Live Attenuated Influenza Vaccine contains Substantial and Unexpected Amounts of Defective Viral Genomic RNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. RNA Isolation
2.3. Reverse Transcription-polymerase Chain Reaction (RT-PCR)
2.4. Growth of Virus in Embryonated Chicken’s Eggs
2.5. Haemagglutination Assay
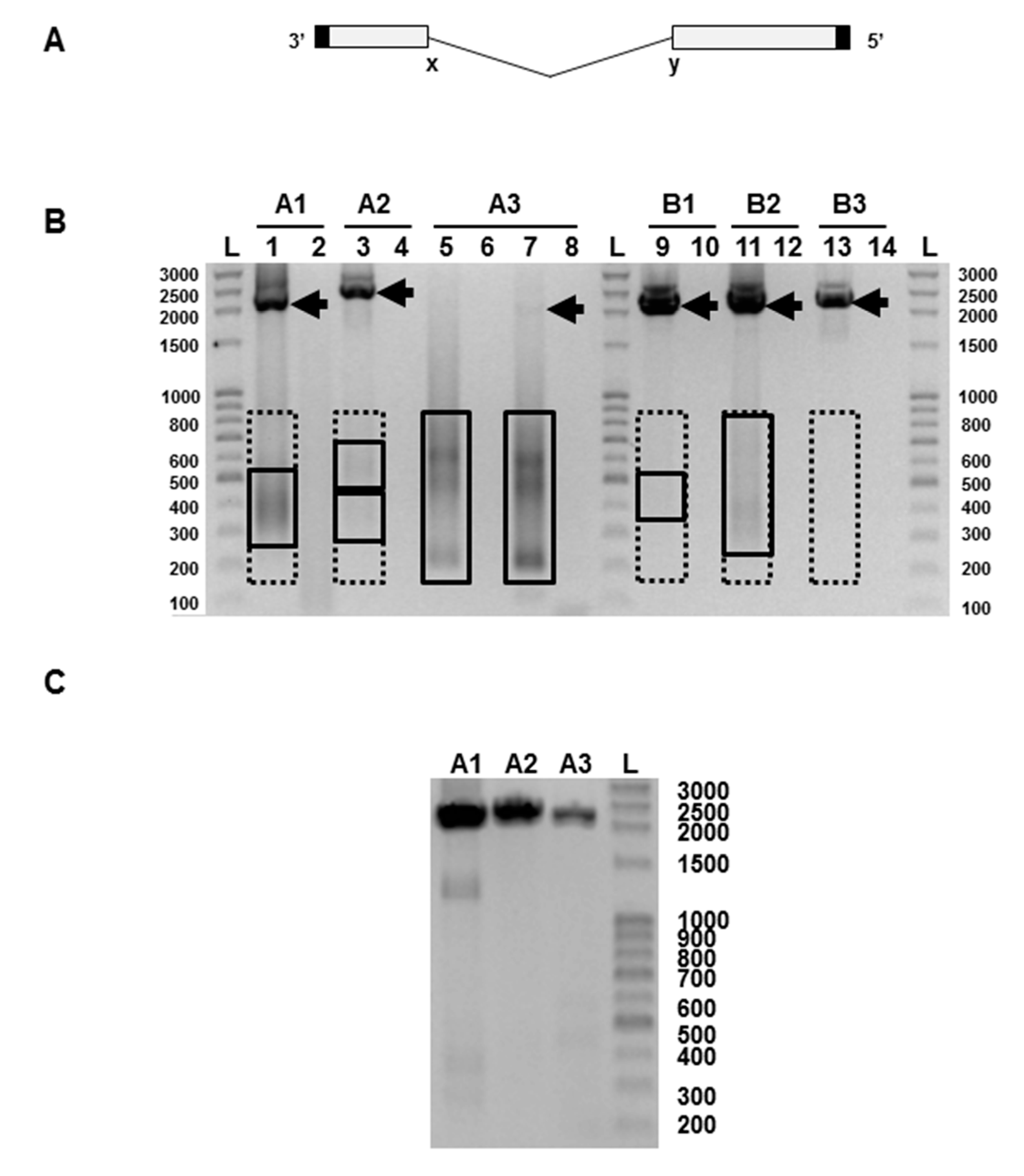
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, I.A.; Cox, N.J. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 1990, 8, 737–771. [Google Scholar] [CrossRef] [PubMed]
- Molinari, N.A.M.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring Disease Burden and Costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Cimons, M. FDA okays live-attenuated nasal spray form of flu vaccine. Am. Soc. Microbiol. News 2003, 69, 426–427. [Google Scholar]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Olsen, S.J.; Karron, R.A.; Jernigan, D.B.; Bresee, J.S. Prevention and control of seasonal influenza with vaccines. MMWR Recomm. Rep. 2016, 65, 1–54. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Fluenz Tetra. Available online: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002617/human_med_001713.jsp&mid=WC0b01ac058001d124 (accessed on 20 September 2017).
- Murphy, B.R.; Coelingh, K. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 2002, 15, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Von Magnus, P. Incomplete forms of influenza virus. Adv. Virus Res. 1954, 21, 59–79. [Google Scholar]
- Frensing, T.; Heldt, F.S.; Pflugmacher, A.; Behrendt, I.; Jordan, I.; Flockerzi, D.; Genzel, Y.; Reichl, U. Continuous influenza virus production in cell culture shows a periodic accumulation of defective interfering particles. PLoS ONE 2013, 8, e72288. [Google Scholar] [CrossRef] [PubMed]
- Frensing, T.; Pflugmacher, A.; Bachmann, M.; Peschel, B.; Reichl, U. Impact of defective interfering particles on virus replication and antiviral host response in cell culture-based influenza vaccine production. Appl. Microbiol. Biot. 2014, 98, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Saira, K.; Lin, X.; DePasse, J.V.; Halpin, R.; Twaddle, A.; Stockwell, T.; Angus, B.; Cozzi-Lepri, A.; Delfino, M.; Dugan, V.; et al. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J. Virol. 2013, 87, 8064–8074. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, N.J. Antiviral activity of defective interfering influenza virus in vivo. In Viral and Other Infections of the Respiratory Tract; Myint, S., Taylor-Robinson, D., Eds.; Chapman and Hall: London, UK, 1996; pp. 421–445. [Google Scholar]
- Huang, A.S. Defective interfering viruses. Annu. Rev. Microbiol. 1973, 27, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Jennings, P.A.; Finch, J.T.; Winter, G.; Robertson, J.S. Does the higher order of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA. Cell 1983, 34, 619–627. [Google Scholar] [CrossRef]
- Duhaut, S.D.; Dimmock, N.J. Heterologous protection of mice from a lethal human H1N1 influenza A virus infection by H3N8 equine defective interfering virus: Comparison of Defective RNA Sequences Isolated from the DI Inoculum and Mouse Lung. Virology 1998, 248, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.P.; Chambers, T.M.; Akkina, R.K. Defective-interfering (DI) RNAs of influenza viruses: Origin, Structure, Expression and Interference. Curr. Top. Microbiol. 1985, 114, 103–151. [Google Scholar]
- Lamb, R.A.; Krug, R.M. Orthomyxoviridae: The viruses and their replication. In Fields Virology; Fields, B.N., Knipe, D.M., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; pp. 1353–1395. [Google Scholar]
- Dimmock, N.J.; Easton, A.J. Defective interfering influenza virus RNAs: Time to Re-evaluate Their Clinical Potential as Broad Spectrum Antivirals? J. Virol. 2014, 88, 5217–5227. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Bentley, K.; Marriott, A.C.; Scott, P.; Dimmock, N.J.; Easton, A.J. Unexpected complexity in the interference activity of a cloned influenza defective interfering RNA. Virol. J. 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.J.; Kitame, F.; Kendal, A.P.; Maassab, H.F.; Naeve, C. Identification of sequence changes in the cold-adapted, live attenuated influenza vaccine strain, A/Ann Arbor/6/60 (H2N2). Virology 1988, 167, 554–567. [Google Scholar] [CrossRef]
- DeBorde, D.C.; Donabedian, A.M.; Herlocher, M.L.; Naeve, C.W.; Maasab, H.F. Sequence Comparison of Wild-Type and Cold-Adapted B/Ann Arbor/l/66 Influenza Virus Genes. Virology 1988, 163, 429–443. [Google Scholar] [CrossRef]
- Xue, J.; Chambers, B.S.; Hensley, S.E.; López, C.B. Propagation and characterization of influenza virus stocks that lack high levels of defective viral genomes and hemagglutinin mutations. Front. Microbiol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McLaren, L.C.; Holland, J.J. Defective interfering particles of poliovirus vaccine and vaccine reference strains. Virology 1974, 60, 579–583. [Google Scholar] [CrossRef]
- Calain, P.; Roux, L. Generation of measles virus defective interfering particles and their presence in a preparation of attenuated live-virus vaccine. J. Virol. 1988, 62, 2859–2866. [Google Scholar] [PubMed]
- Shingai, M.; Ebihara, T.; Begum, N.A.; Kato, A.; Honma, T.; Matsumoto, K.; Saito, H.; Ogura, H.; Matsumoto, M.; Seya, T. Differential type I interferon-inducing abilities of wild-type versus vaccine strains of measles virus. J. Immunol. 2007, 179, 6123–6133. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, C.; Mottet, G.; Roux, L. Wide occurrence of measles virus subgenomic RNAs in attenuated live-virus vaccines. Biologicals 1990, 18, 337–343. [Google Scholar] [CrossRef]
- Ho, T.H.; Kew, C.; Lui, P.Y.; Chan, C.P.; Satoh, T.; Akira, S.; Jin, D.Y.; Kok, K.H. PACT- and RIG-I-dependent activation of type I interferon production by a defective interfering RNA derived from measles virus vaccine. J. Virol. 2016, 90, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Victoria, J.G.; Wang, C.; Jones, M.S.; Jaing, C.; McLoughlin, K.; Gardner, S.; Delwart, E.L. Viral nucleic acids in live-attenuated vaccines: Detection of Minority Variants and an Adventitious Virus. J. Virol. 2010, 84, 6033–6040. [Google Scholar] [CrossRef] [PubMed]
- Fazekas de St Groth, S.; Graham, D.M. The production of incomplete influenza virus particles among influenza strains. Experiments in eggs. Br. J. Exp. Pathol. 1954, 35, 60–74. [Google Scholar] [PubMed]
- Meier-Ewert, H.; Dimmock, N.J. The role of the neuraminidase of the infecting virus in the generation of noninfectious (von Magnus) interfering virus. Virology 1970, 42, 794–798. [Google Scholar] [CrossRef]
- Fodor, E.; Mingay, L.J.; Crowe, M.; Deng, T.; Brownlee, G.G. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase promotes the generation of defective interfering RNAs. J. Virol. 2003, 77, 5017–5020. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Tobita, K. Mutation in NS2, a non-structural protein of influenza A virus, extragenically causes aberrant replication and expression of the PA gene, and leads to the generation of defective interfering particles. Proc. Natl. Acad. Sci. USA. 1990, 87, 5988–5992. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Tominaga, K.; Tobita, K.; Ohta, S. An amino acid change in the non-structural NS2 protein of an influenza A virus mutant is responsible for the generation of defective interfering (DI) particles by amplifying DI RNAs and suppressing complementary RNA synthesis. J. Gen. Virol. 1994, 75, 43–53. [Google Scholar] [CrossRef] [PubMed]
- López, C.B. Defective Viral Genomes: Critical Danger Signals of Viral Infections. J. Virol. 2014, 16, 8720–8723. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.J.; Scott, P.D.; Edworthy, N.L.; Meng, B.; Marriott, A.C.; Dimmock, N.J. A novel broad-spectrum treatment for respiratory virus infections: Influenza-based Defective Interfering Virus Provides Protection against Pneumovirus Infection in vivo. Vaccine 2011, 29, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.D.; Meng, B.; Marriott, A.C.; Easton, A.J.; Dimmock, N.J. DI influenza A virus protects in vivo against disease caused by a heterologous influenza B virus. J. Gen. Virol. 2011, 92, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
| Virus | Segment | Full-Length Segment (nts) a | Total Number of DI RNAs Sequenced | Complex DI RNAs b | Range in Position of First Breakpoint c | Range in Position of Last Breakpoint | Range in Size of DI RNAs (nts) | Median DI RNA (nts) | Mean DI RNA (nts) |
|---|---|---|---|---|---|---|---|---|---|
| A/Ann Arbor/6/60 | 1 | 2341 | 24 (23) d | 3/23 | 102–264 | 1980–2154 | 311–587 | 413 | 438.3 |
| 2 | 2341 | 13 | 8/13 | 54–468 | 1876–2259 | 206–786 | 379 | 454.6 | |
| 3 | 2233 | 17 | 5/17 | 82–319 | 1829–2257 | 257–713 | 447 | 430.6 | |
| B/Ann Arbor/1/66 | 1 | 2369 | 12 | 1/12 | 90–511 | 1730–2218 | 361–809 | 537.5 | 538.6 |
| 2 | 2396 | 11 | 0/11 | 136–429 | 1936–2203 | 387–786 | 543 | 573.2 | |
| 3 | 2308 | 11 | 0/11 | 73–326 | 1954–2139 | 496–658 | 540 | 546.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, P.S.; Easton, A.J.; Dimmock, N.J. Live Attenuated Influenza Vaccine contains Substantial and Unexpected Amounts of Defective Viral Genomic RNA. Viruses 2017, 9, 269. https://doi.org/10.3390/v9100269
Gould PS, Easton AJ, Dimmock NJ. Live Attenuated Influenza Vaccine contains Substantial and Unexpected Amounts of Defective Viral Genomic RNA. Viruses. 2017; 9(10):269. https://doi.org/10.3390/v9100269
Chicago/Turabian StyleGould, Philip S., Andrew J. Easton, and Nigel J. Dimmock. 2017. "Live Attenuated Influenza Vaccine contains Substantial and Unexpected Amounts of Defective Viral Genomic RNA" Viruses 9, no. 10: 269. https://doi.org/10.3390/v9100269




