction Effects of CCYV, Sexes and Biotypes
Two-Way ANOVA Analyses
3.5. Multivariate Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fereres, A.; Moreno, A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009, 141, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.; Bosqueperez, N.A.; Eigenbrode, S.D.; de Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Opin. Virol. 2015, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Blas, C.; Barrios, L.; Fereres, A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of Tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000, 93, 573–579. [Google Scholar] [CrossRef]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Preisser, E.L.; Chu, D.; Pan, H.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; Zhou, X.G.; Zhang, Y.J. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and Tomato yellow leaf curl virus. J. Virol. 2013, 87, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.M.; Lee, G.S.; Lee, S.; Lee, K. Upregulation of probing- and feeding-related behavioural frequencies in Bemisia tabaci upon acquisition of Tomato yellow leaf curl virus. Pest Manag. Sci. 2014, 70, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- He, W.B.; Li, J.; Liu, S.S. Differential profiles of direct and indirect modification of vector feeding behaviour by a plant virus. Sci. Rep. 2015, 5, 7682. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.B.; Li, P.; Han, Y.Q.; Gong, S.L.; Yang, L.; Hou, M.L. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci. Rep. 2016, 6, 30240. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.; Michalakis, Y. Manipulation of hosts and vectors by plant viruses and impact of the environment. Curr. Opin. Insect Sci. 2016, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.; Omongo, C.A.; Govindappa, M.R.; Stevenson, P.C.; Maruthi, M.N.; Gibson, G.; Seal, S.E.; Muniyappa, V. Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: Management and epidemiological implications. Adv. Virus Res. 2006, 67, 419–452. [Google Scholar] [PubMed]
- Luan, J.B.; Wang, X.W.; Colvin, J.; Liu, S.S. Plant-mediated whitefly–begomovirus interactions: Research progress and future prospects. Bull. Entomol. Res. 2014, 104, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Gyoutoku, Y.; Okazaki, S.; Furuta, A.; Etoh, T.; Mizobe, M.; Kuno, K.; Hayashida, S.; Okuda, M. Chlorotic yellows disease of melon caused by Cucurbit chlorotic yellows virus, a new crinivirus. Jpn. J. Phytopathol. 2009, 75, 109–111. (In Japanese) [Google Scholar] [CrossRef]
- Huang, L.H.; Tseng, H.H.; Li, J.T.; Chen, T.C. First report of Cucurbit chlorotic yellows virus infecting cucurbits in Taiwan. Plant Dis. 2010, 94, 1168. [Google Scholar] [CrossRef]
- Zeng, R.; Dai, F.M.; Chen, W.J.; Lu, J.P. First report of Cucurbit chlorotic yellows virus infecting melon in China. Plant Dis. 2011, 95, 354. [Google Scholar] [CrossRef]
- Gu, Q.S.; Liu, Y.H.; Wang, Y.H.; Huangfu, H.F.; Gu, L.; Xu, F.M.; Song, J.K. First report of Cucurbit chlorotic yellows virus in cucumber, melon, and watermelon in China. Plant Dis. 2011, 95, 73. [Google Scholar] [CrossRef]
- Hamed, K.; Menzel, W.; Dafalla, G.; Gadelseed, A.M.A.; Winter, S. First report of Cucurbit chlorotic yellows virus in infecting muskmelon and cucumber in Suda. Plant Dis. 2011, 95, 1321. [Google Scholar] [CrossRef]
- Abrahamian, P.E.; Sobh, H.; Abou-Jawdah, Y. First report of Cucurbit chlorotic yellows virus in cucumber in Lebanon. Plant Dis. 2012, 96, 1704. [Google Scholar] [CrossRef]
- Bananej, K.; Menzel, W.; Kianfar, N.; Vahdat, A.; Winter, S. First report of Cucurbit chlorotic yellows virus in cucumber, melon, and squash in Iran. Plant Dis. 2013, 97, 1005. [Google Scholar] [CrossRef]
- Orfanidou, C.; Maliogka, V.I.; Katis, N.I. First Report of Cucurbit chlorotic yellows virus in Cucumber, Melon, and Watermelon in Greece. Plant Dis. 2014, 98, 1446. [Google Scholar] [CrossRef]
- Al-Saleh, M.A.; Al-Shahwan, I.M.; Amer, M.A.; Shakeel, M.T.; Abdalla, O.A.; Orfanidou, C.G.; Katis, N.I. First report of Cucurbit chlorotic yellows virus in cucumber in Saudi Arabia. Plant Dis. 2015, 99, 734. [Google Scholar] [CrossRef]
- Okuda, M.; Okazaki, S.; Yamasaki, S.; Okuda, S.; Sugiyama, M. Host range and complete genome sequence Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology 2010, 100, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.M.; de Barro, P.J. A practical guide to identifying members of the Bemisia tabaci species complex: And other morphologically identical species. Front. Ecol. Evol. 2014, 2, 45. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yao, Y.; Wang, R.J.; Yan, F.M.; Hu, D.X.; Zhang, Z.L. The use of mitochondrial cytochrome oxidase I (mtCOI) gene sequences for the identification of biotype of Bemisia tabaci (Gennadius) in China. Acta Entomol. Sin. 2002, 45, 759–763. [Google Scholar]
- Chu, D.; Zhang, Y.J.; Brown, J.K.; Cong, B.; Xu, B.Y.; Wu, Q.J.; Zhu, G.R. The introduction of the exotic Q biotype of Bemisia tabaci (Gennadius) from the Mediterranean region into China on ornamental crops. Fla. Entomol. 2006, 89, 168–174. [Google Scholar] [CrossRef]
- Pan, H.P.; Chu, D.; Ge, D.Q.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Liu, B.M.; Yang, X.; Yang, N.N.; et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J. Econ. Entomol. 2011, 104, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R. Whitefly infestations: The Christmas Invasion. Nature 2006, 443, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Czosnek, H. Whitefly transmission of plant viruses. In Advanced in Botanical Research. Plant Virus Vector Interactions; Plumb, R.T., Ed.; Academic Press: New York, NY, USA, 2002; Volume 36, pp. 65–76. [Google Scholar]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; MacFarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Polston, J.E.; de Barro, P.J.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.L.; Kinsey, M.G. A technique for electrical recording aphid feeding and salivation. Nature 1964, 202, 1358–1359. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 1978, 24, 521–530. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphid, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishing B.V.: Amsterdam, The Netherlands, 1988; Volume B, pp. 95–108. [Google Scholar]
- Mayoral, A.M.; Tjallingii, W.F.; Castañera, P. Probing behaviour of Diuraphis noxia on five cereal species with different hydroxamic acid levels. Entomol. Exp. Appl. 1996, 78, 341–348. [Google Scholar] [CrossRef]
- Garzo, E.; Soria, C.; Gomez-Guillamon, M.L.; Fereres, A. Feeding behavior of Aphis gossypii on resistant accessions of different melon genotypes (Cucumis melo). Phytoparasitica 2002, 30, 129–140. [Google Scholar] [CrossRef]
- Xue, K.; Wang, X.Y.; Huang, C.H.; Wang, R.J.; Liu, B.; Yan, F.M.; Xu, C.R. Stylet penetration behaviors of the cotton aphid Aphis gossypii on transgenic Bt cotton. Insect Sci. 2009, 16, 137–146. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Y.J.; Gu, Q.S.; Yan, F.M.; Sun, X.Y.; Li, H.L.; Chen, L.L.; Sun, B.J.; Wang, Z.Y. Infectious clones of the crinivirus Cucurbit chlorotic yellows virus are competent for plant systemic infection and vector transmission. J. Gen. Virol. 2016, 97, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Khasdan, V.; Levin, I.; Rosner, A.; Morin, S.; Kontsedalov, S.; Maslenin, L.; Horowitz, A.R. DNA markers for identifying biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) and studying population dynamics. Bull. Entomol. Res. 2005, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Shatters, R.G., Jr.; Power, C.A.; Boykin, L.M.; He, L.S.; McKenzie, C.L. Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: Development of universal and Bemisia tabaci biotype-specific mitochondrial cytochrome c oxidase I polymerase chain reaction primers. J. Econ. Entomol. 2009, 102, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Lei, H.; Collar, J.L.; Martin, B.; Muniz, M.; Fereres, A. Probing and feeding behaviour of two distinct biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on tomato plants. J. Econ. Entomol. 1999, 92, 357–366. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereresb, A. Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Liu, B.M.; Yan, F.M.; Chu, D.; Pan, H.P.; Jiao, X.G.; Xie, W.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Zhou, X.G.; et al. Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: Implication for competitive displacement. Int. J. Biol. Sci. 2012, 8, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Liu, J.; Jiu, M.; Qian, Y.J.; Liu, S.S. Low frequency of horizontal and vertical transmission of two begomoviruses through whiteflies exhibits little relevance to the vector infectivity. Ann. Appl. Biol. 2010, 157, 125–133. [Google Scholar] [CrossRef]
- Li, J.J.; Liang, X.X.; Wang, X.L.; Shi, Y.; Gu, Q.S.; Kuo, Y.W.; Falk, B.W.; Yan, F.M. Direct evidence for the semipersistent transmission of Cucurbit chlorotic yellows virus by a whitefly vector. Sci. Rep. 2016, 6, 36604. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.X.; Shi, X.B.; Liu, B.M.; Pan, H.P.; Wei, W.T.; Zeng, Y.; Sun, X.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; et al. Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci as Affected by Whitefly Sex and Biotype. Sci. Rep. 2015, 29, 10744. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, V.; Reddy, D.V.R. Transmission of cowpea mild mottle virus by Bemisia tabaci in a nonpersistent manner. Plant Dis. 1983, 67, 391–393. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosqueperez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3655. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.; Brault, A. The role of environmental, virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. Adv. Virus Res. 2014, 89, 39–83. [Google Scholar] [PubMed]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus–insect vector interactions: Current and potential future research directions. Viruses 2016, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Rajabaskar, D.; Bosqueperez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.B.; Resende, R.O.; De Avila, A.C. The plant virus Tomato spotted wilt tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. J. Virol. 2004, 78, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.B.; Li, J.M.; Varela, N.; Wang, Y.J.; Li, F.F.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S.; Wang, X.W. Global analysis of the transcriptional response of whitefly to Tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 2011, 85, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, L.; Lu, H.; Chen, S.L.; Kang, L.; Cui, F. Angiotensin-converting enzymes modulate aphid–plant interactions. Sci. Rep. 2015, 5, 8885. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P.; Groen, S.C.; Carr, J.P. The Rumsfeld paradox: Some of the things we know that we don’t know about plant virus infection. Curr. Opin. Plant Biol. 2013, 16, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Martinez, A.J. How resident microbes modulate ecologically important traits of insects. Curr. Opin. Insect Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pan, H.P.; Liu, B.M.; Chu, D.; Xie, W.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Zhang, Y.Y. Insect symbiont facilitates vector acquisition, retention, and transmission of plant virus. Sci. Rep. 2013, 3, 1367. [Google Scholar] [CrossRef] [PubMed]
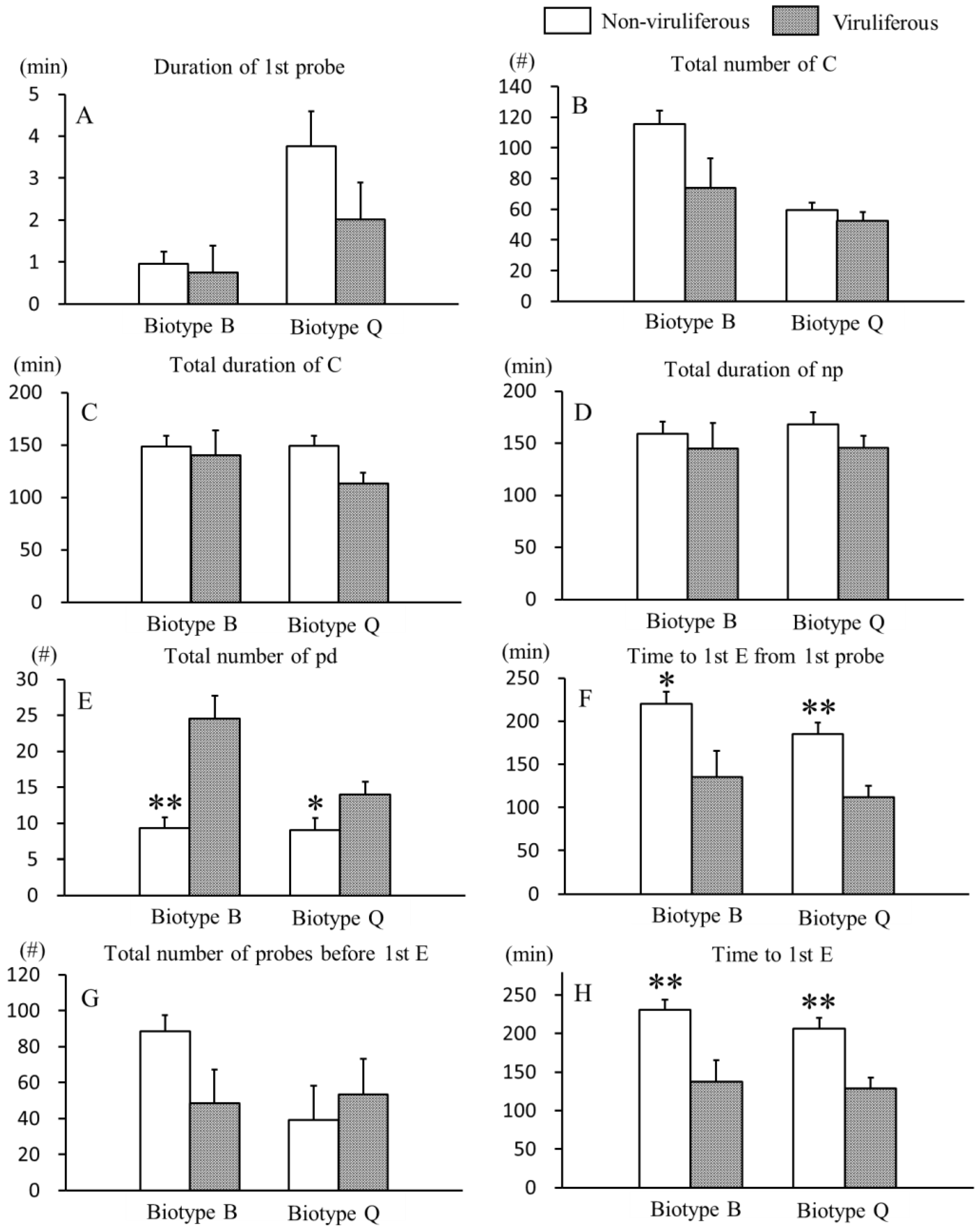
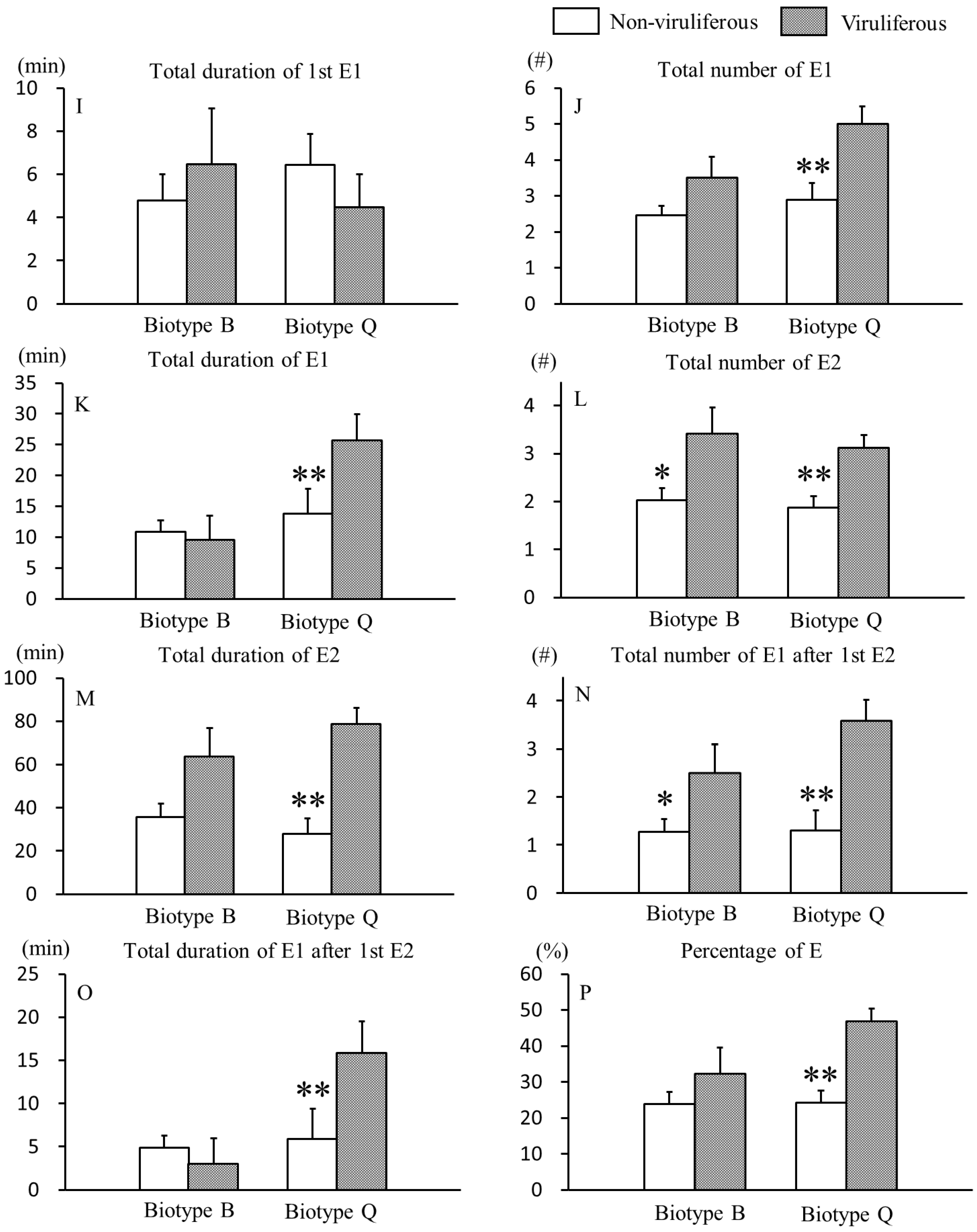
| Variables | Biotype | Non-Viruliferous Male | Viruliferous Male | p 3 Value | Non-Viruliferous Famale | Viruliferous Famale | p Value |
|---|---|---|---|---|---|---|---|
| A, duration of 1st probe (min) | B | 0.96 ± 0.42a 1 | 0.29 ± 1.02a 2 | 0.550 | 0.95 ± 0.42a | 1.21 ± 0.75a | 0.761 |
| Q | 6.02 ± 1.67a | 1.63 ± 1.59a | 0.064 | 1.49 ± 0.70a | 2.40 ± 0.83a | 0.406 | |
| B, total number of C 4 (#) | B | 123.67 ± 14.77a | 88.00 ± 36.17a | 0.373 | 106.84 ± 10.48a | 60.17 ± 18.65a | 0.040 |
| Q | 55.35 ± 7.03b | 56.64 ± 6.70a | 0.895 | 63.50 ± 6.77b | 49.19 ± 8.09a | 0.181 | |
| C, total duration of C (min) | B | 148.94 ± 16.23a | 170.37 ± 39.77a | 0.624 | 147.36 ± 14.45a | 110.48 ± 25.72a | 0.224 |
| Q | 168.21 ± 13.87a | 131.18 ± 13.23a | 0.061 | 129.60 ± 12.55a | 95.36 ± 15.00a | 0.086 | |
| D, total duration of np (min) | B | 164.86 ± 13.15a | 70.41 ± 32.20a | 0.014 | 153.84 ± 18.32a | 218.72 ± 32.60a | 0.096 |
| Q | 157.01 ± 15.31a | 107.93 ± 14.60a | 0.026 | 179.66 ± 15.41a | 183.13 ± 18.42a | 0.886 | |
| E, total number of pd (#) | B | 8.11 ± 1.76a | 34.00 ± 4.30a | <0.001 | 10.58 ± 2.34a | 15.00 ± 4.16a | 0.364 |
| Q | 9.65 ± 2.39a | 15.18 ± 2.28b | 0.102 | 8.43 ± 2.18a | 12.76 ± 2.61a | 0.019 | |
| F, time to 1st E from 1st probe (min) | B | 246.30 ± 16.33a | 89.38 ± 39.99a | 0.002 | 194.33 ± 22.05a | 181.27 ± 39.24a | 0.774 |
| Q | 171.49 ± 19.17b | 94.76 ± 18.28a | 0.006 | 198.78 ± 17.15a | 128.51 ± 20.50a | 0.011 | |
| G, total number of probes before 1st E (#) | B | 103.50 ± 14.88a | 46.00 ± 36.44a | 0.160 | 73.68 ± 9.94a | 51.00 ± 17.68a | 0.275 |
| Q | 33.10 ± 5.56b | 24.45 ± 5.30a | 0.267 | 45.44 ± 31.85b | 82.12 ± 38.07a | 0.463 | |
| H, time to 1st E (min) | B | 251.85 ± 16.90a | 92.13 ± 41.39a | 0.002 | 209.43 ± 20.06a | 182.18 ± 35.70a | 0.512 |
| Q | 198.24 ± 20.07a | 111.47 ± 19.13a | 0.003 | 214.74 ± 17.67a | 145.51 ± 21.13a | 0.015 |
| Variables | Biotype | Non-Viruliferous Male | Viruliferous Male | p 3 Value | Non-Viruliferous Famale | Viruliferous Famale | p Value |
|---|---|---|---|---|---|---|---|
| I, total duration of 1st E1 (min) | B | 2.88 ± 0.89a 1 | 6.93 ± 2.19a 2 | 0.103 | 6.72 ± 2.12a | 6.03 ± 3.78a | 0.875 |
| Q | 5.84 ± 2.65b | 5.03 ± 2.53a | 0.826 | 7.05 ± 1.45a | 3.94 ± 1.73a | 0.025 | |
| J, total number of E1 5 (#) | B | 2.33 ± 0.40a | 4.00 ± 0.97a | 0.129 | 2.58 ± 0.37a | 3.00 ± 0.65a | 0.579 |
| Q | 3.05 ± 0.65a | 6.09 ± 0.62a | 0.002 | 2.73 ± 0.62a | 3.90 ± 0.74a | 0.233 | |
| K, total duration of E1 (min) | B | 9.18 ± 2.46a | 10.89 ± 6.02a | 0.795 | 12.49 ± 2.68a | 8.13 ± 4.77a | 0.434 |
| Q | 11.65 ± 3.66a | 23.78 ± 3.49a | 0.021 | 16.00 ± 6.32a | 27.58 ± 7.55a | 0.245 | |
| L, total number of E2 (#) | B | 2.00 ± 0.35a | 4.00 ± 0.86a | 0.044 | 2.05 ± 0.36a | 2.83 ± 0.65a | 0.304 |
| Q | 1.80 ± 0.43a | 4.09 ± 0.41a | <0.001 | 1.93 ± 0.29a | 2.14 ± 0.35a | 0.646 | |
| M, total duration of E2 (min) | B | 35.55 ± 8.13a | 106.03 ± 19.90a | 0.004 | 36.01 ± 9.00a | 21.22 ± 16.02a | 0.429 |
| Q | 22.13 ± 11.89a | 95.49 ± 11.33a | <0.001 | 33.72 ± 8.18a | 61.87 ± 9.77a | 0.032 | |
| N, total number of E1 after 1st E2 (#) | B | 1.06 ± 0.40a | 3.00 ± 0.98a | 0.082 | 1.47 ± 0.37a | 2.00 ± 0.66a | 0.496 |
| Q | 1.40 ± 0.60a | 4.55 ± 0.57a | <0.001 | 1.20 ± 0.56a | 2.62 ± 0.67a | 0.110 | |
| O, total duration of E1 after 1st E2 (min) | B | 4.45 ± 1.78a | 3.96 ± 4.37a | 0.918 | 5.30 ± 2.06a | 2.10 ± 3.66a | 0.455 |
| Q | 4.68 ± 2.60a | 15.35 ± 2.48a | 0.001 | 7.14 ± 5.65a | 16.36 ± 6.75a | 0.300 | |
| P, percentage of E (%) 4 | B | 24.58 ± 5.24a | 41.15 ± 12.84a | 0.247 | 22.99 ± 4.44a | 23.29 ± 7.90a | 0.891 |
| Q | 18.05 ± 4.78a | 46.63 ± 4.56a | <0.001 | 30.35 ± 4.47a | 47.21 ± 5.34b | 0.019 |
| Variables | B. tabaci B (p Value) 1 | B. tabaci Q (p Value) | ||||
|---|---|---|---|---|---|---|
| Sex | Virus | Sex * Virus | Sex | Virus | Sex * Virus | |
| Non-phloem variables | ||||||
| A, duration of 1st probe (min) | 0.522 | 0.776 | 0.511 | 0.447 | 0.153 | 0.111 |
| B, total number of C 3 (#) | 0.267 | 0.057 | 0.533 | 0.730 | 0.373 | 0.237 |
| C, total duration of C (min) | 0.237 | 0.764 | 0.262 | 0.024 | 0.127 | 0.983 |
| D, total duration of np (min) | 0.016 | 0.591 | 0.006 | 0.003 | 0.166 | 0.111 |
| E, total number of pd (#) | 0.024 | <0.001 | 0.004 | 0.45 | 0.043 | 0.802 |
| F, time to 1st E from 1st probe (min) | 0.550 | 0.014 | 0.036 | 0.085 | <0.001 | 0.906 |
| G, total number of probes before 1st E (#) | 0.747 | 0.060 | 0.580 | 0.160 | 0.082 | 0.396 |
| H, Time to 1st E (min) | 0.453 | 0.005 | 0.041 | 0.202 | <0.001 | 0.657 |
| Phloem variables | ||||||
| I, total duration of 1st E1 (min) | 0.721 | 0.558 | 0.135 | 0.978 | 0.347 | 0.580 |
| J, total number of E1 (#) | 0.558 | 0.110 | 0.335 | 0.006 | 0.001 | 0.084 |
| K, total duration of E1 (min) | 0.950 | 0.762 | 0.488 | 0.484 | 0.001 | 0.146 |
| L, total number of E2 (#) | 0.361 | 0.026 | 0.246 | 0.019 | <0.001 | 0.006 |
| M, total duration of E2 (min) | 0.006 | 0.061 | 0.005 | 0.989 | <0.001 | 0.003 |
| N, total number of E1 after 1st E2 (#) | 0.655 | 0.049 | 0.279 | 0.031 | 0.001 | 0.094 |
| O, total duration of E1 after 1st E2 (min) | 0.493 | 0.575 | 0.351 | 0.116 | 0.007 | 0.049 |
| P, percentage of E (%) 2 | 0.235 | 0.302 | 0.319 | 0.189 | <0.001 | 0.231 |
| Variables | p Value 1 | ||||||
|---|---|---|---|---|---|---|---|
| Biotype | Sex | Virus | Biotype * Sex | Biotype * Virus | Sex * Virus | Biotype * Sex * Virus | |
| Non-phloem variables | |||||||
| A, duration of 1st probe (min) | 0.167 | 0.932 | 0.796 | 0.511 | 0.634 | 0.972 | 0.111 |
| B, total number of C 3 (#) | <0.001 | 0.271 | 0.193 | 0.443 | 0.205 | 0.272 | 0.957 |
| C, total duration of C (min) | 0.367 | 0.021 | 0.139 | 0.824 | 0.340 | 0.342 | 0.296 |
| D, total duration of np (min) | 0.766 | 0.001 | 0.262 | 0.555 | 0.810 | 0.002 | 0.112 |
| E, total number of pd (#) | 0.024 | 0.035 | <0.001 | 0.177 | 0.033 | 0.018 | 0.035 |
| F, time to 1st E from 1st probe (min) | 0.136 | 0.201 | <0.001 | 0.788 | 0.770 | 0.058 | 0.083 |
| G, total number of probes before 1st E (#) | 0.001 | 0.687 | 0.060 | 0.340 | 0.837 | 0.946 | 0.377 |
| H, time to 1st E (min) | 0.411 | 0.219 | <0.001 | 0.971 | 0.697 | 0.061 | 0.151 |
| Phloem variables | |||||||
| I, total duration of 1st E1 (min) | 0.932 | 0.707 | 0.945 | 0.728 | 0.369 | 0.386 | 0.764 |
| J, total number of E1 (#) | 0.120 | 0.190 | 0.012 | 0.480 | 0.391 | 0.210 | 0.801 |
| K, total duration of E1 (min) | 0.072 | 0.681 | 0.321 | 0.720 | 0.215 | 0.755 | 0.794 |
| L, total number of E2 (#) | 0.538 | 0.051 | 0.001 | 0.638 | 0.851 | 0.028 | 0.563 |
| M, total duration of E2 (min) | 0.976 | 0.041 | 0.002 | 0.042 | 0.330 | 0.001 | 0.634 |
| N, total number of E1 after 1st E2 (#) | 0.462 | 0.199 | 0.001 | 0.359 | 0.710 | 0.132 | 0.848 |
| O, total duration of E1 after 1st E2 (min) | 0.190 | 0.187 | 0.128 | 0.797 | 0.213 | 0.087 | 0.788 |
| P, percentage of E (%) 2 | 0.130 | 0.741 | 0.002 | 0.106 | 0.152 | 0.161 | 0.819 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Li, J.; Wang, X.; Song, D.; Bai, R.; Shi, Y.; Gu, Q.; Kuo, Y.-W.; Falk, B.W.; Yan, F. A Semipersistent Plant Virus Differentially Manipulates Feeding Behaviors of Different Sexes and Biotypes of Its Whitefly Vector. Viruses 2017, 9, 4. https://doi.org/10.3390/v9010004
Lu S, Li J, Wang X, Song D, Bai R, Shi Y, Gu Q, Kuo Y-W, Falk BW, Yan F. A Semipersistent Plant Virus Differentially Manipulates Feeding Behaviors of Different Sexes and Biotypes of Its Whitefly Vector. Viruses. 2017; 9(1):4. https://doi.org/10.3390/v9010004
Chicago/Turabian StyleLu, Shaohua, Jingjing Li, Xueli Wang, Danyang Song, Rune Bai, Yan Shi, Qinsheng Gu, Yen-Wen Kuo, Bryce W. Falk, and Fengming Yan. 2017. "A Semipersistent Plant Virus Differentially Manipulates Feeding Behaviors of Different Sexes and Biotypes of Its Whitefly Vector" Viruses 9, no. 1: 4. https://doi.org/10.3390/v9010004






