Echovirus 6 Infects Human Exocrine and Endocrine Pancreatic Cells and Induces Pro-Inflammatory Innate Immune Response
Abstract
:1. Introduction
2. Material and Methods
2.1. Viruses
2.2. Tissue Source and Purity
2.3. Virus Replication and Cytopathic Effect
2.4. Gene Expression
2.5. Glucose Stimulation Test
2.6. Immunohistochemistry
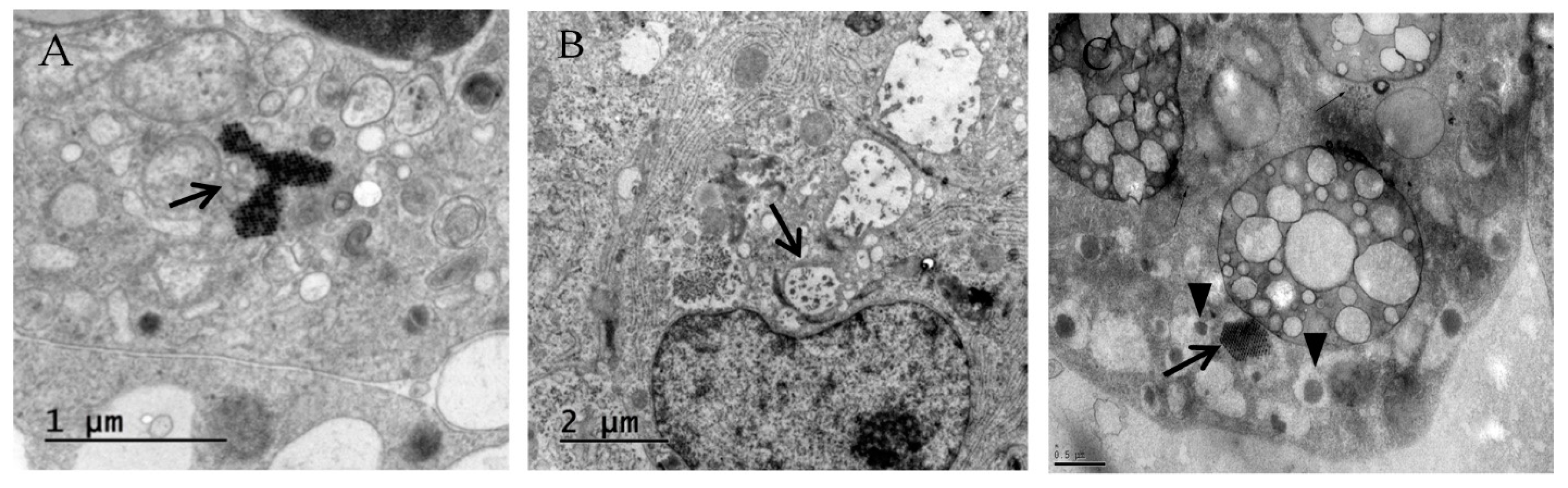
2.7. Electron Microscopy
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
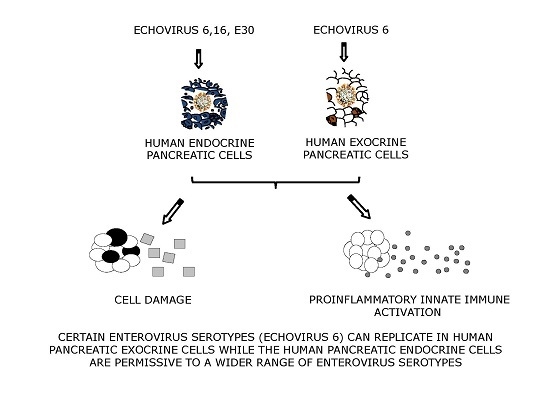
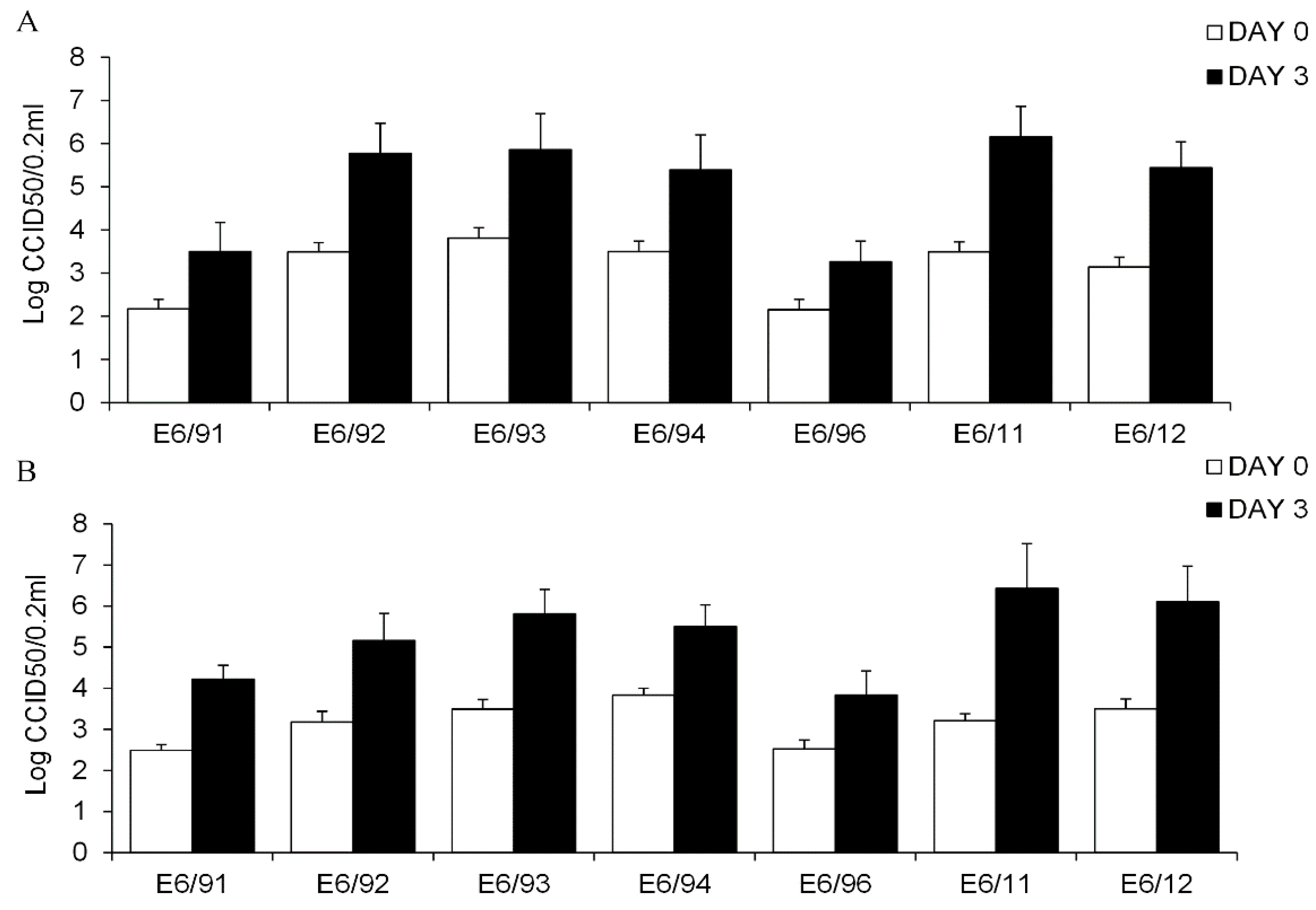
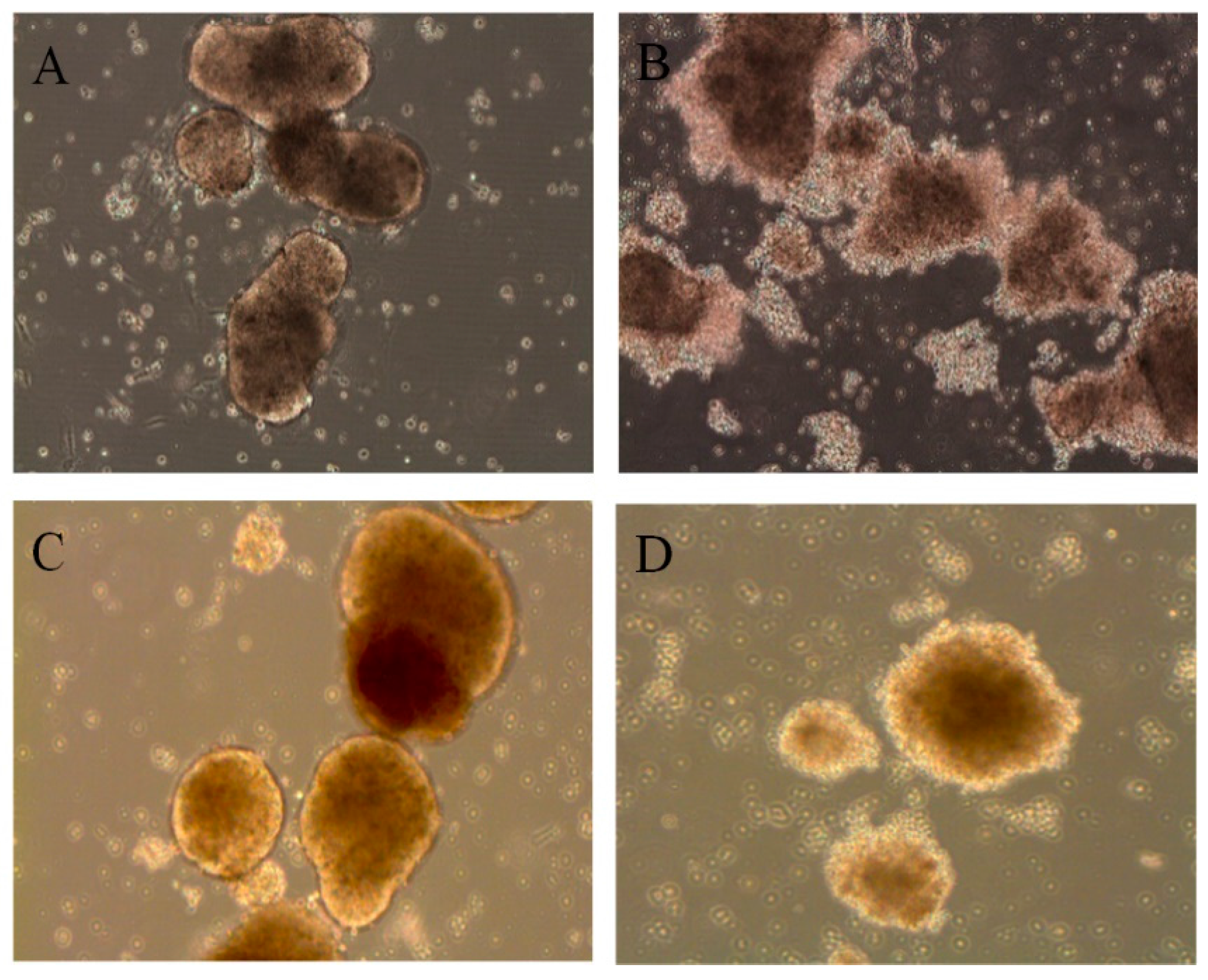
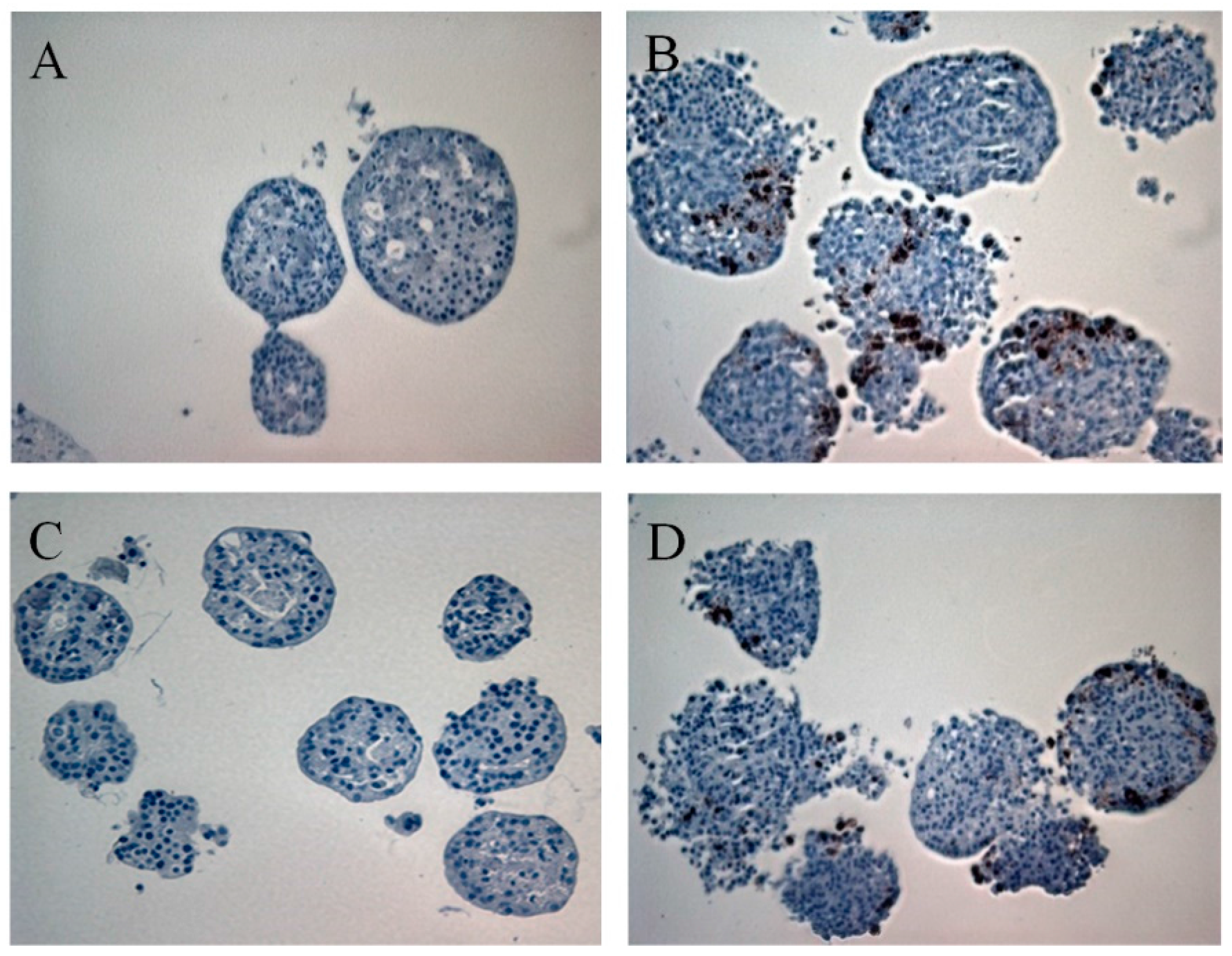
3.1. Primary Human Pancreatic Exocrine and Endocrine Cells Are Productively Infected by Echovirus 6
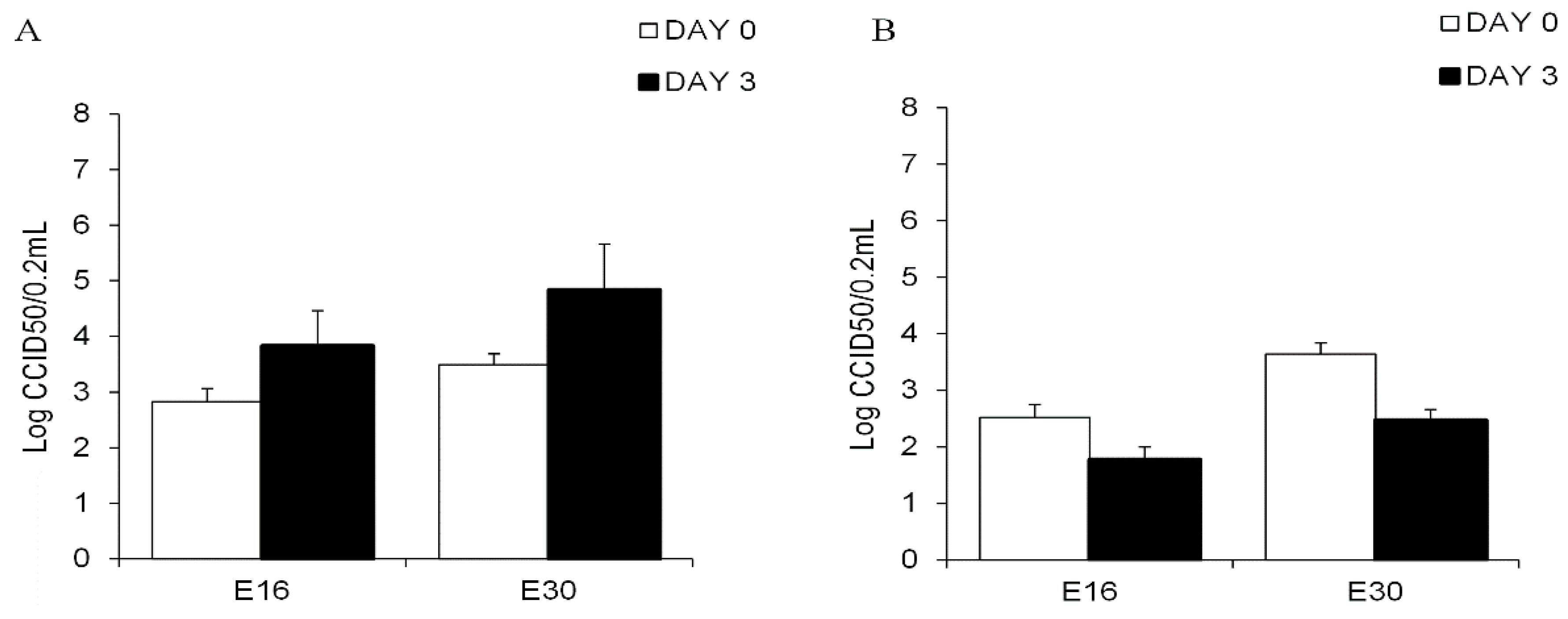
3.2. Echovirus 16 or Echovirus 30 Can Replicate in Human Islets but Not in Human Exocrine Cells
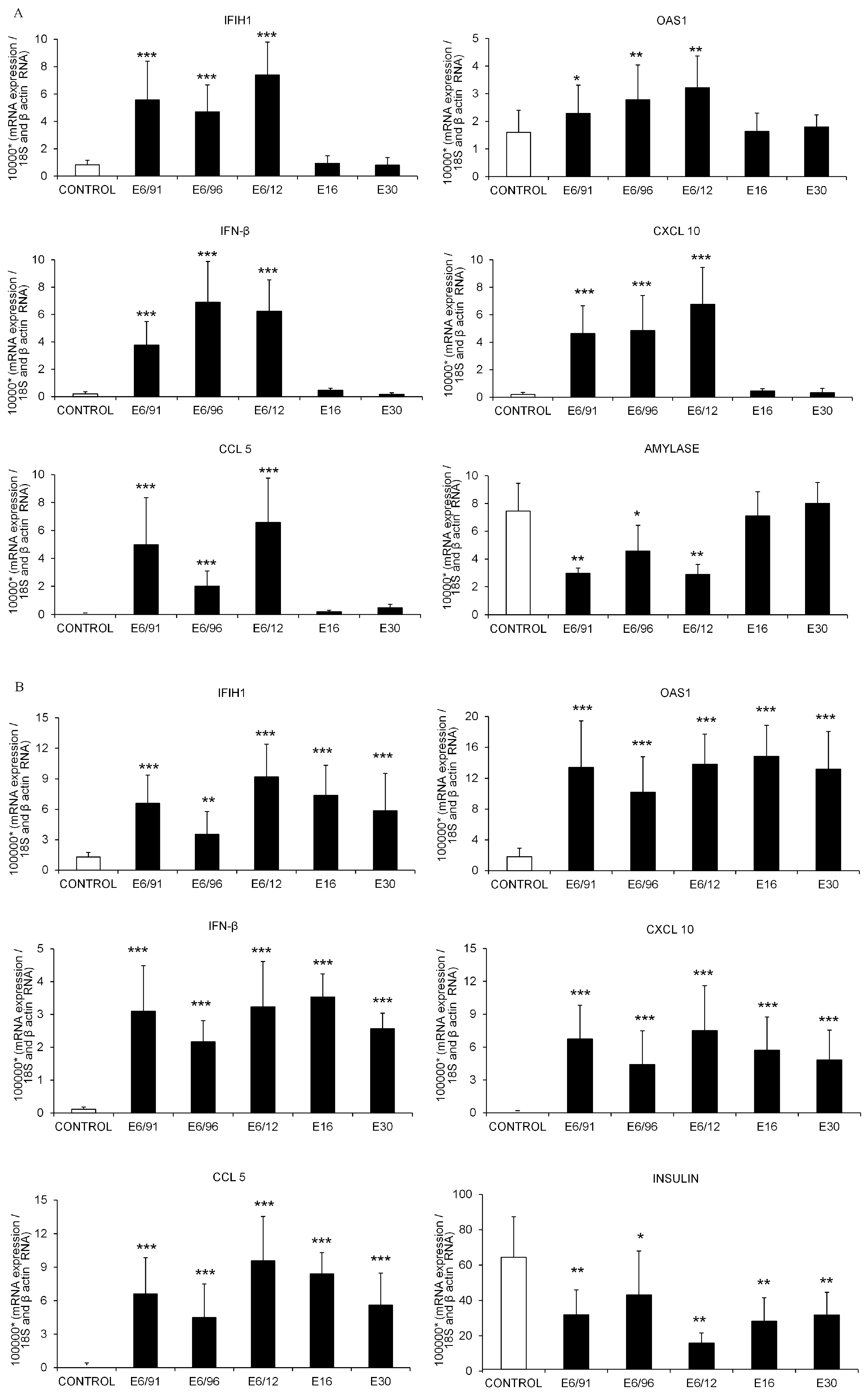
3.3. Pancreatic Exocrine and Endocrine Cells Express Inflammatory and Antiviral Genes in Response to Echovirus 6 Infection
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huber, S.; Ramsingh, A.I. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004, 17, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.C.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef] [PubMed]
- Ylipaasto, P.; Klingel, K.; Lindberg, A.M.; Otonkoski, T.; Kandolf, R.; Hovi, T.; Roivainen, M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Ylipaasto, P.; Kutlu, B.; Rasilainen, S.; Rasschaert, J.; Salmela, K.; Teerijoki, H.; Korsgren, O.; Lahesmaa, R.; Hovi, T.; Eizirik, D.L.; et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 2005, 48, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Elshebani, A.; Olsson, A.; Westman, J.; Tuvemo, T.; Korsgren, O.; Frisk, G. Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res. 2007, 124, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Anagandula, M.; Richardson, S.J.; Oberste, M.S.; Sioofy-Khojine, A.B.; Hyöty, H.; Morgan, N.G.; Korsgren, O.; Frisk, G. Infection of human islets of Langerhans with two strains of Coxsackie B virus serotype 1: Assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J. Med. Virol. 2014, 86, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Ozsvár, Z.; Deákm, J.; Pap, A. Possible role of Coxsackie-B virus infection in pancreatitis. Int. J. Pancreatol. 1992, 11, 105–108. [Google Scholar] [PubMed]
- Arnesjö, B.; Eden, T.; Ihse, I.; Nordenfelt, E.; Ursing, B. Enterovirus infections in acute pancreatitis. A possible etiological connection. Scand. J. Gastroenterol. 1976, 11, 645–649. [Google Scholar] [PubMed]
- Gooby Toedt, D.M.; Byrd, J.C.; Omori, D. Coxsackievirus-associated pancreatitis mimicking metastatic carcinoma. South. Med. J. 1996, 89, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Akuzawa, N.; Harada, N.; Hatori, T.; Imai, K.; Kitahara, Y.; Sakurai, S.; Kurabayashi, M. Myocarditis, hepatitis, and pancreatitis in a patient with coxsackievirus A4 infection: A case report. Virol. J. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Deng, H.L.; Fu, J.; Zhang, Y.; Wei, J.Q. Pancreatitis in hand-foot-and-mouth disease caused by enterovirus 71. World J. Gastroenterol. 2016, 22, 2149–2152. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.; Fischer, C.; Gebhard, J.R.; Perry, C.M.; Harkins, S.; Whitton, J.L. Coxsackievirus infection of the pancreas: Evaluation of receptor expression, pathogenesis, and immunopathology. Virology 2000, 271, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Ramsingh, A.I. Coxsackieviruses and pancreatitis. Front. Biosci. 1997, 2, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Burch, G.E.; Harb, J.M. Electron microscopic studies of viral pancreatitis in coxsackie B4 virus infected mice. Exp. Mol. Pathol. 1979, 31, 23–35. [Google Scholar] [CrossRef]
- Caggana, M.; Chan, P.; Ramsingh, A. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 1993, 67, 4797–4803. [Google Scholar] [PubMed]
- Vella, C.; Easton, A.J.; Eglin, R.P.; Brown, C.L.; Perry, L. Coxsackie virus B4 infection of the mouse pancreas. Detection of virus-specific RNA in the pancreas by in situ hybridisation. J. Med. Virol. 1991, 35, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Foulis, A.K.; Farquharson, M.A.; Cameron, S.O.; McGill, M.; Schönke, H.; Kandolf, R. A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 1990, 33, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Willcox, A.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009, 52, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodik, M.; Lukinius, A.; Korsgren, O.; Frisk, G. Tropism analysis of two Coxsackie B5 strains reveals virus growth in human primary pancreatic islets but not in exocrine cell clusters in vitro. Open Virol. J. 2013, 7, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Galvan, J.A.; Cabrera-Rode, E.; Aira, L.; Correa, C.; Sariego, S.; Fonseca, M.; Cubas-Dueñas, I.; Hung, L.H.; Resik, S.; et al. Type 1 diabetes associated and tissue transglutaminase autoantibodies in patients without diabetes and coeliac disease with confirmed viral infections. J. Med. Virol. 2012, 84, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Frisk, G.; Anagandula, M.; Cabrera-Rode, E.; Roivainen, M.; Cilio, C.M. Expression of innate immunity genes and damage of primary human pancreatic islets by epidemic strains of echovirus: Implication for post-virus islet autoimmunity. PLoS ONE 2013, 8, e77850. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Medina, A.; Aziz, K.; Anagandula, M.; Cabrera-Rode, E.; Fex, M.; Frisk, G.; Cilio, C.M. Differential effects of three echovirus strains on cell lysis and insulin secretion in beta cell derived lines. J. Med. Virol. 2015, 8, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Otonkoski, T.; Roivainen, M.; Vaarala, O.; Dinesen, B.; Leipälä, J.A.; Hovi, T.; Knip, M. Neonatal Type I diabetes associated with maternal echovirus 6 infection: A case report. Diabetologia 2000, 43, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L. Enteroviral meningitis and emergence of rare enterovirus types: Cuban experience. In Focus on Meningitis Research; Strong, P.V., Ed.; Nova: New York, NY, USA, 2004; pp. 1–14. [Google Scholar]
- Cabrera-Rode, E.; Sarmiento, L.; Tiberti, C.; Molina, G.; Barrios, J.; Hernández, D.; Díaz-Horta, O.; Di Mario, U. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia 2003, 46, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rode, E.; Sarmiento, L.; Molina, G.; Pérez, C.; Arranz, C.; Galvan, J.A.; Prieto, M.; Barrios, J.; Palomera, R.; Fonseca, M.; et al. Islet cell related antibodies and type 1 diabetes associated with echovirus 30 epidemic: A case report. J. Med. Virol. 2005, 76, 373–377. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Isolation and Identification of Polioviruses. In WHO Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Lennette, E.H. General principles underlying laboratory diagnosis of viral and rickettsial infections. In Diagnostic Procedures for Viral and Rickettsial Infections; Lennette, E.H., Schmidt, N.J., Eds.; American Public Health Association: New York, NY, USA, 1969; pp. 1–63. [Google Scholar]
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Flemister, M.R.; Brown, B.A.; Pallansch, M.A. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 1999, 37, 1288–1293. [Google Scholar] [PubMed]
- Human Tissue Laboratory. Available online: http://www.ludc.med.lu.se/platforms/human-tissue-laboratory/ (accessed on 6 may 2016).
- Goto, M.; Eich, T.M.; Felldin, M.; Foss, A.; Källen, R.; Salmela, K.; Tibell, A.; Tufveson, G.; Fujimori, K.; Engkvist, M.; et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004, 78, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Sane, F.; Caloone, D.; Gmyr, V.; Engelmann, I.; Belaich, S.; Kerr-Conte, J.; Pattou, F.; Desailloud, R.; Hober, D. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell. Mol. Life Sci. 2013, 70, 4169–4180. [Google Scholar] [CrossRef] [PubMed]
- Smura, T.; Natri, O.; Ylipaasto, P.; Hellman, M.; Al-Hello, H.; Piemonti, L.; Roivainen, M. Enterovirus strain and type-specific differences in growth kinetics and virus-induced cell destruction in human pancreatic duct epithelial HPDE cells. Virus Res. 2015, 210, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Skog, O.; Korsgren, S.; Wiberg, A.; Danielsson, A.; Edwin, B.; Buanes, T.; Krogvold, L.; Korsgren, O.; Dahl-Jørgensen, K. Expression of human leukocyte antigen class in endocrine and exocrine pancreatic tissue at onset of type 1 diabetes. Am. J. Pathol. 2015, 185, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kemball, C.C.; Alirezaei, M.; Flynn, C.T.; Wood, M.R.; Harkins, S.; Kiosses, W.B.; Whitton, J.L. Coxsackievirus infection induces autophagy like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J. Virol. 2010, 84, 12110–12124. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, M.; Rasilainen, S.; Ylipaasto, P.; Nissinen, R.; Ustinov, J.; Bouwens, L.; Eizirik, D.L.; Hovi, T.; Otonkoski, T. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J. Clin. Endocrinol. Metab. 2000, 85, 432–440. [Google Scholar] [PubMed]
- Yin, H.; Berg, A.K.; Westman, J.; Hellerström, C.; Frisk, G. Complete nucleotide sequence of a Coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: Effects on insulin release, proinsulin synthesis, and cell morphology. J. Med. Virol. 2002, 68, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Hodik, M.; Skog, O.; Lukinius, A.; Isaza-Correa, J.M.; Kuipers, J.; Giepmans, B.N.; Frisk, G. Enterovirus infection of human islets of Langerhans affects β-cell function resulting in disintegrated islets, decreased glucose stimulated insulin secretion and loss of Golgi structure. BMJ Open Diabetes Res. Care 2016, 4, e000179. [Google Scholar] [CrossRef] [PubMed]
- Marroqui, L.; Lopes, M.; dos Santos, R.S.; Grieco, F.A.; Roivainen, M.; Richardson, S.J.; Morgan, N.G.; de Beeck, A.O.; Eizirik, D.L. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic α and β cells. eLife 2015, 4, e06990. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Kalimo, H.; Dahllund, L.; Santti, J.; Hughes, P.; Hyypiä, T.; Stanway, G. Mapping of tissue tropism determinants in coxsackievirus genomes. J. Gen. Virol. 2002, 83, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Marjomäki, V.; Turkki, P.; Huttunen, M. Infectious Entry Pathway of Enterovirus B Species. Viruses 2015, 7, 6387–6399. [Google Scholar] [CrossRef] [PubMed]
- Paananen, A.; Ylipaasto, P.; Smura, T.; Lempinen, M.; Galama, J.; Roivainen, M. A single amino acid substitution in viral VP1 protein alters the lytic potential of clone-derived variants of echovirus 9 DM strain in human pancreatic islets. J. Med. Virol. 2013, 87, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Narayanan, B.; Shah, S.; Yoder, J.D.; Cifuente, J.O.; Hafenstein, S.; Bergelson, J.M. Single amino acid changes in the virus capsid permit coxsackievirus B3 to bind decay-accelerating factor. J. Virol. 2011, 85, 7436–7443. [Google Scholar] [CrossRef] [PubMed]
- Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965, 14, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Lankisch, P.G.; Manthey, G.; Otto, J.; Koop, H.; Talaulicar, M.; Willms, B.; Creutzfeldt, W. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion 1982, 25, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hardt, P.D.; Hauenschild, A.; Nalop, J.; Marzeion, A.M.; Jaeger, C.; Teichmann, J.; Bretzel, R.G.; Hollenhorst, M.; Kloer, H.U.; S2453112/S2453113 Study Group. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1021 diabetic patients. Pancreatology 2003, 3, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W.; Gleichmann, D.; Otto, J.; Stöckmann, F.; Maisonneuve, P.; Lankisch, P.G. Follow-up of exocrine pancreatic function in type 1 diabetes mellitus. Digestion 2005, 72, 71–75. [Google Scholar] [PubMed]
- Sarkar, S.A.; Lee, C.E.; Victorino, F.; Nguyen, T.T.; Walters, J.A.; Burrack, A.; Eberlein, J.; Hildemann, S.K.; Homann, D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 2012, 61, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Calvo, T.; Ekwall, O.; Amirian, N.; Zapardiel-Gonzalo, J.; von Herrath, M.G. Increased immune cell infiltration of the exocrine pancreas: A possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014, 63, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, A.; Granstam, A.; Ingvast, S.; Härkönen, T.; Knip, M.; Korsgren, O.; Skog, O. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin. Exp. Immunol. 2015, 182, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Thompson, M.; Rodriguez-Calvo, T.; Battaglia, M. Abnormalities of the Exocrine Pancreas in Type 1 Diabetes. Curr. Diabetes Rep. 2015, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Atkinson, M.A. The streetlight effect in type 1 diabetes. Diabetes 2015, 64, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.A.; von Herrath, M.G. Potential viral pathogenic mechanism in human type 1 diabetes. Diabetologia 2014, 57, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Cabrera-Rode, E.; Lekuleni, L.; Cuba, I.; Molina, G.; Fonseca, M.; Heng-Hung, L.; Borroto, A.; Gonzalez, P.; Mas, P.; et al. Occurrence of enterovirus RNA in serum of children with newly diagnosed type 1 diabetes and islet cell autoantibody-positive subjects in a population with a low incidence of type 1 diabetes. Autoimmunity 2007, 40, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.; Cubas-Dueñas, I.; Cabrera-Rode, E. Evidence of association between exposure to enterovirus and type 1 diabetes in Cuban children and adolescents. MEDICC Rev. 2013, 15, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Smura, T.; Kakkola, L.; Blomqvist, S.; Klemola, P.; Parsons, A.; Kallio-Kokko, H.; Savolainen-Kopra, C.; Kainov, D.E.; Roivainen, M. Molecular evolution and epidemiology of echovirus 6 in Finland. Infect. Genet. Evol. 2013, 16, 234–247. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento, L.; Frisk, G.; Anagandula, M.; Hodik, M.; Barchetta, I.; Netanyah, E.; Cabrera-Rode, E.; Cilio, C.M. Echovirus 6 Infects Human Exocrine and Endocrine Pancreatic Cells and Induces Pro-Inflammatory Innate Immune Response. Viruses 2017, 9, 25. https://doi.org/10.3390/v9020025
Sarmiento L, Frisk G, Anagandula M, Hodik M, Barchetta I, Netanyah E, Cabrera-Rode E, Cilio CM. Echovirus 6 Infects Human Exocrine and Endocrine Pancreatic Cells and Induces Pro-Inflammatory Innate Immune Response. Viruses. 2017; 9(2):25. https://doi.org/10.3390/v9020025
Chicago/Turabian StyleSarmiento, Luis, Gun Frisk, Mahesh Anagandula, Monika Hodik, Ilaria Barchetta, Eitan Netanyah, Eduardo Cabrera-Rode, and Corrado M. Cilio. 2017. "Echovirus 6 Infects Human Exocrine and Endocrine Pancreatic Cells and Induces Pro-Inflammatory Innate Immune Response" Viruses 9, no. 2: 25. https://doi.org/10.3390/v9020025