Antibody Cross-Reactivity between Porcine Cytomegalovirus (PCMV) and Human Herpesvirus-6 (HHV-6)
Abstract
:1. Introduction
2. Materials and Methods
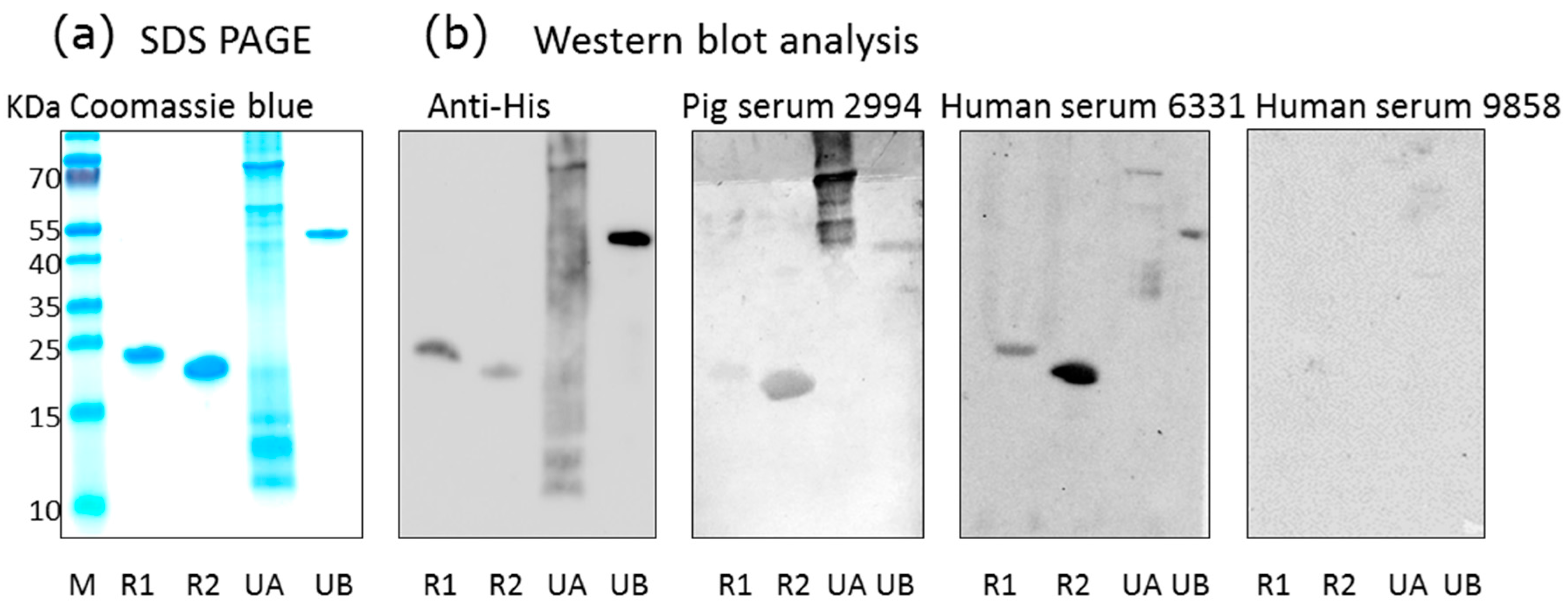
2.1. Recombinant Antigens and Western Blot Analysis for the Detection of PCMV-Specific Antibodies
2.2. Sera
2.3. Nucleotide Sequence Alignment
2.4. Assay for the Detection of Antibodies against HCMV
2.5. Immunofluorescence Assays for the Detection of Antibodies against HHV-6 and HHV-7
2.6. Real-Time PCR for the Detection of PCMV
3. Results
3.1. Detection of Antibodies against PCMV in Pig and Human Sera
3.2. Sequence Comparison of PCMV and Other Herpesviruses
3.3. Human Antibodies against PCMV and HCMV
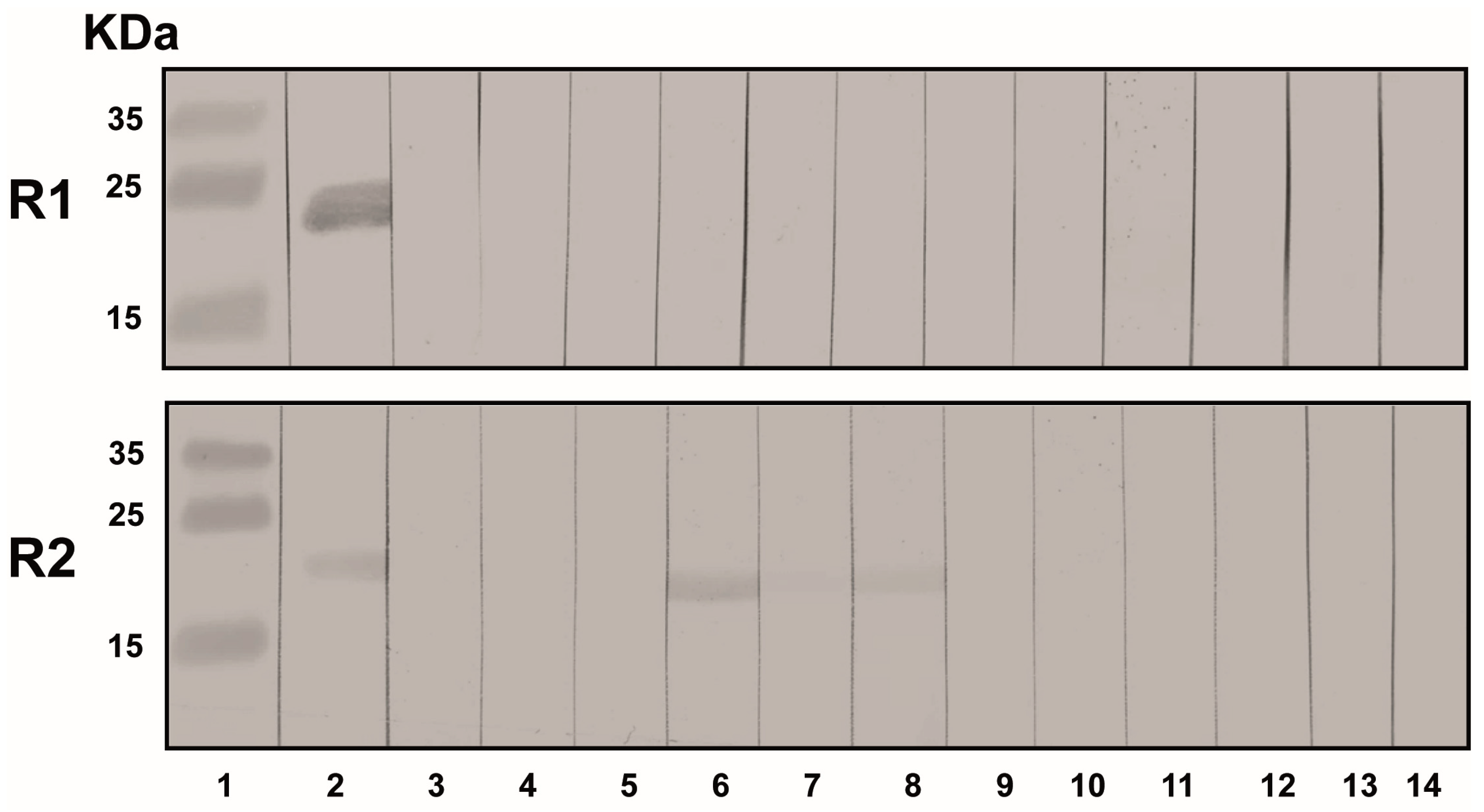
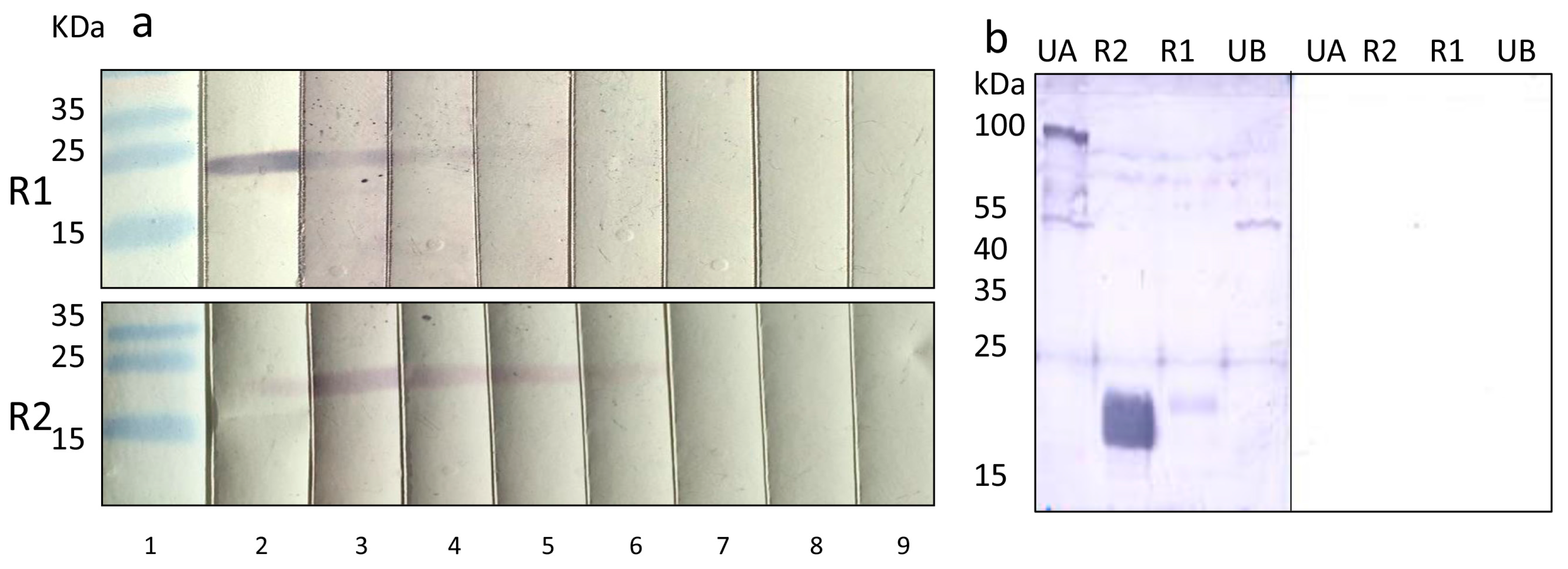
3.4. Pig Sera Reacting with PCMV Reacted with HHV-6
3.5. Human Sera Reacting with HHV-6 Reacted with PCMVand Vice Versa
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davison, A.J. Herpesvirus systematics. Vet. Microbiol. 2010, 143, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C.; Keil, G.M.; Fuchs, W. Molecular Biology of Animal Herpesviruses. In Animal Viruses: Molecular Biology; Mettenleiter, T.C., Sobrino, F., Eds.; Caister Academic Press: Poole, UK, 2008. [Google Scholar]
- Gu, W.; Zeng, N.; Zhou, L.; Ge, X.; Guo, X.; Yang, H. Genomic organization and molecular characterization of porcine cytomegalovirus. Virology 2014, 460–461, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Xenotransplantation and porcine cytomegalovirus. Xenotransplantation 2015, 22, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Puga Yung, G.L.; Rieben, R.; Bühler, L.; Schuurman, H.J.; Seebach, J. Xenotransplantation: Where do we stand in 2016. Swiss Med. Wkly. 2017, 147, w14403. [Google Scholar] [PubMed]
- Denner, J. Recent Progress in Xenotransplantation, with Emphasis on Virological Safety. Ann. Transplant. 2016, 21, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, P.; Razonable, R.R. Cytomegalovirus infections in solid organ transplantation: A review. Infect. Chemother. 2013, 45, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Jurak, I.; Brune, W. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 2006, 25, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Whitteker, J.L.; Dudani, A.K.; Tackaberry, E.S. Human fibroblasts are permissive for porcine cytomegalovirus in vitro. Transplantation 2008, 86, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.W.; Galbraith, D.; McEwan, P.; Onions, D. Evaluation of porcine cytomegalovirus as a potential zoonotic agent in Xenotransplantation. Transplant. Proc. 1999, 31, 915. [Google Scholar] [CrossRef]
- Yamada, K.; Tasaki, M.; Sekijima, M.; Wilkinson, R.A.; Villani, V.; Moran, S.G.; Cormack, T.A.; Hanekamp, I.M.; Hawley, R.J.; Arn, J.S.; et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation 2014, 98, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, M.; Waki, S.; Sahara, H.; Tasaki, M.; Wilkinson, R.A.; Villani, V.; Shimatsu, Y.; Nakano, K.; Matsunari, H.; Nagashima, H.; et al. Results of life-supporting galactosyltransferase knockout kidneys in cynomolgus monkeys using two different sources of galactosyltransferase knockout swine. Transplantation 2014, 98, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.J.; Kuwaki, K.; Dor, F.J.; Knosalla, C.; Gollackner, B.; Wilkinson, R.A.; Sachs, D.H.; Cooper, D.K.; Fishman, J.A. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation 2004, 78, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Plotzki, E.; Keller, M.; Ivanusic, D.; Denner, J. A new Western blot assay for the detection of porcine cytomegalovirus (PCMV). J. Immunol. Methods 2016, 437, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Hiller, K.; Scheer, M.; Munch, R.; Nortemann, B.; Hempel, D.C.; Jahn, D. JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, W526–W531. [Google Scholar] [CrossRef] [PubMed]
- Plotzki, E.; Keller, M.; Ehlers, B.; Denner, J. Immunological methods for the detection of porcine lymphotropic herpesviruses (PLHV). J. Virol. Methods 2016, 233, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Plotzki, E.; Heinrichs, G.; Kubícková, B.; Ulrich, R.G.; Denner, J. Microbiological characterization of a newly established pig breed, Aachen Minipigs. Xenotransplantation 2016, 23, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tacke, S.J.; Bodusch, K.; Berg, A.; Denner, J. Sensitive and specific immunological detection methods for porcine endogenous retroviruses applicable to experimental and clinical xenotransplantation. Xenotransplantation 2001, 8, 125–135. [Google Scholar] [CrossRef] [PubMed]
- DNASTAR. Available online: www.dnastar.com (accessed on 23 October 2017).
- Neipel, F.; Ellinger, K.; Fleckenstein, B. Gene for the major antigenic structural protein (p100) of human herpesvirus-6. J. Virol. 1992, 66, 3918–3924. [Google Scholar] [PubMed]
- Mueller, N.J.; Livingston, C.; Knosalla, C.; Barth, R.N.; Yamamoto, S.; Gollackner, B.; Dor, F.J.; Buhler, L.; Sachs, D.H.; Yamada, K.; et al. Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in a pig-to-baboon xenotransplantation. J. Infect. Dis. 2004, 189, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J. Virol. Methods 2005, 306, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. (Eds.) Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Miller, C.S.; Berger, J.R.; Mootoor, Y.; Avdiushko, S.A.; Zhu, H.; Kryscio, R.J. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J. Clin. Microbiol. 2006, 44, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Widen, F.; Goltz, M.; Wittenbrink, N.; Ehlers, B.; Banks, M.; Belak, S. Identification and sequence analysis of the glycoprotein B gene of porcine cytomegalovirus. Virus Genes 2001, 23, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rea, F.; Potena, L.; Yonan, N.; Wagner, F.; Calabrese, F. Cytomegalovirus Hyper Immunoglobulin for CMV Prophylaxis in Thoracic Transplantation. Transplantation 2016, 100 (Suppl. 3), S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Buxmann, H.; Stackelberg, O.M.; Schlößer, R.L.; Enders, G.; Gonser, M.; Meyer-Wittkopf, M.; Hamprecht, K.; Enders, M. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: A retrospective analysis. J. Perinat. Med. 2012, 40, S439–S446. [Google Scholar] [CrossRef] [PubMed]
- Hamel, A.L.; Lin, L.; Sachvie, C.; Grudeski, E.; Nayar, G.P. PCR assay for detecting porcine cytomegalovirus. J. Clin. Microbiol. 1999, 37, 3767–3768. [Google Scholar] [PubMed]
- Widen, B.F.; Lowings, J.P.; Belak, S.; Banks, M. Development of a PCR system for porcine cytomegalovirus detection and determination of the putative partial sequence of its DNA polymerase gene. Epidemiol. Infect. 1999, 123, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Fryer, D.; Fishman, J.A.; Emery, V.C.; Clark, D.A. Quantitation of porcine cytomegalovirus in pig tissues by PCR. J. Clin. Microbiol. 2001, 39, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Moon, H.J.; Yang, J.S.; Park, S.J.; Song, D.S.; Kang, B.K.; Park, B.K. Multiplex PCR for the simultaneous detection of pseudorabies virus, porcine cytomegalovirus, and porcine circovirus in pigs. J. Virol. Methods 2007, 139, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Morozov, A.V.; Denner, J. New PCR diagnostic systems for the detection and quantification of porcine cytomegalovirus (PCMV). Arch. Virol. 2016, 161, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, L.; Shi, X.; Xu, Z.; Mei, M.; Xu, W.; Zhou, Y.; Guo, W.; Wang, X. Indirect-blocking ELISA for detecting antibodies against glycoprotein B (gB) of porcine cytomegalovirus (PCMV). J. Virol. Methods 2012, 186, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Xenotransplantation and Hepatitis E virus. Xenotransplantation 2015, 22, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Roque Afonso, A.M.; Pavio, N. Foodborne Transmission of Hepatitis E Virus from Raw Pork Liver Sausage, France. Emerg. Infect. Dis. 2014, 20, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.K.; Dominguez, G.; Pellett, P.E. Human herpesvirus 6. Clin. Microbiol. Rev. 1997, 10, 521–567. [Google Scholar] [PubMed]
- Kaulitz, D.; Fiebig, U.; Eschricht, M.; Wurzbacher, C.; Kurth, R.; Denner, J. Generation of neutralising antibodies against porcine endogenous retroviruses (PERVs). Virology 2011, 411, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tesini, B.L.; Epstein, L.G.; Caserta, M.T. Clinical impact of primary infection with roseoloviruses. Curr. Opin. Virol. 2014, 9, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Zerr, D.M. Roseoloviruses in transplant recipients: Clinical consequences and prospects for treatment and prevention trials. Curr. Opin. Virol. 2014, 9, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.W.; McNeilly, F.; Meehan, B.; Galbraith, D.; McArdle, P.D.; Allan, G.; Patience, C. Methods for the exclusion of circoviruses and gammaherpesviruses from pigs. Xenotransplantation 2003, 10, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Developing a Vaccine against Congenital Cytomegalovirus (CMV) Infection: What Have We Learned from Animal Models? Where Should We Go Next? Future Virol. 2013, 8, 1161–1182. [Google Scholar] [CrossRef] [PubMed]
- Fryer, J.F.; Griffiths, P.D.; Emery, V.C.; Clark, D.A. Susceptibility of porcine cytomegalovirus to antiviral drugs. J. Antimicrob. Chemother. 2004, 53, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.J.; Sulling, K.; Gollackner, B.; Yamamoto, S.; Knosalla, C.; Wilkinson, R.A.; Kaur, A.; Sachs, D.H.; Yamada, K.; Cooper, D.K.; et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am. J. Transplant. 2003, 3, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Denner, J.; Robert Koch Institute, Berlin, Germany. Personal communication, 2017.


| Number Tested | PCR | Western Blot Analysis Using PCMV Proteins | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | UA | UB | |||
| Human sera | ||||||
| Butcher and workers in the meat production industry | 24 | n.t. | 1 | 3 | n.t. | n.t. |
| Blood donors | 24 | n.t. | 1 | 0 | n.t. | n.t. |
| Pig sera | ||||||
| Aachen minipigs | 12 | 2 | 3 | 12 | 6 | 6 |
| Göttingen minipigs | 11 | 5 | 2 | 4 | 4 | 9 |
| Slaughterhouse pigs | 12 | 2 | 4 | 10 | 12 | 9 |
| Serum Number | Western Blot Analysis PCMV (1) | Result HCMV (2) | |
|---|---|---|---|
| R1 | R2 | ||
| 1 (3) | − | +/− | >250 |
| 2 (3) | − | + | >250 |
| 3 | − | + | - |
| 4 | − | +/− | - |
| 5 | − | + | - |
| 6 | − | − | >250 |
| 7 | − | − | 6.1 |
| 8 | − | − | >250 |
| 9 | − | − | 214.1 |
| 10 | − | − | 180.7 |
| Number | Serum Number | HCMV IgG (1) | Western Blot Analysis Using PCMV gB Antigens (2) | |
|---|---|---|---|---|
| R1 | R2 | |||
| 1 | 914,640 | − | − | + |
| 2 (3) | 914,644 | − | − | − |
| 3 (3) | 914,672 | + | − | + |
| 4 | 914,714 | − | − | + |
| 5 | 914,814 | + | − | − |
| 6 | 914,854 | − | + | |
| 7 | 914,855 | + | − | − |
| 8 | 914,887 | − | − | + |
| 9 | 914,978 | + | − | − |
| 10 | 914,984 | + | − | − |
| 11 (3) | 914,986 | + | − | + |
| 12 | 914,998 | + | − | − |
| 13 | 915,159 | + | − | − |
| 14 (3) | 915,164 | + | − | + |
| 15 | 915,274 | + | − | − |
| Number | Serum Number | HHV-6 (1) | PCMV Western Blot Analysis | |||
|---|---|---|---|---|---|---|
| R1 | R2 | U54A | U54B | |||
| 1 | 11,415 | − | − | − | − | − |
| 2 | 10,301 | − | − | − | − | − |
| 3 | 9858 | 32 | − | − | − | − |
| 4 | 7186 | >64 | − | + | − | − |
| 5 | 6515 | 32 | − | + | − | − |
| 6 | 6331 | >64 | − | + | + | + |
| Number | Serum Number | HHV-6 (1) | PCMV Western Blot Analysis | |||
|---|---|---|---|---|---|---|
| R1 | R2 | U54A | U54B | |||
| PCMV-positive sera | ||||||
| 1 | G2 | − | − | + | − | + |
| 2 | G13 | >64 | − | + | − | + |
| 3 | G15 | 16 | − | + | + | − |
| 4 | G23 | >>64 | +/− | + | + | + |
| 5 | G24 | 16 | − | + | − | − |
| PCMV-negative sera | ||||||
| 1 | G16 | >64 | − | − | − | − |
| 2 | G17 | >64 | − | − | − | − |
| 3 | G18 | >64 | − | − | − | − |
| 4 | G19 | >>64 | − | − | − | − |
| 5 | G20 | − | − | − | − | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiebig, U.; Holzer, A.; Ivanusic, D.; Plotzki, E.; Hengel, H.; Neipel, F.; Denner, J. Antibody Cross-Reactivity between Porcine Cytomegalovirus (PCMV) and Human Herpesvirus-6 (HHV-6). Viruses 2017, 9, 317. https://doi.org/10.3390/v9110317
Fiebig U, Holzer A, Ivanusic D, Plotzki E, Hengel H, Neipel F, Denner J. Antibody Cross-Reactivity between Porcine Cytomegalovirus (PCMV) and Human Herpesvirus-6 (HHV-6). Viruses. 2017; 9(11):317. https://doi.org/10.3390/v9110317
Chicago/Turabian StyleFiebig, Uwe, Angela Holzer, Daniel Ivanusic, Elena Plotzki, Hartmut Hengel, Frank Neipel, and Joachim Denner. 2017. "Antibody Cross-Reactivity between Porcine Cytomegalovirus (PCMV) and Human Herpesvirus-6 (HHV-6)" Viruses 9, no. 11: 317. https://doi.org/10.3390/v9110317





