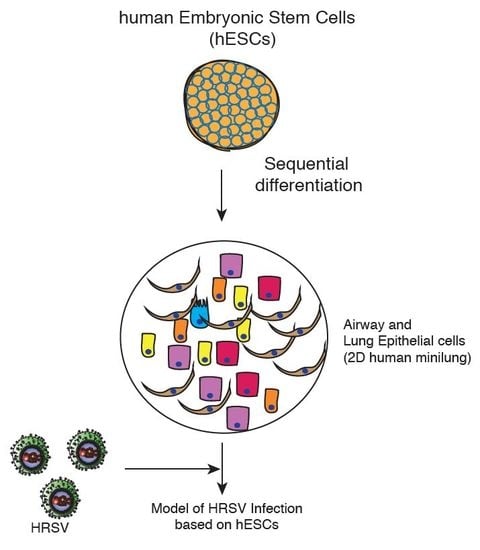
A Two-Dimensional Human Minilung System (Model) for Respiratory Syncytial Virus Infections
Abstract
:- (i)
- Standard methods of expansion and maintenance of hESCs
- (ii)
- Use of NOG (NOGGIN) instead of dorsomorphin
- (iii)
- Cell density in replatings
- (iv)
- Sizes of multiwell plates used
- (v)
- Final assembly of the minilungs on glass chamber slides
- (vi)
- Addition of primary lung fibroblasts to the generated epithelial cells
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACTA2 | Alpha Smooth Muscle Actin (α-SMA) |
| AFE | Anterior Foregut Endoderm |
| AQP5 | Aquaporin 5 |
| ATI | Alveolar Type I Cells |
| ATII | Alveolar Type II Cells |
| BMP | Bone Morphogenic Protein |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 (IL8) |
| CX3CR1 | C-X3-C Motif Chemokine Receptor 1 |
| DE | Definitive Endoderm |
| dpi | Days Post-Infection |
| DXD58 | DExd/H-Box Helicase 58 (RIG-1: Retinoic Acid Inducible Gene 1) |
| EBs | Embryoid Bodies |
| FGF | Fibroblast Growth Factor |
| FGFR | Fibroblast Growth Factor Receptor |
| FOXA2 | Forkhead Box A2 |
| FOXJ1 | Forkhead Box J1 |
| hESC | Human Embryonic Stem Cells |
| HRSV | Human Respiratory Syncytial Virus |
| IL1B IL6 | Interleukin 1 β Interleukin 6 |
| ISG15 | ISG15 Ubiquitin-Like Modifier |
| mAb | Monoclonal Antibody |
| MEFs | Mouse Embryonic Fibroblasts |
| moi | Multiplicity of Infection |
| MUCIN5AC | Mucin 5AC, Oligomeric Mucus/Gel-Forming |
| NKX2-1 | NK2 Homeobox 1 |
| NOG | Noggin |
| PDPN | Podoplanin |
| pfu | Plaque Forming Unit |
| PSC | Pluripotent Stem Cells |
| qRT-PCR | Quantitative Real-Time RT-PCR (Reverse Transcription Polymerase Chain Reaction) |
| SCGB1A1 | Secretoglobin Family 1A Member 1; CC10 |
| SFTPA | Surfactant Protein A |
| SFTPB | Surfactant Protein B |
| SFTPC | Surfactant Protein C |
| SFTPD | Surfactant Protein D |
| SOX2 | SRY (Sex Determining Region Y)-box 2 |
| TBP | TATA Box Binding Protein |
| TGFB1 | Tumor Growth Factor β |
| TNF | Tumor Necrosis Factor (TNF α) |
| TP63 | Tumor Protein p63 |
| WNT | Wingless-Related Integration Site |
| μm | Micrometers |
References
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Karron, R.A. Respiratory Syncytial Virus and Metapneumovirus, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 1086–1123. [Google Scholar]
- Touzelet, O.; Power, U. Cellular and molecular characteristics of RSV-induced disease in humans. In Human Respiratory Syncytial Virus Infection; Tech, I., Benhard, R., Eds.; InTech: Rijeka, Croatia, 2011; pp. 97–122. [Google Scholar]
- Guo-Parke, H.; Canning, P.; Douglas, I.; Villenave, R.; Heaney, L.G.; Coyle, P.V.; Lyons, J.D.; Shields, M.D.; Power, U.F. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 2013, 188, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Villenave, R.; Shields, M.D.; Power, U.F. Respiratory syncytial virus interaction with human airway epithelium. Trends Microbiol. 2013, 21, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, L.; Villenave, R.; Guo-Parke, H.; Douglas, I.; Shields, M.D.; Power, U.F. In Vitro Modeling of RSV Infection and Cytopathogenesis in Well-Differentiated Human Primary Airway Epithelial Cells (WD-PAECs). Methods Mol. Biol. 2016, 1442, 119–139. [Google Scholar] [PubMed]
- Green, M.D.; Huang, S.X.; Snoeck, H.W. Stem cells of the respiratory system: From identification to differentiation into functional epithelium. Bioessays 2013, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Huang, S.X.; de Carvalho, A.; Ho, S.H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Green, M.D.; de Carvalho, A.T.; Mumau, M.; Chen, Y.W.; D’Souza, S.L.; Snoeck, H.W. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 2015, 10, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.G.; Chen, Y.W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Magro-Lopez, E.; Institute of Health Carlos III, Madrid. Q-RT-PCR result. 2017. [Google Scholar]
- Guo, X.; Liu, T.; Shi, H.; Wang, J.; Ji, P.; Wang, H.; Hou, Y.; Tan, R.X.; Li, E. Respiratory Syncytial Virus Infection Upregulates NLRC5 and Major Histocompatibility Complex Class I Expression through RIG-I Induction in Airway Epithelial Cells. J. Virol. 2015, 89, 7636–7645. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jamaluddin, M.; Li, K.; Garofalo, R.P.; Casola, A.; Brasier, A.R. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007, 81, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanz, R.; Mata, M.; Bermejo-Martin, J.; Alvarez, A.; Cortijo, J.; Melero, J.A.; Martinez, I. ISG15 Is Upregulated in Respiratory Syncytial Virus Infection and Reduces Virus Growth through Protein ISGylation. J. Virol. 2016, 90, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Lombardia, L.; Garcia-Barreno, B.; Dominguez, O.; Melero, J.A. Distinct gene subsets are induced at different time points after human respiratory syncytial virus infection of A549 cells. J. Gen. Virol. 2007, 88, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38. [Google Scholar] [PubMed]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, T.; Lin, S.; Oomens, A.G.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.C.; Alva-Ornelas, J.A.; Sucre, J.M.; Vijayaraj, P.; Durra, A.; Richardson, W.; Jonas, S.J.; Paul, M.K.; Karumbayaram, S.; Dunn, B.; et al. Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem. Cells Transl. Med. 2017, 6, 622–633. [Google Scholar] [CrossRef] [PubMed]
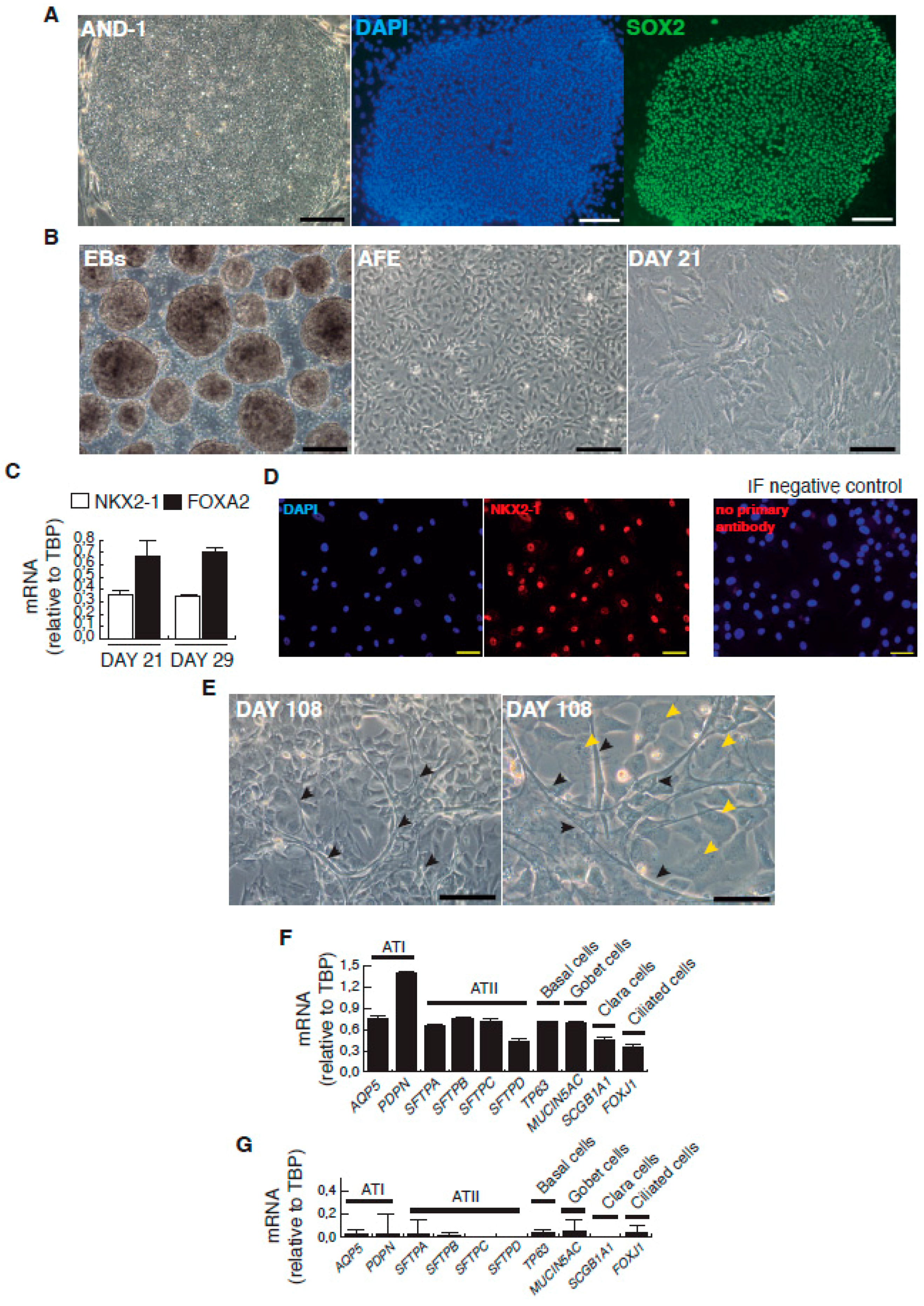
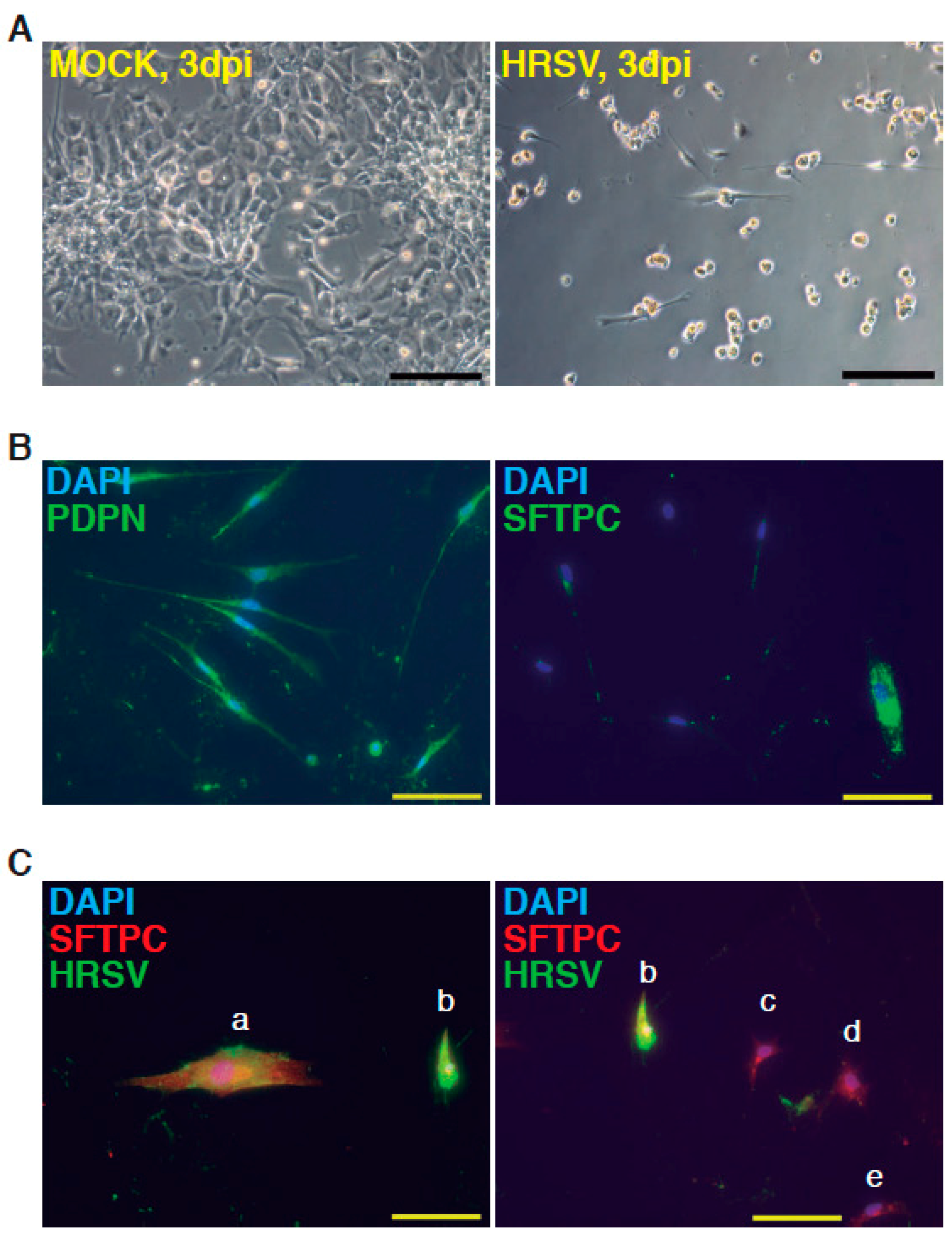

| Day | Process | Atmosphere/Observations | Factors, Inhibitors and Hormones Used | Targeted Patwways Activation Inhibition |
|---|---|---|---|---|
| Matrigel depletion of MEFs | 95% air/5% CO2 | |||
| 0 | Primitive streak induction | 5% O2/95% N2/5% CO2 Low attachment plates | Wnt3a, BMP4, ROCK inhibitor | WNTs BMP apoptosis |
| 1–3 | Definitive endoderm induction | 5% O2/95% N2/5% CO2 Low attachment plates | Activin A, BMP4, hbFGF, ROCK inhibitor | ACVR BMP FGFR apoptosis |
| 4–6 | Anterior foregut induction | 5% O2/95% N2/5% CO2 fibronectin coated-plants | Dorsomorphin or NOGGIN, SB431542, IWP2 | BMP TGF-β WNTs |
| 6–15 | Lung progenitor induction and expansion | 5% O2/95% N2/5% CO2 (intermediate incubation under 95% air/5% CO2) fibronectin coated-plates | CHIR99021, KGF, FGF10, BMP4, EGF, all-trans retinoic acid | Wnts BMP FGFR2b (Ventralization) |
| 16–25 | Lung progenitor induction and expansion | 5% O2/95% N2/5% CO2 fibronectin coated-plates | CHIR99021, KGF, FGF10 | Wnts FGFR2b |
| 26–~1 year | Lung and airway epithelial maturation | 5% O2/95% N2/5% CO2 fibronectin coated-plates | CHIR99021, KGF, FGF10, Dexamethasone, IBMX, cAMP | Wnts FGFR2b (Alveolar maturation) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magro-Lopez, E.; Guijarro, T.; Martinez, I.; Martin-Vicente, M.; Liste, I.; Zambrano, A. A Two-Dimensional Human Minilung System (Model) for Respiratory Syncytial Virus Infections. Viruses 2017, 9, 379. https://doi.org/10.3390/v9120379
Magro-Lopez E, Guijarro T, Martinez I, Martin-Vicente M, Liste I, Zambrano A. A Two-Dimensional Human Minilung System (Model) for Respiratory Syncytial Virus Infections. Viruses. 2017; 9(12):379. https://doi.org/10.3390/v9120379
Chicago/Turabian StyleMagro-Lopez, Esmeralda, Trinidad Guijarro, Isidoro Martinez, Maria Martin-Vicente, Isabel Liste, and Alberto Zambrano. 2017. "A Two-Dimensional Human Minilung System (Model) for Respiratory Syncytial Virus Infections" Viruses 9, no. 12: 379. https://doi.org/10.3390/v9120379