A Review of the Strain Diversity and Pathogenesis of Chicken Astrovirus
Abstract
:1. Introduction
2. Identification and Genomic Structure of Chicken Astrovirus
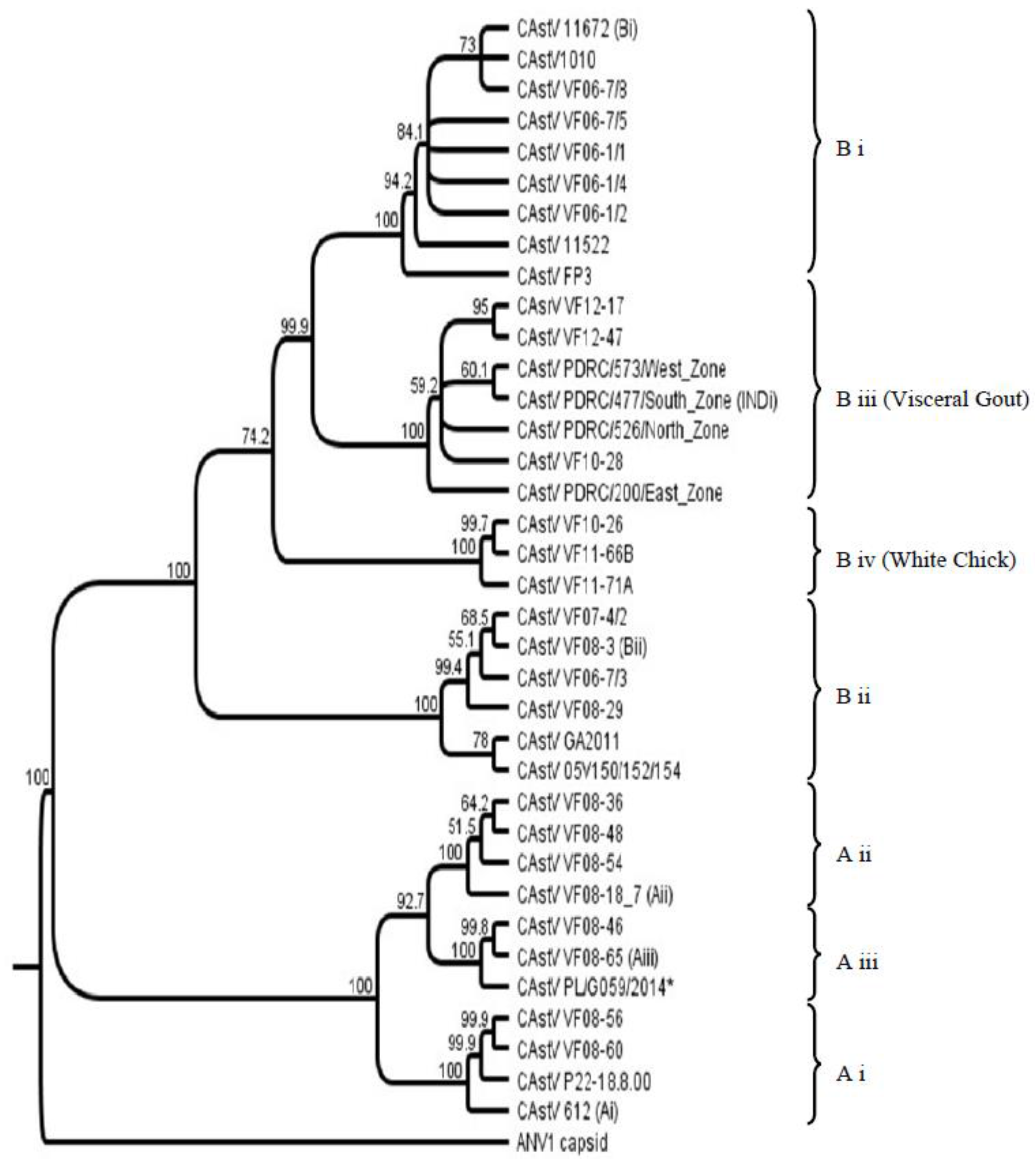
3. Infection, Transmission and Strain Diversity of Chicken Astrovirus
4. Pathogenesis of Chicken Astrovirus
4.1. Runting Stunting Syndrome and Uneven Flock Performance
4.2. Kidney Disease and Visceral Gout
4.3. White Chicks Hatchery Disease
5. Immunity, Treatments and Future Developments
Acknowledgments
Conflicts of Interest
References
- Kang, K.; Icard, A.H.; Linnemann, E.; Sellers, H.; Mundt, E. Determination of the full length sequence of a chicken astrovirus suggests a different replication mechanism. Virus Genes 2012, 44, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Méndez, E.; Arias, C.F. Astrovirus. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 611. [Google Scholar]
- Madeley, C.R.; Cosgrove, B.P. 28 nm particles in faeces in infantile gastroenteritis. Lancet 1975, 6, 451–452. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Spackman, E.; Woolcock, P.R. Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: Implications for diagnostics. Avian Dis. 2006, 50, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Asplin, F.D. Duck hepatitis: Vaccination against two serological types. Vet. Rec. 1965, 77, 1529–1530. [Google Scholar] [PubMed]
- Gough, R.E.; Collins, M.S.; Borland, E.; Keymer, L.F. Astrovirus-like particles associated with hepatitis in ducklings. Vet. Rec. 1984, 114, 279. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Borland, E.D.; Keymer, L.F.; Stuart, J.C. An outbreak of duck hepatitis type II in commercial ducks. Avian Pathol. 1985, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.A.; Calnek, B.W. In Vitro isolation, propagation and characterization of duck hepatitis virus type III. Avian Dis. 1979, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; Smyth, V.J.; Ball, N.W.; Donnelly, B.M.; Wylie, M.; Knowles, N.J.; Adair, B.M. Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol. 2009, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.S.; Curran, W.L.; McFerran, J.B. Detection of astroviruses in turkey faeces by direct electron microscopy. Vet. Rec. 1980, 106, 561. [Google Scholar] [CrossRef] [PubMed]
- Koci, M.D.; Seal, B.S.; Schultz-Cherry, S. Molecular characterization of an avian astrovirus. J. Virol. 2000, 74, 6173–6177. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Imada, T.; Kawamura, H. Characterization of a picornavirus isolated from broiler chicks. Avian Dis. 1979, 23, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Yamaguchi, S.; Kawamura, H. Pathogenicity for baby chicks of the G-4260 strain of the picornavirus “Avian Nephritis Virus”. Avian Dis. 1979, 23, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Yamaguchi, S.; Mase, M.; Tsukamoto, K.; Kubo, M.; Marooka, A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J. Virol. 2000, 74, 8487–8493. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, W.; Metbatsion, T. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 2004, 33, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Spackman, D.; Gough, R.E.; Collins, M.S.; Lanning, D. Isolation of an enterovirus-like agent from the meconium of dead-in-shell chicken embryos. Vet. Rec. 1984, 114, 216–218. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, F.; Connor, T.J.; Calvert, V.M.; Smyth, J.A.; Curran, W.L.; Morley, A.J.; Thompson, D.; Singh, S.; McFerran, J.B.; Adair, B.M.; McNulty, S. Studies on a new enterovirus-like virus isolated from chickens. Avian Pathol. 1994, 23, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Monroe, S.; Koonin, E.V.; Stine, S.E.; Glass, R.I. RNA sequence of astrovirus: Distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA 1993, 90, 10539–10543. [Google Scholar] [CrossRef] [PubMed]
- Monceyron, C.; Grinde, B.; Jonassen, T.Ø. Molecular characterisation of the 3′-end of the astrovirus genome. Arch. Virol. 1997, 142, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J. Darkling beetles as vectors for bacterial and viral pathogens found in poultry litter. In Proceedings of the 45th National Meeting on Poultry Health and Processing, Ocean City, MD, USA, 4–6 October 2010.
- Smyth, V.J.; Trudgett, J.; Jewhurst, H.L.; Todd, D. Intercrop carryover contamination of avian astroviruses in poultry houses. Unpublished work, manuscript in preparation. 2017. [Google Scholar]
- Smyth, V.J.; Todd, D.; Trudgett, J.; Lee, A.; Welsh, M.D. Capsid protein sequence diversity of chicken astrovirus. Avian Path. 2012, 39, 151–159. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.S.; Connor, T.J.; McNeilly, F.; McFerran, J.B. Biological characterisation of avian enteroviruses and enterovirus-like viruses. Avian Pathol. 1990, 19, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; Wilkinson, D.S.; Jewhurst, H.L.; Wylie, M.; Gordon, A.W.; Adair, B.M. A seroprevalence investigation of chicken astrovirus infections. Avian Pathol. 2009, 38, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Canelli, E.; Cordioli, P.; Barbieri, I.; Catella, C.; Pennelli, D.; Ceruti, R.; Moreno, A.; Lavazza, A. Astroviruses as causative agents of poultry enteritis: Genetic characterization and longitudinal studies on field conditions. Avian Dis. 2012, 56, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.I.; El-Gazzar, M.; Sellers, H.S.; Dorea, F.; Willliams, S.M.; Kim, T.; Collett, S.; Mundt, E. Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathol. 2012, 41, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J. Update on the runting-stunting syndrome. Ceva Eggs Program Online. 2012. Available online: http://fs-1.5mpublishing.com/images/ceva/EPO_No3-May2012.pdf (accessed on 29 December 2016).
- Kouwenhoven, B.; Vertommen, M.; van Eck, J.H.H. Runting and leg weakness in broilers; involvement of infectious factors. Vet. Sci. Commun. 1978, 2, 253–259. [Google Scholar] [CrossRef]
- Tierlynck, E.; Gussem, M.D.E.; Dewulf, J.; Haesebrouck, F.; Ducatelle, R.; van Immerseel, F. Morphometric evaluation of “dysbacteriosis” in broilers. Avian Pathol. 2011, 40, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Connor, T.J.; McNeilly, F.; Moffet, D.A.; Calvert, V.M.; McNulty, M.S. Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathol. 2007, 36, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.S.; Lee, H.R.; Jeon, E.O.; Jang, H.S.; Han, M.S.; Mo, I.P. An unusual case of concomitant infection with chickens astrovirus and group A rotavirus in broilers with a history of severe clinical signs. J. Vet. Sci. 2013, 14, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Smyth, V.J.; Jewhurst, H.L.; Wilkinson, D.S.; Adair, B.M.; Gordon, A.W.; Todd, D. Development and evaluation of real-time TaqMan® RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathol. 2010, 39, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Spackman, E.; Pantin-Jackwood, M. A multiplex RT-PCR test for the differential identification of turkey astrovirus type-1, turkey astrovirus type-2, chicken astrovirus, avian nephritis virus and avian rotavirus. Avian Dis. 2007, 51, 681–684. [Google Scholar] [CrossRef]
- Shah, J.D.; Desai, P.T.; Zhang, Y.; Scharber, S.K.; Baller, J.; Xing, Z.S.; Cardona, C.J. Development of the intestinal RNA virus community of healthy broiler chickens. PLoS ONE 2016, 11, e0150094. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Oakley, B.B.; Seal, B.S.; Zsak, L. Comparative Analysis of the Intestinal Bacterial and RNA Viral Communities from Sentinel Birds Placed on Selected Broiler Chicken Farms. PLoS ONE 2015, 1, e0117210. [Google Scholar] [CrossRef] [PubMed]
- Devaney, R.; Trudgett, J.; Trudgett, A.; Meharg, C.; Smyth, V.J. A metagenomic comparison of endemic viruses from broiler chickens with runting-stunting syndrome and from normal birds. Avian Pathol. 2016, 45, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Bulbule, N.R.; Mandakhalikar, K.D.; Kapgate, S.S.; Deshmukh, V.V.; Schat, K.A.; Chawak, M.M. Role of chicken astrovirus as a causative agent of gout in commercial broilers in India. Avian Pathol. 2013, 42, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Smyth, V.J.; Kaukonen, E.; Trudgett, J.; Wylie, M.; Jewhurst, H.; Conway, B.; Welsh, M.D.; Todd, D. Chicken astrovirus detected in hatchability problems associated with “White Chicks”. Vet. Rec. 2013, 173, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, L.F.N.; Santander Parra, S.H.; Carranza, C.; Astolfi-Ferreira, C.S.; Buim, M.R.; Piantino Ferreira, A.J. Detection and molecular characterization of chicken astrovirus associated with chicks that have an unusual condition known as “white chicks” in Brazil. Poult. Sci. 2016, 95, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Sajewicz-Krukowska, J.; Krzysztof, P.; Lisowska, A.; Pikuła, A.; Zenon, M.; Krόliczewska, B.; Domańska-Blicharz, K. Astrovirus-induced “white chicks” condition—Field observation, virus detection and preliminary characterization. Avian Pathol. 2016, 45, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Sellers, H.; Linnemann, E.; Icard, A.H.; Mundt, E. A purified recombinant baculovirus expressed capsid protein of a new astrovirus provides partial protection to runting-stunting syndrome in chickens. Vaccine 2010, 28, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Wylie, M.; Smyth, V.J.; Skibinska, A.; Patterson, I.A.; Forster, F.; Welsh, M.D.; Todd, D. Chicken astrovirus capsid proteins produced by recombinant baculoviruses: Potential use for diagnosis and vaccination. Avian Pathol. 2013, 42, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Skibinska, A.; Lee, A.; Wylie, M.; Smyth, V.J.; Welsh, M.D.; Todd, D. Development of an ELISA test for detecting antibodies to chicken astrovirus in chicken sera. Avian Pathol. 2015, 44, 436–442. [Google Scholar] [CrossRef] [PubMed]
| Avian Species | Virus | Disease/Condition | Major Tissue Distribution |
|---|---|---|---|
| Turkey (ICTV designation: Avastrovirus 1) | Turkey astrovirus type 1 (TAstV-1) | Enteritis, growth retardation | Intestine |
| Turkey astrovirus type 2 (TAstV-2) | Enteritis, growth retardation, (PEC: poult enteritis complex) | Intestine, bursa of Fabricius, thymus | |
| Chicken (ICTV designation: Avastrovirus 2) | Avian nephritis virus (ANV) | Nephritis, baby chick nephropathy, growth retardation | Intestine, kidney |
| Chicken astrovirus (CAstV) | Growth retardation, kidney disease, White Chicks hatchery disease | Intestine, kidney, liver pancreas, spleen | |
| Duck (ICTV designation: Avastrovirus 3) | Duck astrovirus type 1 (DAstV-1) | Hepatitis and variable mortality in young ducks | Liver, kidney, spleen |
| Duck astrovirus type 2 (DAstV-2) | Hepatitis and variable mortality in ducklings | Liver, kidney, spleen |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyth, V.J. A Review of the Strain Diversity and Pathogenesis of Chicken Astrovirus. Viruses 2017, 9, 29. https://doi.org/10.3390/v9020029
Smyth VJ. A Review of the Strain Diversity and Pathogenesis of Chicken Astrovirus. Viruses. 2017; 9(2):29. https://doi.org/10.3390/v9020029
Chicago/Turabian StyleSmyth, Victoria J. 2017. "A Review of the Strain Diversity and Pathogenesis of Chicken Astrovirus" Viruses 9, no. 2: 29. https://doi.org/10.3390/v9020029





