A3R Phage and Staphylococcus aureus Lysate Do Not Induce Neutrophil Degranulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phage
2.2. Bacterial Lysate
2.3. Blood Samples
2.4. Neutrophil Degranulation
2.5. Statistical Analysis
3. Results
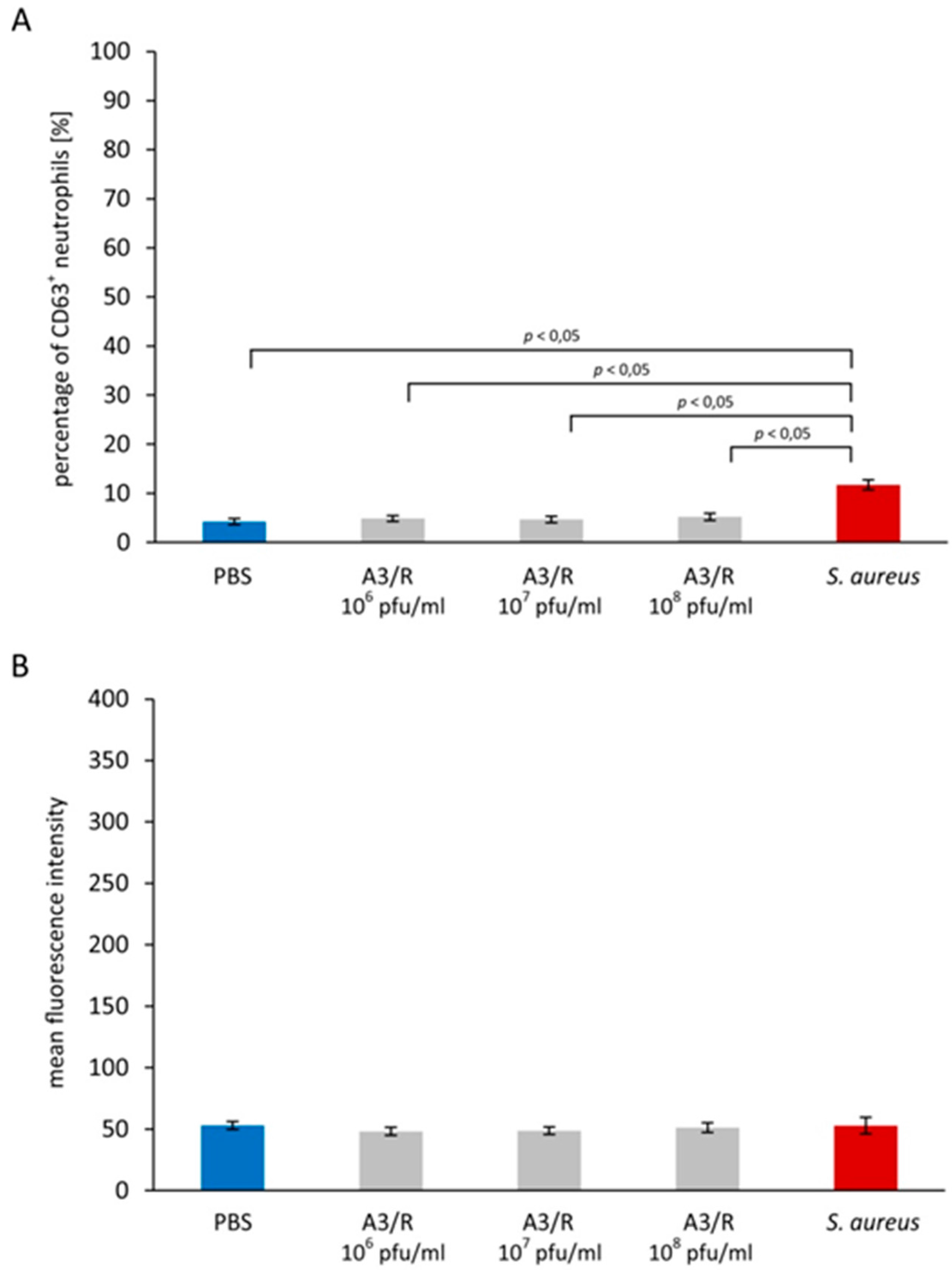
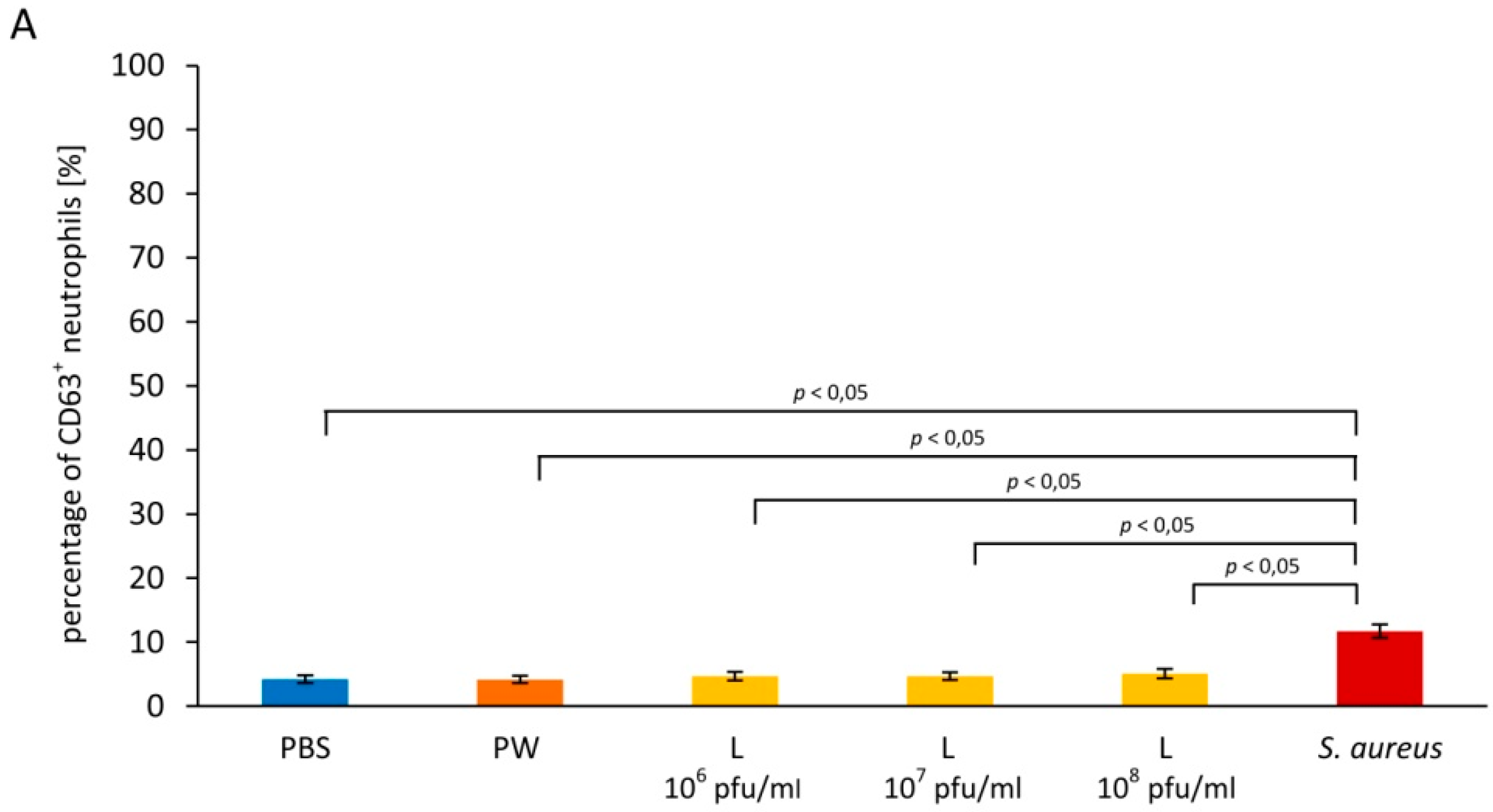
3.1. Effects of A3R Phage on Neutrophil Degranulation
3.1.1. Primary Granules
3.1.2. Secondary Granules
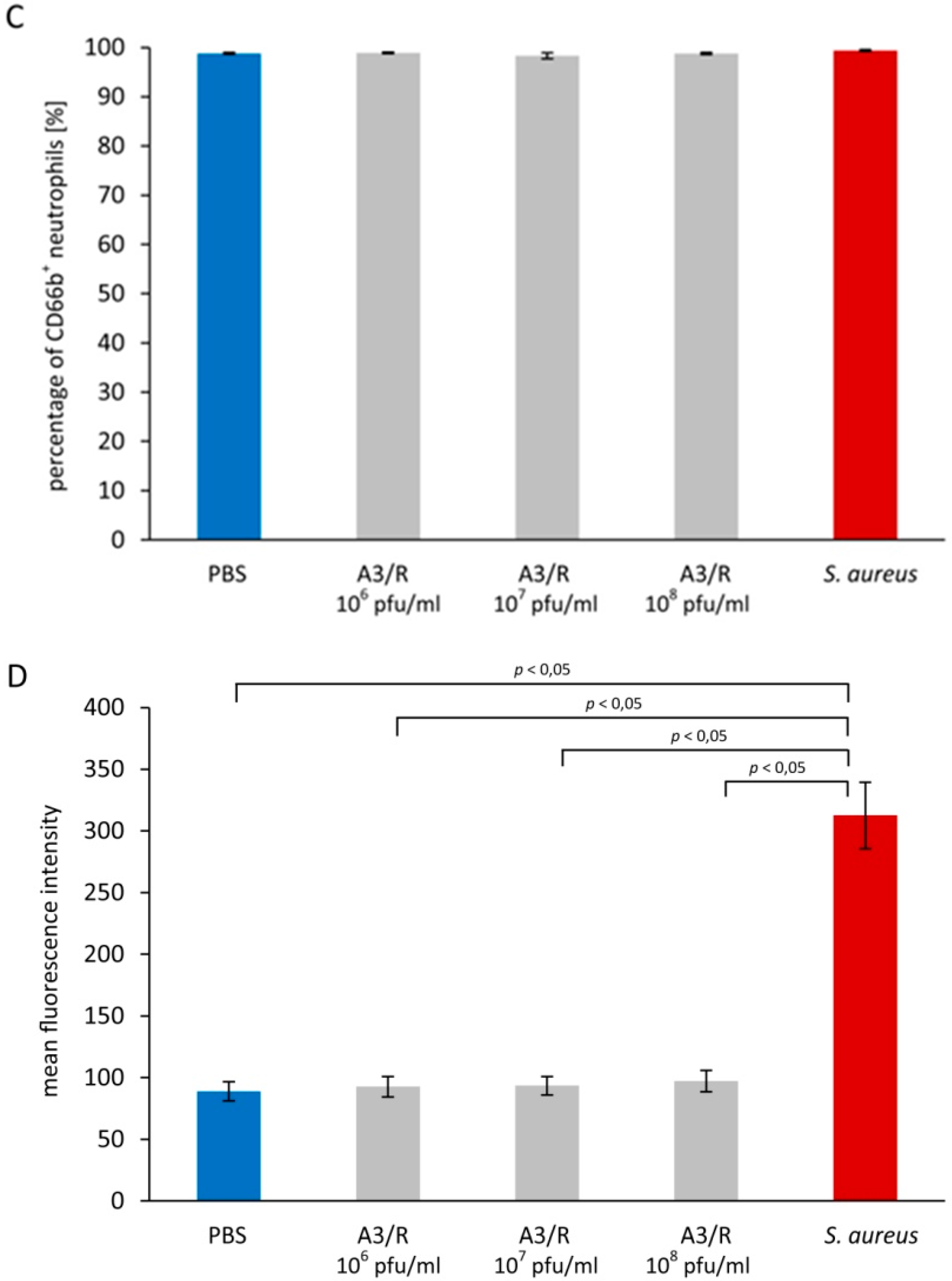
3.2. Influence of Staphylococcus aureus Lysate on Neutrophil Degranulation
3.2.1. Primary Granules
3.2.2. Secondary Granules
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An, T.W.; Kim, S.J.; Lee, Y.D.; Park, J.H.; Chang, H.I. The immune-enhancing effect of the Cronobacter sakazakii ES2 phage results in the activation of nuclear factor-κB and dendritic cell maturation via the activation of IL-12p40 in the mouse bone marrow. Immunol. Lett. 2014, 157, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Międzybrodzki, R.; Borysowski, J.; Dąbrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Łusiak-Szelachowska, M.; et al. Phage as a modulator of immune responses: Practical implications for phage therapy. Adv. Virus Res. 2012, 83, 41–71. [Google Scholar] [PubMed]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapała, J.; Drab, M.; Jończyk-Matysiak, E.; Lecion, D.; Kaźmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 14802. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.S.; Kaido, T.; Carrier, E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J. Virol. Methods 2004, 115, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Boudiaf, K.; Hurtado-Nedelec, M.; Belambri, S.A.; Marie, J.C.; Derradji, Y.; Benboubetra, M.; El-Benna, J.; Dang, P.M. Thymoquinone strongly inhibits fMLF-induced neutrophil functions and exhibits anti-inflammatory properties in vivo. Biochem. Pharmacol. 2016, 104, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule protein processing and regulated secretion in neutrophils. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rørvig, S.; Østergaard, O.; Heegaard, N.H.; Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: Correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Jaovisidha, P.; Peeples, M.E.; Brees, A.A.; Carpenter, L.R.; Moy, J.N. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J. Immunol. 1999, 163, 2816–2820. [Google Scholar] [PubMed]
- Bowers, N.L.; Helton, E.S.; Huijbregts, R.P.; Goepfert, P.A.; Heath, S.L.; Hel, Z. Immune suppression by neutrophils in HIV-1 infection: Role of PD-L1/PD-1 pathway. PLoS Pathog. 2014. [Google Scholar] [CrossRef] [PubMed]
- Juffrie, M.; van Der Meer, G.M.; Hack, C.E.; Haasnoot, K.; Sutaryo; Veerman, A.J.; Thijs, L.G. Inflammatory mediators in dengue virus infection in children: Interleukin-8 and its relationship to neutrophil degranulation. Infect. Immun. 2000, 68, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Łobocka, M.; Hejnowicz, M.S.; Dąbrowski, K.; Gozdek, A.; Kosakowski, J.; Witkowska, M.; Ulatowska, M.I.; Weber-Dąbrowska, B.; Kwiatek, M.; Parasion, S.; et al. Genomics of staphylococcal Twort-like phages—Potential therapeutics of the post-antibiotic era. Adv. Virus Res. 2012, 83, 143–216. [Google Scholar] [PubMed]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Slopek, S.; Durlakowa, I.; Weber-Dabrowska, B.; Kucharewicz-Krukowska, A.; Dabrowski, M.; Bisikiewicz, R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch. Immunol. Ther. Exp. 1983, 31, 267–291. [Google Scholar]
- Letkiewicz, S.; Miedzybrodzki, R.; Fortuna, W.; Weber-Dabrowska, B.; Górski, A. Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis—Case report. Folia Microbiol. 2009, 54, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Deree, J.; Lall, R.; Melbostad, H.; Grant, M.; Hoyt, D.B.; Coimbra, R. Neutrophil degranulation and the effects of phosphodiesterase inhibition. J. Surg. Res. 2006, 133, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [PubMed]
- Mollinedo, F.; Martín-Martín, B.; Calafat, J.; Nabokina, S.M.; Lazo, P.A. Role of vesicle-associated membrane protein-2, through Q-soluble N-ethylmaleimide-sensitive factor attachment protein receptor/R-soluble N-ethylmaleimide-sensitive factor attachment protein receptor interaction, in the exocytosis of specific and tertiary granules of human neutrophils. J. Immunol. 2003, 170, 1034–1042. [Google Scholar] [PubMed]
- Wark, P.A.; Johnston, S.L.; Moric, I.; Simpson, J.L.; Hensley, M.J.; Gibson, P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002, 19, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Meddows-Taylor, S.; Pendle, S.; Tiemessen, C.T. Altered expression of CD88 and associated impairment of complement 5a-induced neutrophil responses in human immunodeficiency virus type 1-infected patients with and without pulmonary tuberculosis. J. Infect. Dis. 2001, 183, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Henricks, P.A.; van der Tol, M.E.; Verhoef, J. Interactions between human polymorphonuclear leukocytes and influenza virus. Scand. J. Immunol. 1985, 22, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Wierzbicki, P.; Kłosowska, D.; Korczak-Kowalska, G.; Weber-Dabrowska, B.; Górski, A. The effects of T4 and A3/R phage preparations on whole-blood monocyte and neutrophil respiratory burst. Viral. Immunol. 2010, 23, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R.; Switala-Jelen, K.; Fortuna, W.; Weber-Dabrowska, B.; Przerwa, A.; Lusiak-Szelachowska, M.; Dabrowska, K.; Kurzepa, A.; Boratynski, J.; Syper, D.; et al. Bacteriophage preparation inhibition of reactive oxygen species generation by endotoxin-stimulated polymorphonuclear leukocytes. Virus Res. 2008, 131, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Przerwa, A.; Zimecki, M.; Switała-Jeleń, K.; Dabrowska, K.; Krawczyk, E.; Łuczak, M.; Weber-Dabrowska, B.; Syper, D.; Międzybrodzki, R.; Górski, A. Effects of bacteriophages on free radical production and phagocytic functions. Med. Microbiol. Immunol. 2006, 195, 143–150. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borysowski, J.; Międzybrodzki, R.; Wierzbicki, P.; Kłosowska, D.; Korczak-Kowalska, G.; Weber-Dąbrowska, B.; Górski, A. A3R Phage and Staphylococcus aureus Lysate Do Not Induce Neutrophil Degranulation. Viruses 2017, 9, 36. https://doi.org/10.3390/v9020036
Borysowski J, Międzybrodzki R, Wierzbicki P, Kłosowska D, Korczak-Kowalska G, Weber-Dąbrowska B, Górski A. A3R Phage and Staphylococcus aureus Lysate Do Not Induce Neutrophil Degranulation. Viruses. 2017; 9(2):36. https://doi.org/10.3390/v9020036
Chicago/Turabian StyleBorysowski, Jan, Ryszard Międzybrodzki, Piotr Wierzbicki, Danuta Kłosowska, Grażyna Korczak-Kowalska, Beata Weber-Dąbrowska, and Andrzej Górski. 2017. "A3R Phage and Staphylococcus aureus Lysate Do Not Induce Neutrophil Degranulation" Viruses 9, no. 2: 36. https://doi.org/10.3390/v9020036




