Neurotropism In Vitro and Mouse Models of Severe and Mild Infection with Clinical Strains of Enterovirus 71
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
2.3. Virus Infection and Sample Collection
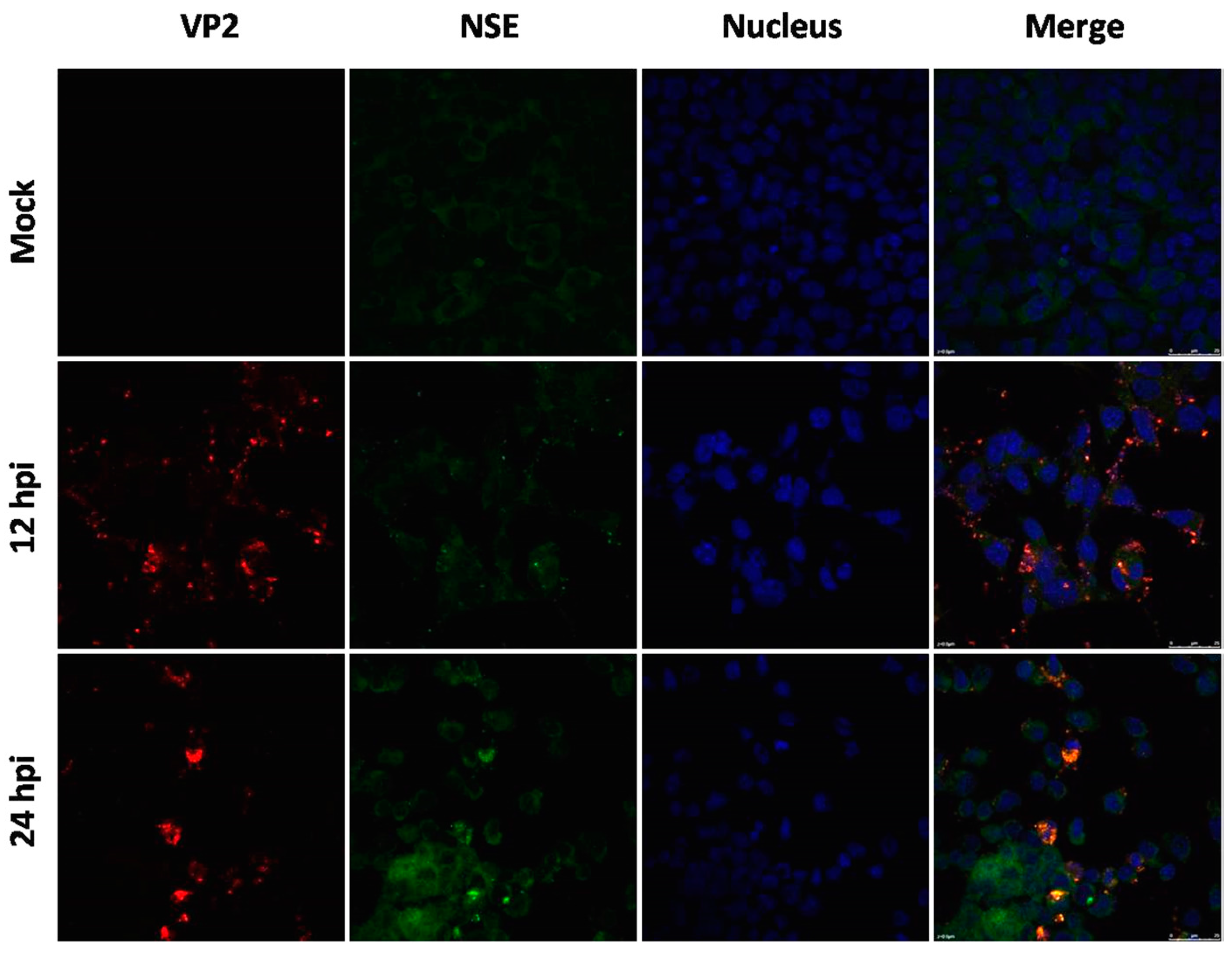
2.4. Double-Label Immunofluorescence Assay
2.5. RNA Extraction and Reverse Transcription Polymerase Chain Reaction
2.6. Nucleotide Sequencing
2.7. Phylogenetic Analysis
2.8. Nucleotide Sequence Accession Number
2.9. Severe and Mild EV71 Mouse Models and Sample Collection
2.10. RNA Extraction and Quantitative Detection of Viral RNA by Reverse Transcription Polymerase Chain Reaction
2.11. Histopathology Examination and Immunohistochemistry
2.12. Cytometric Bead Array (CBA)
2.13. Statistical Analysis
3. Results
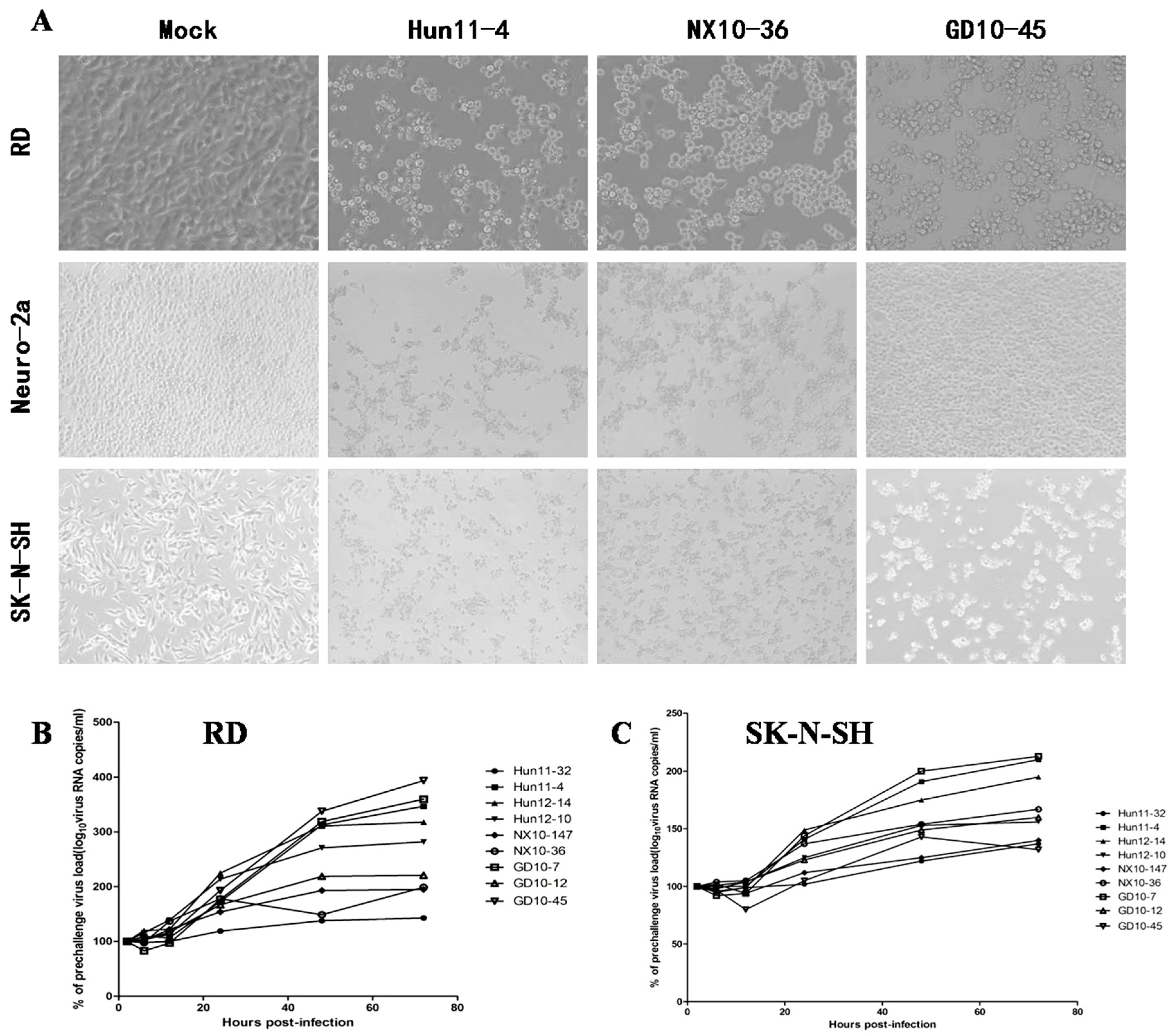
3.1. Virulence and Neurotropism of EV71 In Vitro
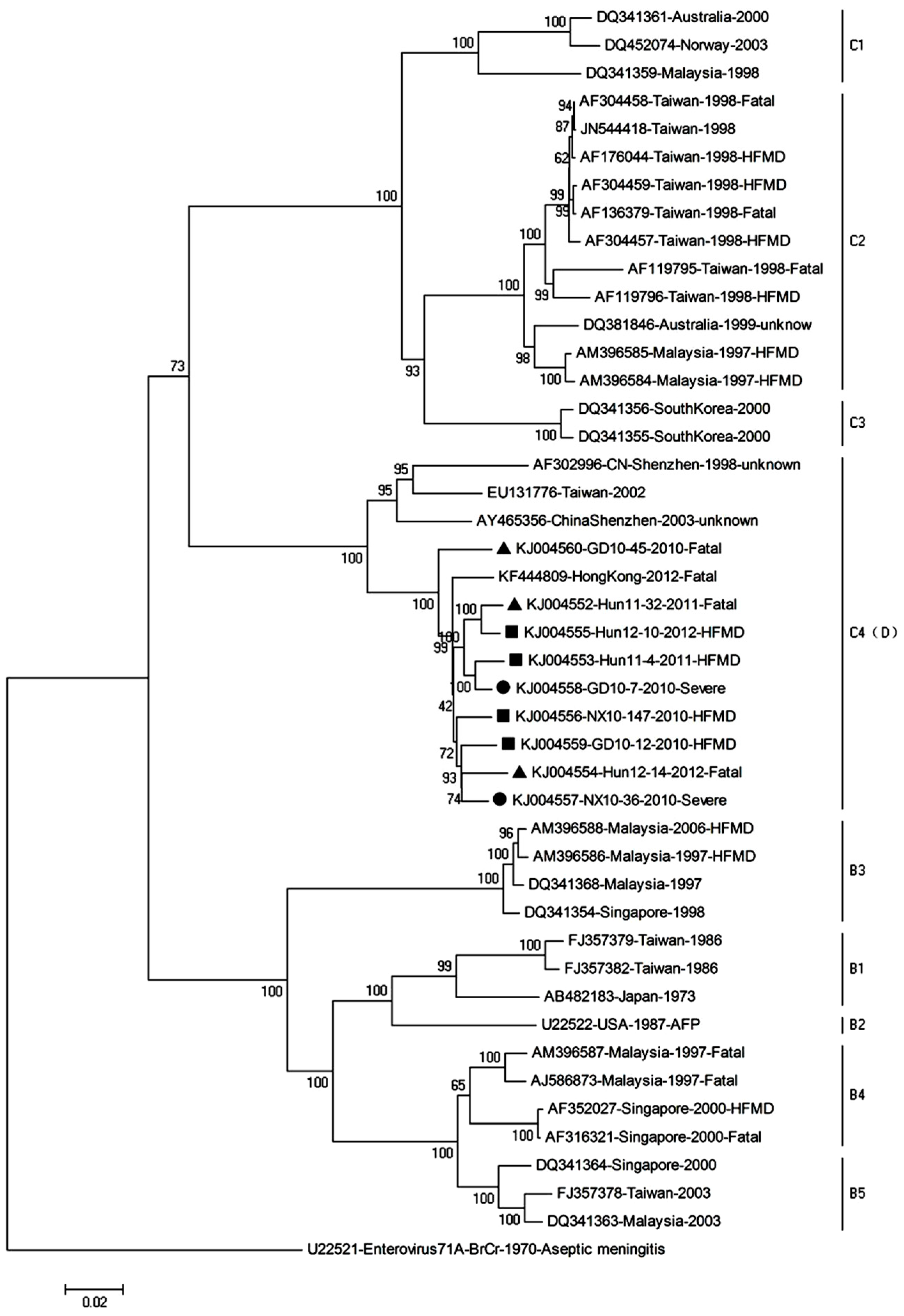
3.2. Phylogenetic Relationships of EV71 Strains
3.3. Establishment of Mouse Models of Severe and Mild EV71 Infection
3.4. EV71 Replication in Severe and Mild EV71 Mouse Models
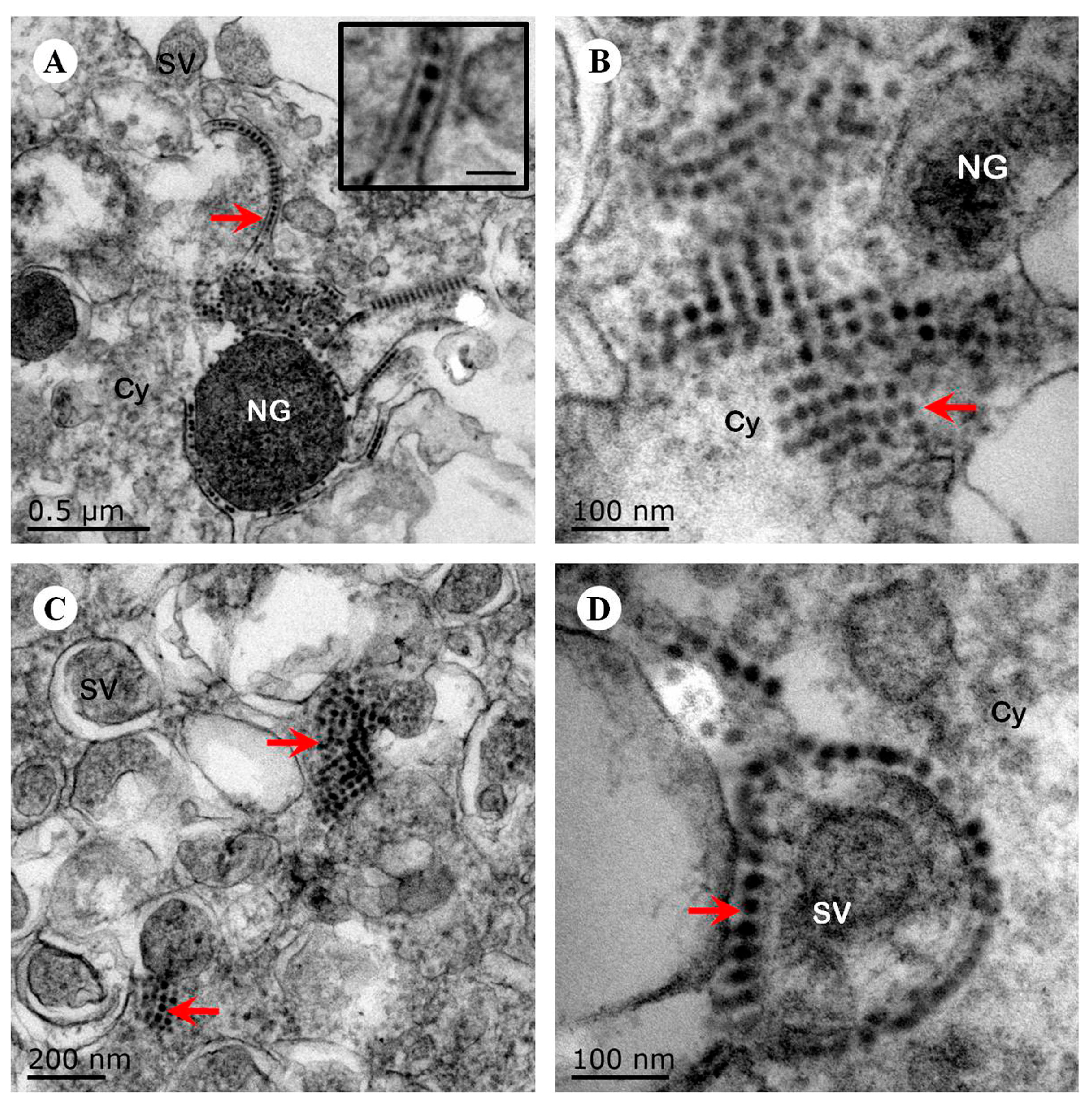
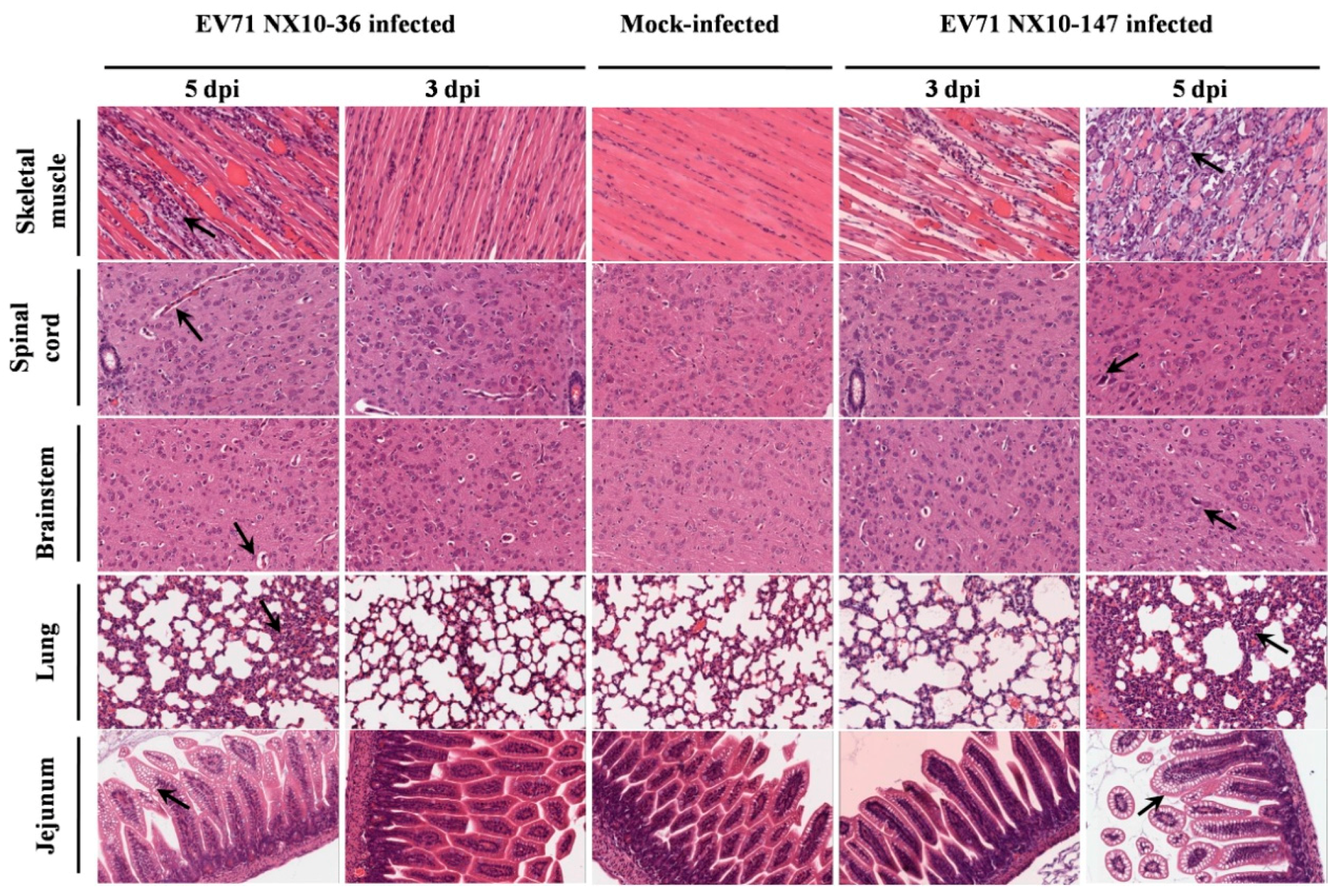
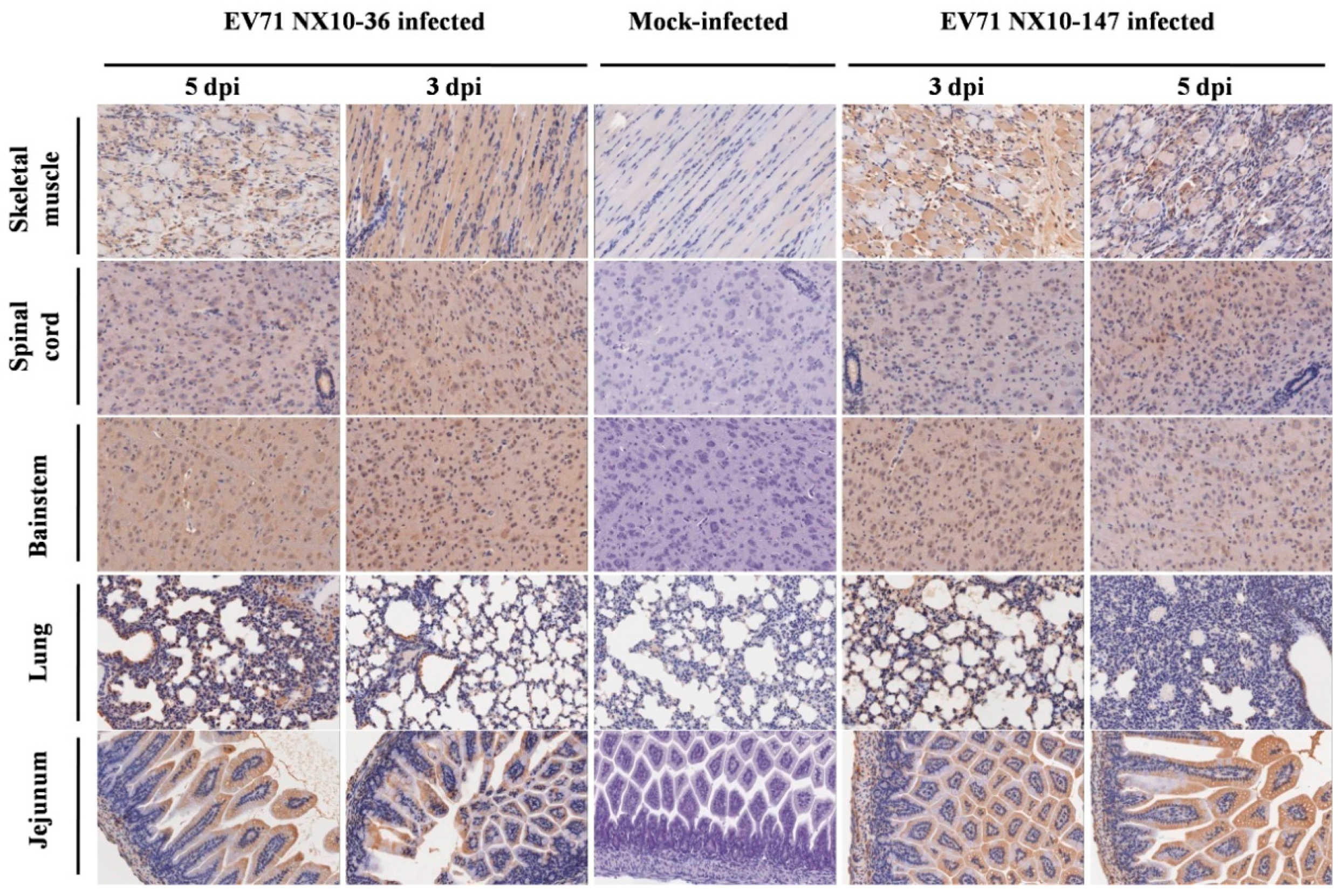
3.5. Pathology in Severe and Mild EV71 Mouse Models
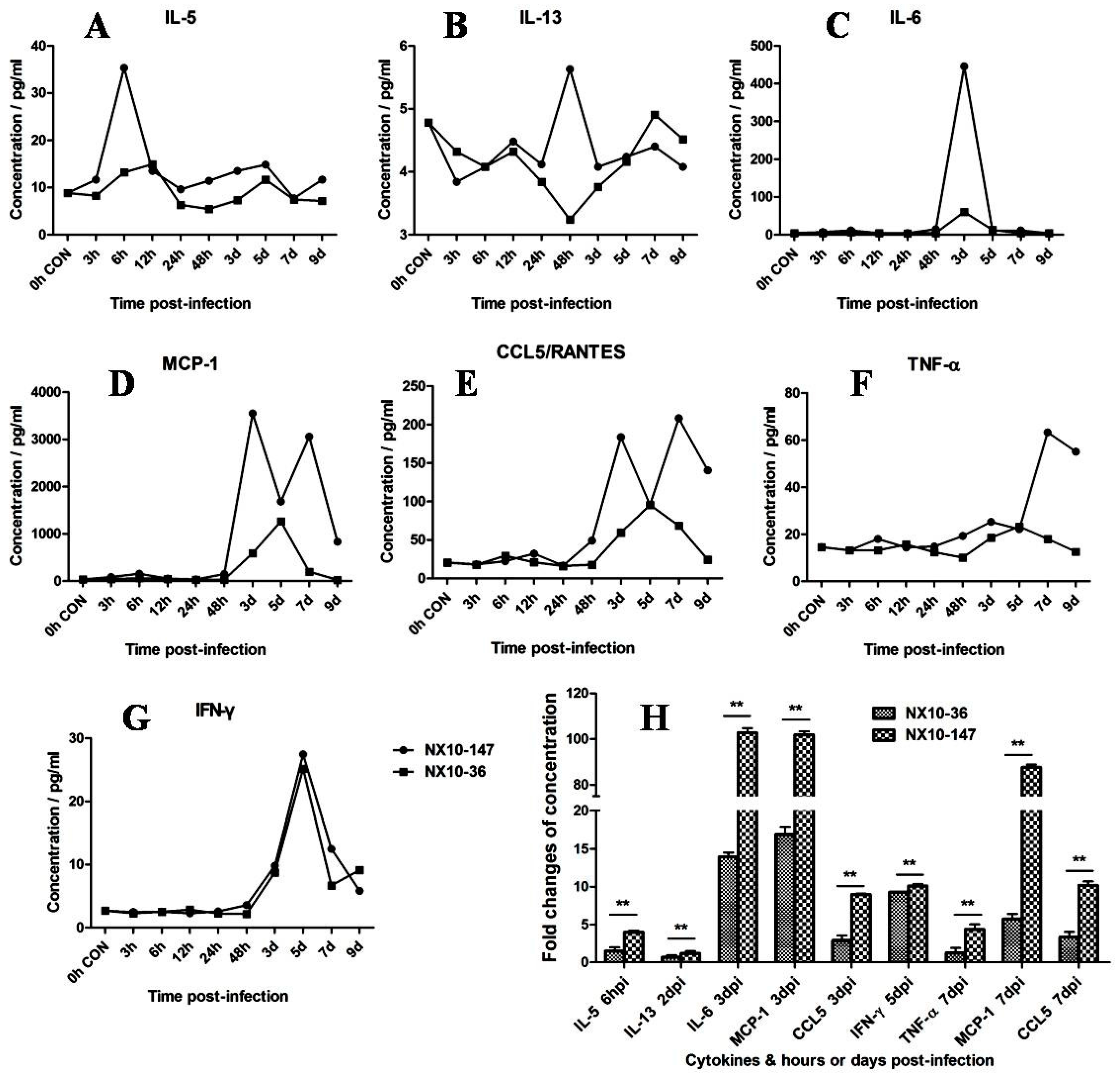
3.6. Detection of Cytokines and Chemokines in the Sera of Severe and Mild EV71 Mouse Models
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Wong, S.S.; Yip, C.C.; Lau, S.K.; Yuen, K.Y. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol. Infect. 2010, 138, 1071–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, M. Enterovirus 71: The virus, its infections and outbreaks. J. Microbiol. Immunol. Infect. 2000, 33, 205–216. [Google Scholar] [PubMed]
- McMinn, P.C. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr. Opin. Virol. 2012, 2, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Pallansch, M.A. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995, 39, 195–205. [Google Scholar] [CrossRef]
- Brown, B.A.; Oberste, M.S.; Alexander, J.P., Jr.; Kennett, M.L.; Pallansch, M.A. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J. Virol. 1999, 73, 9969–9975. [Google Scholar] [PubMed]
- Cardosa, M.J.; Perera, D.; Brown, B.A.; Cheon, D.; Chan, H.M.; Chan, K.P.; Cho, H.; McMinn, P. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: Comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 2003, 9, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Lin, T.L.; Kuo, C.Y.; Lin, M.W.; Yao, C.Y.; Liao, H.W.; Hsu, L.C.; Yang, C.F.; Yang, J.Y.; Chen, P.J.; et al. The circulation of subgenogroups B5 and C5 of enterovirus 71 in Taiwan from 2006 to 2007. Virus Res. 2008, 137, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.J.; Lee, C.S.; Hurrelbrink, R.J.; Chua, B.H.; Chua, K.B.; McMinn, P.C. Molecular epidemiology of enterovirus 71 in peninsular Malaysia, 1997–2000. Arch. Virol. 2003, 148, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.; Jung, S.M.; Shih, S.R.; Kuo, T.T.; Shieh, W.J.; Zaki, S.; Lin, T.Y.; Chang, L.Y.; Ning, H.C.; Yen, D.C. Acute encephalomyelitis during an outbreak of enterovirus type 71 infection in Taiwan: Report of an autopsy case with pathologic, immunofluorescence, and molecular studies. Mod. Pathol. 2000, 13, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.P.; Goh, K.T.; Chong, C.Y.; Teo, E.S.; Lau, G.; Ling, A.E. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 2003, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Utama, A.; Onnimala, N.; Li, C.; Li-Bi, Z.; Yu-Jie, M.; Pongsuwanna, Y.; Miyamura, T. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr. Int. 2004, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, X.J.; Wang, H.Y.; Yan, D.M.; Zhu, S.L.; Wang, D.Y.; Ji, F.; Wang, X.J.; Gao, Y.J.; Chen, L.; et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 2009, 44, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Z.; Yang, W.; Ren, J.; Tan, X.; Wang, Y.; Mao, N.; Xu, S.; Zhu, S.; Cui, A.; et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol. J. 2010, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.C.; Lau, S.K.; Woo, P.C.; Yuen, K.Y. Human enterovirus 71 epidemics: What's next? Emerg. Health Threats J. 2013, 6, 19780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.C.; Lau, S.K.; Lo, J.Y.; Chan, K.H.; Woo, P.C.; Yuen, K.Y. Genetic characterization of EV71 isolates from 2004 to 2010 reveals predominance and persistent circulation of the newly proposed genotype D and recent emergence of a distinct lineage of subgenotype C2 in Hong Kong. Virol. J. 2013, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Lycke, E.; Ahlfors, K.; Johnsson, T.; Wolontis, S.; Von Zeipel, G. Letter: New enterovirus type associated with epidemic of aseptic meningitis and-or hand, foot, and mouth disease. Lancet 1974, 2, 112. [Google Scholar] [CrossRef]
- Hagiwara, A.; Tagaya, I.; Yoneyama, T. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology 1978, 9, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wei, D.; Ou, W.L.; Li, K.X.; Luo, D.Z.; Li, Y.Q.; Chen, E.; Nong, G.M. Autopsy findings in children with hand, foot, and mouth disease. N Engl. J. Med. 2012, 367, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.J.; Jung, S.M.; Hsueh, C.; Kuo, T.T.; Mounts, A.; Parashar, U.; Yang, C.F.; Guarner, J.; Ksiazek, T.G.; Dawson, J.; et al. Pathologic studies of fatal cases in outbreak of hand, foot, and mouth disease, Taiwan. Emerg. Infect. Dis. 2001, 7, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.C.; Wong, K.T.; Lam, S.K.; Chua, K.B.; Goh, A.Y.; Lim, W.L.; Ong, B.B.; Paul, G.; AbuBakar, S.; Lambert, M. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 1998, 133, 795–798. [Google Scholar] [CrossRef]
- Khong, W.X.; Yan, B.; Yeo, H.; Tan, E.L.; Lee, J.J.; Ng, J.K.; Chow, V.T.; Alonso, S. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J. Virol. 2012, 86, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.K.; Chen, C.C.; Chen, C.L.; Wang, J.R.; Liu, C.C.; Yan, J.J.; Su, I.J. Neutralizing antibody provided protection against enterovirus type 71 lethal challenge in neonatal mice. J. Biomed. Sci. 2000, 7, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Chou, C.T.; Lei, H.Y.; Liu, C.C.; Wang, S.M.; Yan, J.J.; Su, I.J.; Wang, J.R.; Yeh, T.M.; Chen, S.H.; et al. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J. Virol. 2004, 78, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Yu, S.L.; Shao, H.Y.; Lin, H.Y.; Liu, C.C.; Hsiao, K.N.; Chitra, E.; Tsou, Y.L.; Chang, H.W.; Sia, C.; et al. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS ONE 2013, 8, e57591. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, I.; Hagiwara, A. Pathogenicity of a poliomyelitis-like disease in monkeys infected orally with enterovirus 71: A model for human infection. Neuropathol. Appl. Neurobiol. 1982, 8, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.P.; Qian, L.; Xia, Y.; Xu, F.; Yang, Z.N.; Xie, R.H.; Li, X.; Liang, W.F.; Huang, X.X.; Zhu, Z.Y.; et al. Enterovirus 71-induced neurological disorders in young gerbils, Meriones unguiculatus: Development and application of a neurological disease model. PLoS ONE 2012, 7, e51996. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–12: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Wang, S.M.; Lei, H.Y.; Huang, K.J.; Wu, J.M.; Wang, J.R.; Yu, C.K.; Su, I.J.; Liu, C.C. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: Roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 2003, 188, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chang, L.Y.; Huang, Y.C.; Hsu, K.H.; Chiu, C.H.; Yang, K.D. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: Implications for early recognition and therapy. Acta. Paediatr. 2002, 91, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lei, H.Y.; Su, L.Y.; Wu, J.M.; Yu, C.K.; Wang, J.R.; Liu, C.C. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin. Microbiol. Infect. 2007, 13, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hsia, S.H.; Huang, Y.C.; Wu, C.T.; Chang, L.Y. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin. Infect. Dis. 2003, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.F.; Chen, L.L.; Huang, P.N.; Shih, S.R. Neural pathogenesis of enterovirus 71 infection. Microbes Infect. 2010, 12, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, C.; Feng, J.; Li, X.; Wang, Y.; Yang, J.; Chen, Z. Peripheral T lymphocyte subset imbalances in children with enterovirus 71-induced hand, foot and mouth disease. Virus Res. 2014, 180, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Shih, S.R.; Pan, M.; Li, C.; Lue, C.F.; Stollar, V.; Li, M.L. hnRNP A1 interacts with the 5′ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 2009, 83, 6106–6114. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Liu, C.C.; Tseng, H.W.; Wang, J.R.; Huang, C.C.; Chen, Y.J.; Yang, Y.J.; Lin, S.J.; Yeh, T.F. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin. Infect. Dis. 1999, 29, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Takatsy, S.; Kukan, E.; Mihaly, I.; Domok, I. Virological diagnosis of enterovirus type 71 infections: Experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch. Virol. 1982, 71, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Van der Sanden, S.; Koopmans, M.; Uslu, G.; Van der Avoort, H. Epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008. J. Clin. Microbiol. 2009, 47, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fan, G.; Xia, W.; He, F.; Hu, M.; Ni, X.; Zhang, Y.; Chen, H. Molecular epidemiology of human enterovirus 71 strains in the Nanchang region of China in 2011. Jpn. J. Infect. Dis. 2013, 66, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lu, J.; Kung, H.F.; He, M.L. The virology and developments toward control of human enterovirus 71. Crit. Rev. Microbiol. 2011, 37, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.L.; Kung, S.H.; Hsu, Y.Y.; Liu, W.T. Infection with enterovirus 71 or expression of its 2A protease induces apoptotic cell death. J. Gen. Virol. 2002, 83 Pt 6, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Hsu, T.A.; Chen, T.C.; Chang, S.C.; Lee, J.C.; Chen, C.C.; Stollar, V.; Shih, S.R. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology 2002, 293, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Chang, C.L.; Wang, P.S.; Tsai, Y.; Liu, H.S. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J. Med. Virol. 2009, 81, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Huang, X.; Zhu, S.; Chen, H.; Yu, Q.; Wang, H.; Huo, X.; Zhou, J.; Wu, Y.; Yan, D.; et al. The persistent circulation of enterovirus 71 in People’s Republic of China: Causing emerging nationwide epidemics since 2008. PLoS ONE 2011, 6, e25662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, S.; Zhu, L.; Huang, Z.; Chen, L.; Yin, H.; Peng, T.; Wang, Y. Clinically isolated enterovirus A71 subgenogroup C4 strain with lethal pathogenicity in 14-day-old mice and the application as an EV-A71 mouse infection model. Antivir. Res. 2017, 137, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Ooi, M.H.; Wong, S.C.; Mohan, A.; Podin, Y.; Perera, D.; Chieng, C.H.; Tio, P.H.; Cardosa, M.J.; Solomon, T. In enterovirus 71 encephalitis with cardio-respiratory compromise, elevated interleukin 1beta, interleukin 1 receptor antagonist, and granulocyte colony-stimulating factor levels are markers of poor prognosis. J. Infect. Dis. 2012, 206, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Khong, W.X.; Foo, D.G.; Trasti, S.L.; Tan, E.L.; Alonso, S. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J. Virol. 2011, 85, 3067–3076. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Bao, L.; Xu, L.; Li, F.; Lv, Q.; Deng, W.; Xu, Y.; Qin, C. Neurotropism In Vitro and Mouse Models of Severe and Mild Infection with Clinical Strains of Enterovirus 71. Viruses 2017, 9, 351. https://doi.org/10.3390/v9110351
Yu P, Bao L, Xu L, Li F, Lv Q, Deng W, Xu Y, Qin C. Neurotropism In Vitro and Mouse Models of Severe and Mild Infection with Clinical Strains of Enterovirus 71. Viruses. 2017; 9(11):351. https://doi.org/10.3390/v9110351
Chicago/Turabian StyleYu, Pin, Linlin Bao, Lili Xu, Fengdi Li, Qi Lv, Wei Deng, Yanfeng Xu, and Chuan Qin. 2017. "Neurotropism In Vitro and Mouse Models of Severe and Mild Infection with Clinical Strains of Enterovirus 71" Viruses 9, no. 11: 351. https://doi.org/10.3390/v9110351



