Venezuelan Equine Encephalitis Virus Capsid—The Clever Caper
Abstract
:1. VEEV Overview
2. VEEV Virion and Genome Structure
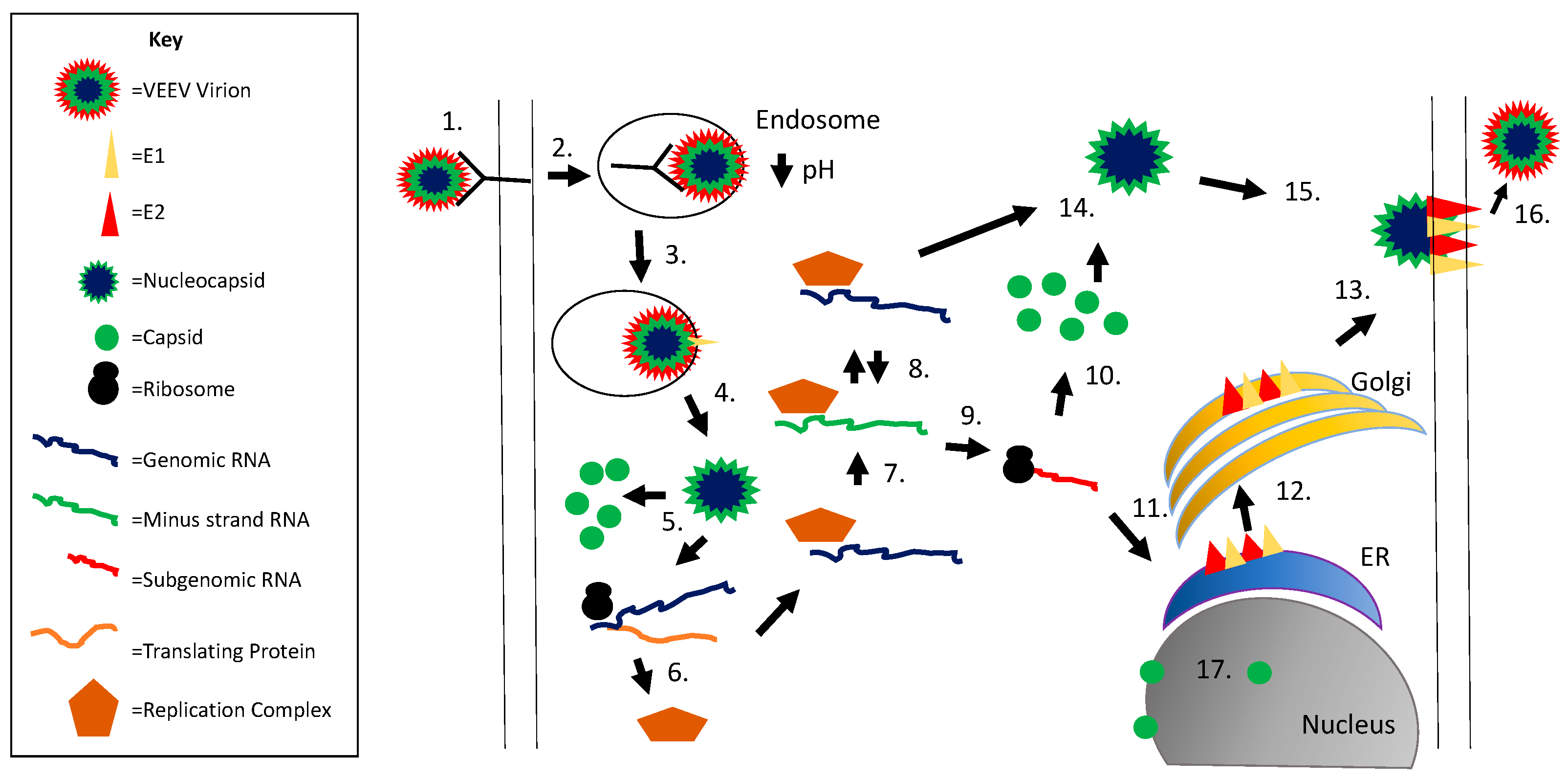
3. Alphavirus Replication Cycle
4. VEEV Capsid Structure and Function
5. Capsid’s Role in Viral Assembly
6. Capsid’s Role in Innate Immune Response Suppression
7. nsP2 Inhibits Innate Immune Responses
8. Capsid as a Target for Therapeutic and Rational Vaccine Design
9. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [PubMed]
- Powers, A.M.; Roehrig, J.T. Alphaviruses. Methods Mol. Biol. 2011, 665, 17–38. [Google Scholar] [PubMed]
- Kubes, V.; Rios, F.A. The causative agent of infectious equine encephalomyelitis in venezuela. Science 1939, 90, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Kubes, V.; Rios, F.A. Equine encephalomyelitis in venezuela: Advance data concerning the causative agent. Can. J. Comp. Med. 1939, 3, 43–44. [Google Scholar] [PubMed]
- Walton, T.E.; Holbrook, F.R.; Bolivar-Raya, R.; Ferrer-Romero, J.; Ortega, M.D. Venezuelan equine encephalomyelitis and african horse sickness. Current status and review. Ann. N. Y. Acad. Sci. 1992, 653, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Anishchenko, M.; Bowen, R.; Brault, A.C.; Estrada-Franco, J.G.; Fernandez, Z.; Greene, I.; Ortiz, D.; Paessler, S.; Powers, A.M. Genetic determinants of venezuelan equine encephalitis emergence. In Emergence and Control of Zoonotic Viral Encephalitides; Springer: Vienna, Austria, 2004; pp. 43–64. [Google Scholar]
- Johnson, K.M.; Martin, D.H. Venezuelan equine encephalitis. Adv. Vet. Sci. Comp. Med. 1974, 18, 79–116. [Google Scholar] [PubMed]
- Weaver, S.C.; Ferro, C.; Barrera, R.; Boshell, J.; Navarro, J.C. Venezuelan equine encephalitis. Annu. Rev. Entomol. 2004, 49, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Zacks, M.A.; Paessler, S. Encephalitic alphaviruses. Vet. Microbiol. 2010, 140, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Barrett, A.D.T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.; Castro, F.; Bonilla, N.J.; Gaskin de Urdaneta, A.; Hutchins, G.M. The systemic pathology of venezuelan equine encephalitis virus infection in humans. Am. J. Trop. Med. Hyg. 1985, 34, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Burke, C.W.; Tesfay, M.Z.; Glass, P.J.; Klimstra, W.B.; Ryman, K.D. Eastern and venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: Impact of altered cell tropism on pathogenesis. J. Virol. 2008, 82, 10634–10646. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Gebhardt, L.P.; Thorpe, B.D. Epidemiological aspects of venezuelan equine encephalitis virus infections. Bacteriol. Rev. 1967, 31, 65–81. [Google Scholar] [PubMed]
- Croddy, E. Chemical and Biological Warfare: A Comprehensive Survey for the Concerned Citizen; Copernicus Books: New York, NY, USA, 2002; p. 306. [Google Scholar]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guillen, J.; Rabah, N.; Blanjoie, A.; Debart, F.; Vasseur, J.J.; Canard, B.; Decroly, E.; Coutard, B. mRNA capping by venezuelan equine encephalitis virus nsp1: Functional characterization and implications for antiviral research. J. Virol. 2015, 89, 8292–8303. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Atasheva, S.; Frolova, E.I.; Frolov, I. Venezuelan equine encephalitis virus nsp2 protein regulates packaging of the viral genome into infectious virions. J. Virol. 2013, 87, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.; Brooks-Faulconer, T.; Lark, T.; Keck, F.; Bailey, C.; Raman, V.; Narayanan, A. Venezuelan equine encephalitis virus non-structural protein 3 (nsp3) interacts with RNA helicases ddx1 and ddx3 in infected cells. Antivir. Res. 2016, 131, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kamer, G.; Argos, P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984, 12, 7269–7282. [Google Scholar] [CrossRef] [PubMed]
- Lemm, J.A.; Rice, C.M. Assembly of functional sindbis virus RNA replication complexes: Requirement for coexpression of p123 and p34. J. Virol. 1993, 67, 1905–1915. [Google Scholar] [PubMed]
- Paessler, S.; Weaver, S.C. Vaccines for venezuelan equine encephalitis. Vaccine 2009, 27, D80–D85. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.Y.-S.; Ng, M.M.-L.; Chu, J.J.H. Replication of alphaviruses: A review on the entry process of alphaviruses into cells. Adv. Virol. 2011, 2011, 249640. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus entry and membrane fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G. The regulation of disassembly of alphavirus cores. Arch. Virol. 2009, 154, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Rumenapf, T.; Weir, R.C.; Kuhn, R.J.; Wang, K.S.; Strauss, E.G. Cellular Receptors for Alphaviruses; Cold Spring Harbor Monograph Series; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1994; Volume 28, pp. 141–164. [Google Scholar]
- Kolokoltsov, A.A.; Fleming, E.H.; Davey, R.A. Venezuelan equine encephalitis virus entry mechanism requires late endosome formation and resists cell membrane cholesterol depletion. Virology 2006, 347, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, T.M.; Moore, A.C.; Kolokoltsov, A.A.; Davey, R.A. Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins rab5 and rab7. Virology 2007, 369, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, D.L.; Ahn, A.; Chatterjee, P.K.; Kielian, M. Formation and characterization of the trimeric form of the fusion protein of Semliki forest virus. J. Virol. 2000, 74, 7772–7780. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G.; Wengler, G. In Vitro analysis of factors involved in the disassembly of sindbis virus cores by 60s ribosomal subunits identifies a possible role of low pH. J. Gen. Virol. 2002, 83, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.G.; de Groot, R.J.; Levinson, R.; Strauss, J.H. Identification of the active site residues in the nsp2 proteinase of sindbis virus. Virology 1992, 191, 932–940. [Google Scholar] [CrossRef]
- Kaariainen, L.; Ahola, T. Functions of alphavirus nonstructural proteins in RNA replication. Prog. Nucleic Acid. Res. Mol. Biol. 2002, 71, 187–222. [Google Scholar] [PubMed]
- Froshauer, S.; Kartenbeck, J.; Helenius, A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J. Cell Biol. 1988, 107, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New world and old world alphaviruses have evolved to exploit different components of stress granules, FXR and g3bp proteins, for assembly of viral replication complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.D.; Varjak, M.; Lulla, A.; Eng, K.E.; Merits, A.; Karlsson Hedestam, G.B.; McInerney, G.M. Sequestration of g3bp coupled with efficient translation inhibits stress granules in Semliki forest virus infection. Mol. Biol. Cell 2012, 23, 4701–4712. [Google Scholar] [CrossRef] [PubMed]
- Scholte, F.E.; Tas, A.; Albulescu, I.C.; Zusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J. Stress granule components g3bp1 and g3bp2 play a proviral role early in chikungunya virus replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Garoff, H.; Huylebroeck, D.; Robinson, A.; Tillman, U.; Liljestrom, P. The signal sequence of the p62 protein of Semliki forest virus is involved in initiation but not in completing chain translocation. J. Cell Biol. 1990, 111, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G. Structure and synthesis of the core protein: Role in regulation of assembly and disassembly of alphavirus and flavivirus cores. In New Aspects of Positive-Strand RNA Viruses; American Society for Microbiology: Washington, DC, USA, 1990; pp. 227–236. [Google Scholar]
- Schlesinger, M.J.; Schlesinger, S. Formation and assembly of alphavirus glycoproteins. In The Togaviridae and Flaviviridae; Springer: Berlin, Germany, 1986; pp. 121–148. [Google Scholar]
- Uchime, O.; Fields, W.; Kielian, M. The role of E3 in pH protection during alphavirus assembly and exit. J. Virol. 2013, 87, 10255–10262. [Google Scholar] [CrossRef] [PubMed]
- Soonsawad, P.; Xing, L.; Milla, E.; Espinoza, J.M.; Kawano, M.; Marko, M.; Hsieh, C.; Furukawa, H.; Kawasaki, M.; Weerachatyanukul, W.; et al. Structural evidence of glycoprotein assembly in cellular membrane compartments prior to alphavirus budding. J. Virol. 2010, 84, 11145–11151. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.M.; Brown, D.T.; Rothnagel, R.; Chiu, W.; Schoepp, R.J.; Johnston, R.E.; Prasad, B. Three-dimensional structure of a membrane-containing virus. Proc. Natl. Acad. Sci. USA 1993, 90, 9095–9099. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Alwell-Warda, K.; Weaver, S.C.; Chiu, W.; Watowich, S.J. Venezuelan equine encephalomyelitis virus structure and its divergence from old world alphaviruses. J. Virol. 2001, 75, 9532–9537. [Google Scholar] [CrossRef] [PubMed]
- Kinney, R.M.; Pfeffer, M.; Tsuchiya, K.R.; Chang, G.-J.J.; Roehrig, J.T. Nucleotide sequences of the 26s mRNAs of the viruses defining the venezuelan equine encephalitis antigenic complex. Am. J. Trop. Med. Hyg. 1998, 59, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hryc, C.F.; Cong, Y.; Liu, X.; Jakana, J.; Gorchakov, R.; Baker, M.L.; Weaver, S.C.; Chiu, W. 4.4 å cryo-em structure of an enveloped alphavirus venezuelan equine encephalitis virus. EMBO J. 2011, 30, 3854–3863. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.M. Understanding the Assembly of Simple SsRNA Virus Nucleocapsids. Ph.D. Thesis, The University of Texas Medical Branch Graduate School of Biomedical Sciences, Ann Arbor, MI, USA, 2010. [Google Scholar]
- Wengler, G.; Würkner, D.; Wengler, G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology 1992, 191, 880–888. [Google Scholar] [CrossRef]
- Owen, K.E.; Kuhn, R.J. Identification of a region in the sindbis virus nucleocapsid protein that is involved in specificity of RNA encapsidation. J. Virol. 1996, 70, 2757–2763. [Google Scholar] [PubMed]
- Dalgarno, L.; Rice, C.M.; Strauss, J.H. Ross river virus 26s RNA: Complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology 1983, 129, 170–187. [Google Scholar] [CrossRef]
- Rice, C.M.; Strauss, J.H. Nucleotide sequence of the 26s mRNA of sindbis virus and deduced sequence of the encoded virus structural proteins. Proc. Natl. Acad. Sci. USA 1981, 78, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-K.; Tong, L.; Minor, W.; Dumas, P.; Boege, U.; Rossmann, M.G.; Wengler, G. Structure of sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature 1991, 354, 37. [Google Scholar] [CrossRef] [PubMed]
- Belyi, V.A.; Muthukumar, M. Electrostatic origin of the genome packing in viruses. Proc. Natl. Acad. Sci. USA 2006, 103, 17174–17178. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Owen, K.E.; Tellinghuisen, T.L.; Gorbalenya, A.E.; Kuhn, R.J. Alphavirus nucleocapsid protein contains a putative coiled coil α-helix important for core assembly. J. Virol. 2001, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lulla, V.; Kim, D.Y.; Frolova, E.I.; Frolov, I. The amino-terminal domain of alphavirus capsid protein is dispensable for viral particle assembly but regulates RNA encapsidation through cooperative functions of its subdomains. J. Virol. 2013, 87, 12003–12019. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, M.S.; Watowich, S.J.; Gorenstein, D.G. Chemiluminescence-based electrophoretic mobility shift assay of rna–protein interactions: Application to binding of viral capsid proteins to RNA. J. Virol. Methods 2006, 131, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, J.M.; Lulla, V.; Kim, D.Y.; Frolova, E.I.; Frolov, I. The sd1 subdomain of venezuelan equine encephalitis virus capsid protein plays a critical role in nucleocapsid and particle assembly. J. Virol. 2016, 90, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Navaratnarajah, C.; Kuhn, R.J. A heterologous coiled coil can substitute for helix I of the sindbis virus capsid protein. J. Virol. 2003, 77, 8345–8353. [Google Scholar] [CrossRef] [PubMed]
- Jelke, J.; Fros Pijlman, G.P. Alphavirus infection: Host cell shut-off and inhibition of antiviral responses. Viruses 2016, 8, 166. [Google Scholar]
- Garmashova, N.; Gorchakov, R.; Volkova, E.; Paessler, S.; Frolova, E.; Frolov, I. The old world and new world alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J. Virol. 2007, 81, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Garmashova, N.; Frolov, I.; Frolova, E. Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in mammalian but not in mosquito cells. J. Virol. 2008, 82, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Kim, D.Y.; Frolova, E.I.; Frolov, I. Venezuelan equine encephalitis virus variants lacking transcription inhibitory functions demonstrate highly attenuated phenotype. J. Virol. 2015, 89, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Garmashova, N.; Atasheva, S.; Kang, W.; Weaver, S.C.; Frolova, E.; Frolov, I. Analysis of venezuelan equine encephalitis virus capsid protein function in the inhibition of cellular transcription. J. Virol. 2007, 81, 13552–13565. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Fish, A.; Fornerod, M.; Frolova, E.I. Venezuelan equine encephalitis virus capsid protein forms a tetrameric complex with crm1 and importin α/β that obstructs nuclear pore complex function. J. Virol. 2010, 84, 4158–4171. [Google Scholar] [CrossRef] [PubMed]
- Becskei, A.; Mattaj, I.W. The strategy for coupling the rangtp gradient to nuclear protein export. Proc. Natl. Acad. Sci. USA 2003, 100, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Görlich, D.; Seewald, M.J.; Ribbeck, K. Characterization of ran-driven cargo transport and the rangtpase system by kinetic measurements and computer simulation. EMBO J. 2003, 22, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, P.B.; Dendy, E.; Eddy, G.A. Correlates to increased lethality of attenuated venezuelan encephalitis virus vaccine for immunosuppressed hamsters. Infect. Immun. 1974, 9, 924–930. [Google Scholar] [PubMed]
- Shope, R.E.; Causey, O.R.; De Andrade, A.H.P.; Theiler, M. The venezuelan equine encephalomyelitis complex of group a arthropod-borne viruses, including mucambo and pixuna from the amazon region of brazil. Am. J. Trop. Med. Hyg. 1964, 13, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-K.; Lu, G.; Lee, S.; Wengler, G.; Rossmann, M.G. Structure of Semliki forest virus core protein. Proteins-Struct. Funct. Genet. 1997, 27, 345–359. [Google Scholar] [CrossRef]
- Tong, L.; Choi, H.-K.; Minor, W.; Rossmann, M. The structure determination of sindbis virus core protein using isomorphous replacement and molecular replacement averaging between two crystal forms. Acta Crystallogr. Sect. A 1992, 48, 430–442. [Google Scholar] [CrossRef]
- Hahn, C.S.; Strauss, J.H. Site-directed mutagenesis of the proposed catalytic amino acids of the sindbis virus capsid protein autoprotease. J. Virol. 1990, 64, 3069–3073. [Google Scholar] [PubMed]
- Ten Dam, E.; Flint, M.; Ryan, M.D. Virus-encoded proteinases of the togaviridae. J. Gen. Virol. 1999, 80, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Owen, K.E.; Choi, H.-K.; Lee, H.; Lu, G.; Wengler, G.; Brown, D.T.; Rossmann, M.G.; Kuhn, R.J. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure 1996, 4, 531–541. [Google Scholar] [CrossRef]
- Mancini, E.J.; Clarke, M.; Gowen, B.E.; Rutten, T.; Fuller, S.D. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki forest virus. Mol. Cell 2000, 5, 255–266. [Google Scholar] [CrossRef]
- Kim, D.Y.; Firth, A.E.; Atasheva, S.; Frolova, E.I.; Frolov, I. Conservation of a packaging signal and the viral genome RNA packaging mechanism in alphavirus evolution. J. Virol. 2011, 85, 8022–8036. [Google Scholar] [CrossRef] [PubMed]
- Tellinghuisen, T.L.; Hamburger, A.E.; Fisher, B.R.; Ostendorp, R.; Kuhn, R.J. In Vitro assembly of alphavirus cores by using nucleocapsid protein expressed in Escherichia coli. J. Virol. 1999, 73, 5309–5319. [Google Scholar] [PubMed]
- Geigenmüller-Gnirke, U.; Nitschko, H.; Schlesinger, S. Deletion analysis of the capsid protein of sindbis virus: Identification of the RNA binding region. J. Virol. 1993, 67, 1620–1626. [Google Scholar] [PubMed]
- Weiss, B.; Schlesinger, S. Defective interfering particles of sindbis virus do not interfere with the homologous virus obtained from persistently infected BHK cells but do interfere with Semliki forest virus. J. Virol. 1981, 37, 840–844. [Google Scholar] [PubMed]
- Anthony, R.P.; Brown, D.T. Protein-protein interactions in an alphavirus membrane. J. Virol. 1991, 65, 1187–1194. [Google Scholar] [PubMed]
- Von Bonsdorff, C.; Harrison, S. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J. Virol. 1975, 16, 141. [Google Scholar] [PubMed]
- Zheng, Y.; Kielian, M. Imaging of the alphavirus capsid protein during virus replication. J. Virol. 2013, 87, 9579–9589. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Alwell-Warda, K.; Weaver, S.C.; Chiu, W.; Watowich, S.J. Structure of isolated nucleocapsids from venezuelan equine encephalitis virus and implications for assembly and disassembly of enveloped virus. J. Virol. 2003, 77, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Lokesh, G.L.; Sherman, M.; Watowich, S. Structure of a venezuelan equine encephalitis virus assembly intermediate isolated from infected cells. Virology 2010, 406, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Garoff, H. The budding mechanisms of enveloped animal viruses. J. Gen. Virol. 1980, 50, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, M.; Liljeström, P.; Garoff, H. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J. Virol. 1992, 66, 4737–4747. [Google Scholar] [PubMed]
- Garmashova, N.; Gorchakov, R.; Frolova, E.; Frolov, I. Sindbis virus nonstructural protein nsp2 is cytotoxic and inhibits cellular transcription. J. Virol. 2006, 80, 5686–5696. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Weaver, S.C.; Basler, C.F. Capsid protein of eastern equine encephalitis virus inhibits host cell gene expression. J. Virol. 2007, 81, 3866–3876. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gardner, C.L.; Burke, C.W.; Ryman, K.D.; Klimstra, W.B. Similarities and differences in antagonism of neuron alpha/beta interferon responses by Venezuelan equine encephalitis and Sindbis alphaviruses. J. Virol. 2009, 83, 10036–10047. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Sun, C.; Metthew Lam, L.K.; Gardner, C.L.; Ryman, K.D.; Klimstra, W.B. Host translation shutoff mediated by non-structural protein 2 is a critical factor in the antiviral state resistance of Venezuelan equine encephalitis virus. Virology 2016, 496, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Levinson, R.S.; Strauss, J.H.; Strauss, E.G. Complete sequence of the genomic RNA of o’nyong-nyong virus and its use in the construction of alphavirus phylogenetic trees. Virology 1990, 175, 110–123. [Google Scholar] [CrossRef]
- Karlsen, M.; Yousaf, M.; Villoing, S.; Nylund, A.; Rimstad, E. The amino terminus of the salmonid alphavirus capsid protein determines subcellular localization and inhibits cellular proliferation. Arch. Virol. 2010, 155, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, L.; Pinkham, C.; Baer, A.; Amaya, M.; Narayanan, A.; Wagstaff, K.M.; Jans, D.A.; Kehn-Hall, K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce venezuelan equine encephalitis virus replication. Antivir. Res. 2013, 100, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, L.; Pinkham, C.; de la Fuente, C.; Brahms, A.; Shafagati, N.; Wagstaff, K.M.; Jans, D.A.; Tamir, S.; Kehn-Hall, K. Selective inhibitor of nuclear export (sine) compounds alter new world alphavirus capsid localization and reduce viral replication in mammalian cells. PLoS Negl. Trop. Dis. 2016, 10, e0005122. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Leung, L.W.; Wang, E.; Weaver, S.C.; Basler, C.F. A five-amino-acid deletion of the eastern equine encephalitis virus capsid protein attenuates replication in mammalian systems but not in mosquito cells. J. Virol. 2008, 82, 6972–6983. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.D.; White, L.J.; Morrison, T.E.; Montgomery, S.A.; Whitmore, A.C.; Johnston, R.E.; Heise, M.T. Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff. J. Virol. 2009, 83, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Peltier, D.C.; Lazear, H.M.; Farmer, J.R.; Diamond, M.S.; Miller, D.J. Neurotropic arboviruses induce interferon regulatory factor 3-mediated neuronal responses that are cytoprotective, interferon independent, and inhibited by western equine encephalitis virus capsid. J. Virol. 2013, 87, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Akhrymuk, I.; Kulemzin, S.V.; Frolova, E.I. Evasion of the innate immune response: The old world alphavirus nsp2 protein induces rapid degradation of rpb1, a catalytic subunit of RNA polymerase II. J. Virol. 2012, 86, 7180–7191. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Liu, W.J.; Prow, N.A.; Geertsema, C.; Ligtenberg, M.; Vanlandingham, D.L.; Schnettler, E.; Vlak, J.M.; Suhrbier, A.; Khromykh, A.A.; et al. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated jak-stat signaling. J. Virol. 2010, 84, 10877–10887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frolov, I.; Akhrymuk, M.; Akhrymuk, I.; Atasheva, S.; Frolova, E.I. Early events in alphavirus replication determine the outcome of infection. J. Virol. 2012, 86, 5055–5066. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Major, L.D.; Scholte, F.E.; Gardner, J.; van Hemert, M.J.; Suhrbier, A.; Pijlman, G.P. Chikungunya virus non-structural protein 2-mediated host shut-off disables the unfolded protein response. J. Gen. Virol. 2015, 96, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Hagenbaugh, A.; Bellew, L.A.; Netesov, S.V.; Volchkov, V.E.; Chang, G.-J.J.; Clarke, D.K.; Gousset, L.; Scott, T.W.; Trent, D.W. A comparison of the nucleotide sequences of eastern and western equine encephalomyelitis viruses with those of other alphaviruses and related RNA viruses. Virology 1993, 197, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.B.; Weaver, S.C. Structure of the recombinant alphavirus western equine encephalitis virus revealed by cryoelectron microscopy. J. Virol. 2010, 84, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Rai, J.; John, L.; Schaefer, S.; Pützer, B.M.; Herchenröder, O. Chikungunya virus capsid protein contains nuclear import and export signals. Virol. J. 2013, 10, 210–269. [Google Scholar] [CrossRef] [PubMed]
- Favre, D.; Studer, E.; Michel, M.R. Two nucleolar targeting signals present in the n-terminal part of Semliki forest virus capsid protein. Arch. Virol. 1994, 137, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.; Sreejith, R.; Gulati, S.; Bharti, I.; Jain, S.; Gupta, S. Deciphering the host-pathogen protein interface in chikungunya virus-mediated sickness. Arch. Virol. 2013, 158, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Rikkonen, M.; Peränen, J.; Kääriäinen, L. Atpase and GTPASE activities associated with Semliki forest virus nonstructural protein nsp2. J. Virol. 1994, 68, 5804–5810. [Google Scholar] [PubMed]
- Gomez de Cedrón, M.; Ehsani, N.; Mikkola, M.L.; Garcı́a, J.A.; Kääriäinen, L. RNA helicase activity of Semliki forest virus replicase protein nsp2. FEBS Lett. 1999, 448, 19–22. [Google Scholar] [CrossRef]
- Hardy, W.R.; Strauss, J.H. Processing the nonstructural polyproteins of sindbis virus: Nonstructural proteinase is in the c-terminal half of nsp2 and functions both in cis and in trans. J. Virol. 1989, 63, 4653–4664. [Google Scholar] [PubMed]
- Suopanki, J.; Sawicki, D.L.; Sawicki, S.G.; Kääriäinen, L. Regulation of alphavirus 26s mrna transcription by replicase component nsp2. J. Gen. Virol. 1998, 79, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, D.L.; Perri, S.; Polo, J.M.; Sawicki, S.G. Role for nsp2 proteins in the cessation of alphavirus minus-strand synthesis by host cells. J. Virol. 2006, 80, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, L.; Ahola, T. Functions of alphavirus nonstructural proteins in RNA replication. In Progress in Nucleic Acid Research and Molecular Biology; Academic Press: Cambridge, MA, USA, 2002; Volume 71, pp. 187–222. [Google Scholar]
- Peränen, J.; Rikkonen, M.; Liljeström, P.; Kääriäinen, L. Nuclear localization of Semliki forest virus-specific nonstructural protein nsp2. J. Virol. 1990, 64, 1888–1896. [Google Scholar] [PubMed]
- Rikkonen, M.; Peranen, J.; Kaariainen, L. Nuclear and nucleolar targeting signals of Semliki forest virus nonstructural protein nsp2. Virology 1992, 189, 462–473. [Google Scholar] [CrossRef]
- Rikkonen, M. Functional significance of the nuclear-targeting and ntp-binding motifs of Semliki forest virus nonstructural protein nsp2. Virology 1996, 218, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, J.K.; Boyd, A.; Mikkola, M.L.; Kääriäinen, L. A single amino acid change in the nuclear localization sequence of the nsp2 protein affects the neurovirulence of Semliki forest virus. J. Virol. 2002, 76, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Johnston, R.E. Nuclear import and export of venezuelan equine encephalitis virus nonstructural protein 2. J. Virol. 2007, 81, 10268–10279. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.I.; Fayzulin, R.Z.; Cook, S.H.; Griffin, D.E.; Rice, C.M.; Frolov, I. Roles of nonstructural protein nsp2 and alpha/beta interferons in determining the outcome of sindbis virus infection. J. Virol. 2002, 76, 11254–11264. [Google Scholar] [CrossRef] [PubMed]
- Bouraï, M.; Lucas-Hourani, M.; Gad, H.H.; Drosten, C.; Jacob, Y.; Tafforeau, L.; Cassonnet, P.; Jones, L.M.; Judith, D.; Couderc, T.; et al. Mapping of chikungunya virus interactions with host proteins identified nsp2 as a highly connected viral component. J. Virol. 2012, 86, 3121–3134. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Gorchakov, R.; English, R.; Frolov, I.; Frolova, E. Development of sindbis viruses encoding nsp2/gfp chimeric proteins and their application for studying nsp2 functioning. J. Virol. 2007, 81, 5046–5057. [Google Scholar] [CrossRef] [PubMed]
- Burnham, A.J.; Gong, L.; Hardy, R.W. Heterogeneous nuclear ribonuclear protein k interacts with sindbis virus nonstructural proteins and viral subgenomic mrna. Virology 2007, 367, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Berglund, P.; Beard, C.W.; Johnston, R.E. Ribosomal protein s6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages. J. Virol. 2006, 80, 7729–7739. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, R.W.; Berge, T.O.; Crozier, D.; Sawyer, W.D.; Tigertt, W.D. Use of an attenuated strain of venezuelan equine encephalomyelitis virus for immunization in man. Am. J. Trop. Med. Hyg. 1963, 12, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kinney, R.M.; Chang, G.J.; Tsuchiya, K.R.; Sneider, J.M.; Roehrig, J.T.; Woodward, T.M.; Trent, D.W. Attenuation of venezuelan equine encephalitis virus strain tc-83 is encoded by the 5′-noncoding region and the e2 envelope glycoprotein. J. Virol. 1993, 67, 1269–1277. [Google Scholar] [PubMed]
- Atasheva, S.; Krendelchtchikova, V.; Liopo, A.; Frolova, E.; Frolov, I. Interplay of acute and persistent infections caused by venezuelan equine encephalitis virus encoding mutated capsid protein. J. Virol. 2010, 84, 10004–10015. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An alphascreen (r)-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sokoloski, K.J.; Nease, L.M.; May, N.A.; Gebhart, N.N.; Jones, C.E.; Morrison, T.E.; Hardy, R.W. Identification of interactions between sindbis virus capsid protein and cytoplasmic vRNA as novel virulence determinants. PLoS Pathog. 2017, 13, e1006473. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.; Keck, F.; Lindquist, M.; Voss, K.; Scavone, L.; Kehn-Hall, K.; Roberts, B.; Bailey, C.; Schmaljohn, C.; Narayanan, A. The ubiquitin proteasome system plays a role in venezuelan equine encephalitis virus infection. PLoS ONE 2015, 10, e0124792. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Willows, S.; Ilkow, C.S.; Hobman, T.C. Phosphorylation and membrane association of the rubella virus capsid protein is important for its anti-apoptotic function. Cell. Microbiol. 2014, 16, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Law, L.M.; Everitt, J.C.; Beatch, M.D.; Holmes, C.F.; Hobman, T.C. Phosphorylation of rubella virus capsid regulates its RNA binding activity and virus replication. J. Virol. 2003, 77, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Ratka, M.; Lackmann, M.; Ueckermann, C.; Karlins, U.; Koch, G. Poliovirus-associated protein kinase: Destabilization of the virus capsid and stimulation of the phosphorylation reaction by Zn2+. J. Virol. 1989, 63, 3954–3960. [Google Scholar] [PubMed]
- Cartier, C.; Sivard, P.; Tranchat, C.; Decimo, D.; Desgranges, C.; Boyer, V. Identification of three major phosphorylation sites within HIV-1 capsid. Role of phosphorylation during the early steps of infection. J. Biol. Chem. 1999, 274, 19434–19440. [Google Scholar] [CrossRef] [PubMed]
| Virus | Viral Protein | Pathway/Responses Modulated | Host Protein Affected a | References |
|---|---|---|---|---|
| VEEV | capsid | Transcription, Nucleocytoplasmic trafficking | CRM1, Importin α/β1 | [58,59,61,62] |
| VEEV | nsPs | Interferon beta and gamma signaling | STAT1 | [93] |
| VEEV | nsP2 | translation | Unknown | [87] |
| EEEV | capsid | Transcription, Translation | eIF2α | [85,92] |
| WEEV | capsid | Pattern recognition receptor pathways | Unknown | [94] |
| SINV, SFV, CHIKV | nsP2 | Transcription | Rpb1 | [95] |
| CHIKV, SINV | nsP2 | Jak/STAT and interferon signaling | STAT1/2 | [96,97] |
| CHIKV | nsP2 | Translation, Transcription, Unfolded Protein Response | eIF2α | [57,98] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundberg, L.; Carey, B.; Kehn-Hall, K. Venezuelan Equine Encephalitis Virus Capsid—The Clever Caper. Viruses 2017, 9, 279. https://doi.org/10.3390/v9100279
Lundberg L, Carey B, Kehn-Hall K. Venezuelan Equine Encephalitis Virus Capsid—The Clever Caper. Viruses. 2017; 9(10):279. https://doi.org/10.3390/v9100279
Chicago/Turabian StyleLundberg, Lindsay, Brian Carey, and Kylene Kehn-Hall. 2017. "Venezuelan Equine Encephalitis Virus Capsid—The Clever Caper" Viruses 9, no. 10: 279. https://doi.org/10.3390/v9100279




