A Student’s Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae
Abstract
:1. Introduction: Defining Giant Viruses
2. Non-Structural Components of the Virion
3. Gauging the Host Range of Giant Viruses in Nature
4. Creating (an) Order from the Chaos: The Nucleocytoplasmic Large DNA Viruses
5. Viruses as a Possible Fourth Domain of Life
6. Giant Viruses in the Environment
7. Intimate Interactions with the Host: Eco-Evolutionary Consequences
8. Virophage
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischer, M.G.; Condit, R.C. Editorial introduction to “giant viruses” special issue of virology. Virology 2014, 466–467, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Durzyriska, J. Giant viruses: Enfants terribles in the microbal world. Future Virol. 2015, 10, 795–806. [Google Scholar] [CrossRef]
- Claverie, J.M.; Ogata, H.; Audic, S.; Abergel, C.; Suhre, K.; Fournier, P.E. Mimivirus and the emerging concept of “giant” virus. Virus Res. 2006, 117, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Giant viruses in the environment: Their origins and evolution. Curr. Opin. Virol. 2011, 1, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Abergel, C.; Legendre, M.; Claverie, J.M. The rapidly expanding universe of giant viruses: Mimivirus, Pandoravirus, Pithovirus and Mollivirus. FEMS Microbiol. Rev. 2015, 39, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Gastrich, M.D.; Leigh-Bell, J.A.; Gobler, C.; Anderson, O.R.; Wilhelm, S.W. Viruses as potential regulators of regional brown tide blooms caused by the alga, Aureococcus anophagefferens: A comparison of bloom years 1999–2000 and 2002. Estuaries 2004, 27, 112–119. [Google Scholar] [CrossRef]
- Serwer, P.; Hayes, S.J.; Thomas, J.A.; Hardies, S.C. Propagating the missing bacteriophages: A large bacteriophage in a new class. Virol. J. 2007, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Swan, B.K.; Wilson, W.H. Marine viruses, a genetic reservoir revealed by targeted viromics. ISME J. 2014, 8, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qin, T.; Xiao, Y.; Qin, F.; Lei, C.; Sun, X. Genomic and biological characterization of a new cypovirus isolated from Dendrolimus punctatus. PLoS ONE 2014, 9, e113201. [Google Scholar] [CrossRef] [PubMed]
- Corradi, N.; Pombert, J.-F.; Farinelli, L.; Didier, E.S.; Keeling, P.J. The complete sequence of the smallest known nuclear genome from the microsporidian Encephalitozoon intestinalis. Nat. Commun. 2010, 1, 77. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cano, D.J.; Reyes-Prieto, M.; Martínez-Romero, E.; Partida-Martínez, L.P.; Latorre, A.; Moya, A.; Delaye, L. Evolution of small prokaryotic genomes. Front. Microbiol. 2015, 5, 742. [Google Scholar] [CrossRef] [PubMed]
- Mesyanzhinov, V.V.; Robben, J.; Grymonprez, B.; Kostyuchenko, V.A.; Bourkaltseva, M.V.; Sykilinda, N.N.; Krylov, V.N.; Volckaert, G. The genome of bacteriophage φKZ of Pseudomonas aeruginosa. J. Mol. Biol. 2002, 317, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.B.; Coleman, M.L.; Weigele, P.; Rohwer, F.; Chisholm, S.W. Three Prochlorococcus cyanophage genomes: Signature features and ecological interpretations. PLoS Biol. 2005, 3, e144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, H.C.; Wilhelm, S.W.; Gobler, C.J.; Bullerjahn, G.; Jacobs, M.A.; McKay, J.; Sims, E.H.; Gillett, W.G.; Zhou, Y.; Haugen, E.; et al. Analyses of the complete chloroplast genome of two members of the pelagophyceae: Aureococcus anophagefferens CCMP1984 and Aureoumbra lagunesis CCMP1507. J. Phycol. 2010, 46, 602–615. [Google Scholar] [CrossRef]
- Orsini, M.; Costelli, C.; Malavasi, V.; Cusano, R.; Alessandro, C.; Angius, A.; Cao, G. Complete sequence and characterization of mitochondrial and chloroplast genome of Chlorella variabilis NC64A. Mitochondrial DNA A 2015, 27, 3128–3130. [Google Scholar]
- Korn, E.D.; Weisman, R.A. Phagocytosis of latex beads by Acanthomoeba. J. Cell Biol. 1967, 34, 219–227. [Google Scholar] [CrossRef]
- Murray, A.G.; Jackson, G.A. Viral dynamics: A model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992, 89, 103–116. [Google Scholar] [CrossRef]
- Klose, T.; Rossmann, M.G. Structure of large dsDNA viruses. Biol. Chem. 2014, 395, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Philippe, N.; Legendre, M.; Doutre, G.; Coute, Y.; Poirot, O.; Lescot, M.; Arslan, D.; Seltzer, V.; Bertaux, L.; Bruley, C.; et al. Pandoraviruses: Amoeba viruses with genomes up to 2.5 mb reaching that of parasitic eukaryotes. Science 2013, 341, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a Pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2014, 111, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Lartigue, A.; Bertaux, L.; Jeudy, S.; Bartoli, J.; Lescot, M.; Alempic, J.M.; Ramus, C.; Bruley, C.; Labadie, K.; et al. In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba. Proc. Natl. Acad. Sci. USA 2015, 112, E5327–E5335. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H. ‘Virophage’ suggests viruses are alive. Nature 2008, 454, 677. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Van Etten, J.L.; Allen, M.J. The phycodnaviridae: The story of how tiny giants rule the world. In Lesser Known Large dsDNA Viruses; VanEtten, J.L., Ed.; Springer Science & Business Media: Berlin, Germany, 2009; Volume 328, pp. 1–42. [Google Scholar]
- Xiao, C.; Rossmann, M.G. Structures of giant icosahedral eukaryotic dsDNA viruses. Curr. Opin. Virol. 2011, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.A.; Chipman, P.R.; Battisti, A.J.; Bowman, V.D.; Renesto, P.; Raoult, D.; Rossmann, M.G. Cryo-electron microscopy of the giant mimivirus. J. Mol. Biol. 2005, 353, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Chothi, M.P.; Duncan, G.A.; Armirotti, A.; Abergel, C.; Gurnon, J.R.; Van Etten, J.L.; Bernardi, C.; Damonte, G.; Tonetti, M. Identification of an l-rhamnose synthetic pathway in two nucleocytoplasmic large DNA viruses. J. Virol. 2010, 84, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.; Chothi, M.P.; Abergel, C.; Seltzer, V.; Gurnon, J.; Van Etten, J.L. Glycosylation in nucleo-cytoplasmic large DNA viruses (NCLDV). FEBS J. 2011, 278, 420. [Google Scholar]
- Piacente, F.; Gaglianone, M.; Laugieri, M.E.; Tonetti, M.G. The autonomous glycosylation of large DNA viruses. Int. J. Mol. Sci. 2015, 16, 29315–29328. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D. Viruses reconsidered. Scientist 2014, 28, 41. [Google Scholar]
- Rodrigues, R.A.L.; Silva, L.K.D.; Dornas, F.P.; de Oliveira, D.B.; Magalhaes, T.F.F.; Santos, D.A.; Costa, A.O.; Farias, L.D.; Magalhaes, P.P.; Bonjardim, C.A.; et al. Mimivirus fibrils are important for viral attachment to the microbial world by a diverse glycoside interaction repertoire. J. Virol. 2015, 89, 11812–11819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Xiang, Y.; Dunigan, D.D.; Klose, T.; Chipman, P.R.; Van Etten, J.L.; Rossmann, M.G. Three-dimensional structure and function of the Paramecium bursaria chlorella virus capsid. Proc. Natl. Acad. Sci. USA 2011, 108, 14837–14842. [Google Scholar] [CrossRef] [PubMed]
- Suzan-Monti, M.; La Scola, B.; Raoult, D. Genomic and evolutionary aspects of mimivirus. Virus Res. 2006, 117, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Maat, D.S.; Bale, N.J.; Hopmans, E.C.; Baudoux, A.C.; Damste, J.S.S.; Schouten, S.; Brussaard, C.P.D. Acquisition of intact polar lipids from the prymnesiophyte Phaeocystis globosa by its lytic virus PgV-07t. Biogeosciences 2014, 11, 185–194. [Google Scholar] [CrossRef]
- Martinez-Martinez, J.; Boere, A.; Gilg, I.C.; van Lent, J.W.M.; Witte, H.J.; van Bleijswijk, J.D.L.; Brussaard, C.P.D. New lipid envelop-containing dsDNA virus isolates infecting Micromonas pusilla reveal a separate phylogenetic group. Aquat. Microb. Ecol. 2015, 74, 17–28. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Worthy, C.A.; Biggi, G.; Hall, M.; Ryan, K.P.; Varsani, A.; Harper, G.M.; Wilson, W.H.; Brownlee, C.; Schroeder, D.C. A unicellular algal virus, Emiliania huxleyi virus 86, exploits an animal-like infection strategy. J. Gen. Virol. 2009, 90, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Frada, M.J.; Schatz, D.; Farstey, V.; Ossolinski, J.E.; Sabanay, H.; Ben-Dor, S.; Koren, I.; Vardi, A. Zooplankton may serve as transmission vectors for viruses infecting algal blooms in the ocean. Curr. Biol. 2014, 24, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.B. Oxidative stress during viral infection: A review. Free Radic. Biol. Med. 1996, 21, 641–649. [Google Scholar] [CrossRef]
- Kang, M.; Duncan, G.A.; Kuszynski, C.; Oyler, G.; Zheng, J.Y.; Becker, D.F.; Van Etten, J.L. Chlorovirus PBCV-1 encodes an active copper-zinc superoxide dismutase. J. Virol. 2014, 88, 12541–12550. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, A.; Burlat, B.; Coutard, B.; Chaspoul, F.; Claverie, J.M.; Abergel, C. The Megavirus chilensis Cu,Zn-superoxide dismutase: The first viral structure of a typical cellular copper chaperone-independent hyperstable dimeric enzyme. J. Virol. 2015, 89, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.G.; Kelly, I.; Foster, L.J.; Suttle, C.A. The virion of Cafeteria roenbergensis virus (CroV) contains a complex suite of proteins for transcription and DNA repair. Virology 2014, 466, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Ezerina, D.; Alon, A.; Vonshak, O.; Fass, D. Exploring ORFan domains in giant viruses: Structure of mimivirus sulfhydryl oxidase R596. PLoS ONE 2012, 7, e50649. [Google Scholar] [CrossRef] [PubMed]
- Apperizeller-Herzog, C.; Ellgaard, L. The human PDI family: Versatility packed into a single fold. Biochim. Biophys. Acta 2008, 1783, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ryser, H.J.P.; Levy, E.M.; Mandel, R.; Disciullo, G.J. Inhibition of human-immunodeficiency-virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. USA 1994, 91, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.; Malmstrom, J.; Pelkmans, L.; Haugstetter, J.; Ellgaard, L.; Grunewald, K.; Helenius, A. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 2007, 131, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.; Brochier-Armanet, C. Giant viruses, giant chimeras: The multiple evolutionary histories of mimivirus genes. BMC Evol. Biol. 2008, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; La Scola, B.; Lepidi, H.; Raoult, D. Pneumonia in mice inoculated experimentally with Acanthamoeba polyphaga mimivirus. Microb. Pathog. 2007, 42, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.M.S.; Ainsworth, T.D.; Rosales, S.M.; Thurber, A.R.; Butler, C.R.; Vega Thurber, R.L. Viral outbreak in corals associated with an in situ bleaching event: Atypical herpes-like viruses and a new megavirus infecting symbiodinium. Front. Microbiol. 2016, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Popgeorgiev, N.; Boyer, M.; Fancello, L.; Monteil, S.; Robert, C.; Rivet, R.; Nappez, C.; Azza, S.; Chiaroni, J.; Raoult, D.; et al. Marseillevirus-like virus recovered from blood donated by asymptomatic humans. J. Infect. Dis. 2013, 208, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Yolken, R.H.; Jones-Brando, L.; Dunigan, D.D.; Kannan, G.; Dickerson, F.; Severance, E.; Sabunciyan, S.; Talbot, C.C.; Prandovszky, E.; Gurnon, J.R.; et al. Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice. Proc. Natl. Acad. Sci. USA 2014, 111, 16106–16111. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Aravind, L.; Koonin, E.V. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 2001, 75, 11720–11734. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, J.L.; Meints, R.H. Giant viruses infecting algae. Annu. Rev. Microbiol. 1999, 53, 447–494. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beato, R.; Salas, M.L.; Vinuela, E.; Salas, J. Role of the host cell nucleus in the replication of african swine fever virus DNA. Virology 1992, 188, 637–649. [Google Scholar] [CrossRef]
- Goorha, R. Frog virus-3 DNA-replication occurs in 2 stages. J. Virol. 1982, 43, 519–528. [Google Scholar] [PubMed]
- Moss, B. Poxviridae: The Viruses and Their Replication; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; pp. 1163–1197. [Google Scholar]
- Koonin, E.V.; Yutin, N. Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses. Intervirology 2010, 53, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Elde, N.C.; Child, S.J.; Eickbush, M.T.; Kitzman, J.O.; Rogers, K.S.; Shendure, J.; Geballe, A.P.; Malik, H.S. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 2012, 150, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Filee, J. Route of NCLDV evolution: The genoic accordion. Curr. Opin. Virol. 2013, 3, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Desnues, C.; La Scola, B.; Yutin, N.; Fournous, G.; Robert, C.; Azza, S.; Jardot, P.; Monteil, S.; Campocasso, A.; Koonin, E.V.; et al. Provirophages and transpovirons as the diverse mobilome of giant viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 18078–18083. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E.; et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008, 455, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Wolf, Y.I.; Raoult, D.; Koonin, E.V. Eukaryotic large nucleo-cytoplasmic DNA viruses: Clusters of orthologous genes and reconstruction of viral genome evolution. Virol. J. 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Claverie, J.M.; Abergel, C. Mimivirus and its virophage. In Annual Review of Genetics; Annual Reviews: Palo Alto, CA, USA, 2009; Volume 43, pp. 49–66. [Google Scholar]
- Aherfi, S.; La Scola, B.; Pagnier, I.; Raoult, D.; Colson, P. The expanding family Marseilleviridae. Virology 2014, 466–467, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; de Lamballerie, X.; Fournous, G.; Raoult, D. Reclassification of giant viruses composing a fourth domain of life in the new order megavirales. Intervirology 2012, 55, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; De Lamballerie, X.; Yutin, N.; Asgari, S.; Bigot, Y.; Bideshi, D.K.; Cheng, X.W.; Federici, B.A.; Van Etten, J.L.; Koonin, E.V.; et al. “Megavirales”, a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses. Arch. Virol. 2013, 158, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Bamford, D.H. Virus evolution: How far does the double beta-barrel viral lineage extend? Nat. Rev. Microbiol. 2008, 6, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Condit, R.C. Vaccinia, Inc.—Probing the functional substructure of poxviral replication factories. Cell Host Microbe 2007, 2, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.; Moffat, K.; Brooks, E.; Wileman, T. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv. Virus Res. 2007, 70, 101–182. [Google Scholar] [PubMed]
- Mutsafi, Y.; Zauberman, N.; Sabanay, I.; Minsky, A. Vaccinia-like cytoplasmic replication of the giant mimivirus. Proc. Natl. Acad. Sci. USA 2010, 107, 5978–5982. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Yutin, N.; Pagnier, I.; Barrassi, L.; Fournous, G.; Espinosa, L.; Robert, C.; Azza, S.; Sun, S.Y.; Rossmann, M.G.; et al. Giant marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc. Natl. Acad. Sci. USA 2009, 106, 21848–21853. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Wileman, T. Virus factories, double membrane vesicles and viroplasm generated in animal cells. Curr. Opin. Virol. 2011, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; LeCleir, G.R.; Brown, C.M.; Gobler, C.J.; Bidle, K.D.; Wilson, W.H.; Wilhelm, S.W. Genome of the brown tide virus (AaV), the little giant of the megaviridae, elucidates NCLDV genome expansion and host-virus coevolution. Virology 2014, 466–467, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Wolf, Y.I.; Koonin, E.V. Origin of giant viruses from smaller DNA viruses not from a fourth domain of cellular life. Virology 2014, 466–467, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Gann, E.R.; LeCleir, G.R.; Kang, Y.; Gobler, C.J.; Wilhelm, S.W. Diversity and dynamics of algal megaviridae members during a harmful brown tide caused by the pelagophyte, Aureococcus anophagefferens. FEMS Microbiol. Ecol. 2016, 92, fiw058. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Koonin, E.V. Pandoraviruses are highly derived phycodnaviruses. Biol. Direct 2013, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Lwoff, A. The concept of a virus. J. Gen. Microbiol. 1957, 17, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 2, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, F. Sur un microbe invisible antagonistic des bacilles dysenterique. C. R. Acad. Sci. Paris 1917, 165, 373–375. [Google Scholar]
- Lwoff, A.; Tournier, P. Classification of viruses. Annu. Rev. Microbiol. 1966, 20, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; La Scola, B.; Birtles, R. The discovery and characterization of mimivirus, the largest known virus and putative pneumonia agent. Clin. Infect. Dis. 2007, 45, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Audic, S.; Robert, C.; Abergel, C.; Renesto, P.; Ogata, H.; La Scola, B.; Suzan, M.; Claverie, J.M. The 1.2-megabase genome sequence of mimivirus. Science 2004, 306, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Madoui, M.A.; Gimenez, G.; La Scola, B.; Raoult, D. Phylogenetic and phyletic studies of informational genes in genomes highlight existence of a 4th domain of life including giant viruses. PLoS ONE 2010, 5, e15530. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Inferring Phylogenies; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Williams, T.A.; Embley, T.M.; Heinz, E. Informational gene phylogenies do not support a fourth domain of life for nucleocytoplasmic large DNA viruses. PLoS ONE 2011, 6, e21080. [Google Scholar] [CrossRef] [PubMed]
- Doutre, G.; Philippe, N.; Abergel, C.; Claverie, J.M. Genome analysis of the first Marseilleviridae representative from Australia indicates that most of its genes contribute to virus fitness. J. Virol. 2014, 88, 14340–14349. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.-E.T.; Winget, D.M.; White, R.A.; Hallam, S.J.; Suttle, C.A. Combining genomic sequencing methods to explore viral diversity and reveal potential virus-host interactions. Front. Microbiol. 2015, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; de Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Dornas, F.P.; Silva, L.C.; Almeida, G.M.; Boratto, P.V.; Colson, P.; La Scola, B.; Kroon, E.G. Acanthamoeba polyphaga mimivirus and other giant viruses: An open field to outstanding discoveries. Virol. J. 2014, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, E.J.; Roh, S.W.; Bae, J.W. Diversity and abundance of single-stranded DNA viruses in human feces. Appl. Environ. Microbiol. 2011, 77, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.P.; Al-Ameeli, Z.; Duncan, G.; Van Etten, J.L.; Dunigan, D.D. Predators catalyze an increase in chloroviruses by foraging on the symbiotic hosts of zoochlorellae. Proc. Natl. Acad. Sci. USA 2016, 113, 13780–13784. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.M.; Mushegian, A.R.; Dolja, V.V.; Koonin, E.V. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 2010, 18, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.J.; Allen, L.Z.; Lorenzi, H.A.; Fadrosh, D.W.; Brami, D.; Thiagarajan, M.; McCrow, J.P.; Tovchigrechko, A.; Yooseph, S.; Venter, J.C. Metagenomic exploration of viruses throughout the Indian Ocean. PLoS ONE 2012, 7, e42047. [Google Scholar] [CrossRef] [PubMed]
- Khalil, J.Y.B.; Langlois, T.; Andreani, J.; Sorraing, J.-M.; Raoult, D.; Carmoin, L.; La Scola, B. Flow cytometry sorting to separate viable giant viruses from amoeba co-culture supernatants. Front. Cell. Infect. Microbiol. 2016, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Martinez Martinez, J.; Poulton, N.J.; Stepanauskas, R.; Sieracki, M.E.; Wilson, W.H. Targeted sorting of single virus-infected cells of the coccolithophore Emiliania huxleyi. PLoS ONE 2011, 6, e22520. [Google Scholar] [CrossRef] [PubMed]
- Zablocki, O.; van Zyl, L.; Adriaenssens, E.M.; Rubagotti, E.; Tuffin, M.; Cary, S.C.; Cowana, D. High-level diversity of tailed phages, eukaryote-associated viruses, and virophage-like elements in the metaviromes of Antarctic soils. Appl. Environ. Microbiol. 2014, 80, 10. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.M.; Welsh, R.M.; Vega Thurber, R.L. Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 2013, 7, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lysholm, F.; Wetterbom, A.; Lindau, C.; Darban, H.; Bjerkner, A.; Fahlander, K.; Lindberg, A.M.; Persson, B.; Allander, T.; Andersson, B. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS ONE 2012, 7, e30875. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.D.; Kuipers, B.; Veldhuis, M.J.W. A mesocosm study of Phaeocystis globosa population dynamics. Harmful Algae 2005, 4, 859–874. [Google Scholar] [CrossRef]
- Castberg, T.; Thyrhaug, R.; Larsen, A.; Sandaa, R.-A.; Heldal, M.; Van Etten, J.L.; Bratbak, G. Isolation and characterization of a virus that infects Emiliania huxleyi (Haptophyta). J. Phycol. 2002, 38, 767–774. [Google Scholar] [CrossRef]
- Gobler, C.J.; Anderson, O.R.; Gastrich, M.D.; Wilhelm, S.W. Ecological aspects of viral infection and lysis in the harmful brown tide alga Aureococcus anophagefferens. Aquat. Microb. Ecol. 2007, 47, 25–36. [Google Scholar] [CrossRef]
- Nagasaki, K.; Tarutani, K.; Yamaguchi, M. Growth characteristics of Heterosigma akashiwo virus and its possible use as a microbiological agent for red tide control. Appl. Environ. Microbiol. 1999, 65, 898–902. [Google Scholar] [PubMed]
- Brussaard, C.P.D.; Gast, G.J.; van Duyl, F.C.; Riegmen, R. Impact of phytoplankton bloom magnitude on a pelagic microbial food web. Mar. Ecol. Prog. Ser. 1996, 144, 211–221. [Google Scholar] [CrossRef]
- Weitz, J.S.; Wilhelm, S.W. Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Suttle, C.A. Viruses and nutrient cycles in the sea. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Nagasaki, K.; Bratbak, G. Isolation of viruses infecting photosynthetic and nonphotosynthetic protists. In Manual of Aquatic Viral Ecology; Wilhelm, S.W., Weinbauer, M.G., Suttle, C.A., Eds.; ASLO: Waco, TX, USA, 2010; pp. 92–101. [Google Scholar]
- Brodie, J.; Zuccarello, G.C. Systematics of the species rich algae: Red algal classificiation, phylogeny and speciation. In Reconstructing the Tree of Life. Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; CRC Press: New York, NY, USA, 2006; pp. 323–336. [Google Scholar]
- Short, S.M. The ecology of viruses that infect eukaryotic algae. Environ. Microbiol. 2012, 14, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, T.V.; Bratbak, G.; Larsen, A.; Ogata, H.; Egge, E.S.; Edvardsen, B.; Eikrem, W.; Sandaa, R.A. Characterisation of three novel giant viruses reveals huge diversity among viruses infecting Prymnesiales (Haptophyta). Virology 2015, 476, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Coy, S.R.; Gann, E.R.; Moniruzzaman, M.; Stough, J.M.A. Standing on the shoulders of giant viruses: 5 lessons learned about large viruses infecting small eukaryotes and the opportunities they create. PLoS Pathog. 2016, 12, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Wurch, L.L.; Alexander, H.; Dyhrman, S.T.; Gobler, C.J.; Wilhelm, S.W. Virus-host infection dynamics of marine single-celled eukaryotes resolved from metatranscriptomics. bioRxiv 2016. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Lane, L.C.; Meints, R.H. Viruses and viruslike particles of eukaryotic algae. Microbiol. Rev. 1991, 55, 586–620. [Google Scholar] [PubMed]
- Reteno, D.G.; Benamar, S.; Kahlil, J.B.; Andreani, J.; Armstrong, N.; Klose, T.; Rossmann, M.G.; Colson, P.; Raoult, D.; La Scola, B. Faustovirus, an asfarvirus-related new lineage of giant viruses infecting amoebae. J. Virol. 2015, 89, 6585–6594. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.G.; Allen, M.J.; Wilson, W.H.; Suttle, C.A. Giant virus with a remarkable complement of genes infects marine zooplankton. Proc. Natl. Acad. Sci. USA 2010, 107, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E.; Soeder, C.J.; Hegewald, E. Polyhedral virus-like particles lysing the aquatic phycomycete Aphedlidium sp., a parasite of the grean algae Scenedesmus armatus. Virology 1970, 42, 482–487. [Google Scholar] [CrossRef]
- Pickett-Heaps, J.D. A possible virus infection in the green alga Oedogonium. J. Phycol. 1972, 8, 44–47. [Google Scholar] [CrossRef]
- Toth, R.; Wilce, R.T. Viruslike particles in the marine alga Chorda tomentosa lyngye (Phaeophyceae). J. Phycol. 1972, 8, 126–130. [Google Scholar]
- Baker, J.R.J.; Evans, L.V. The ship fouling alga Ectocarpus. Protoplasma 1973, 77, 1–13. [Google Scholar] [CrossRef]
- Clitheroe, S.B.; Evans, L.V. Virus like particles in the brown algae Ectocarpus. J. Ultrastruct. Res. 1974, 49, 211–217. [Google Scholar] [CrossRef]
- Swale, E.M.F.; Belcher, J.H. A light and electron microscope study of the colourless flagellate Aulacomonas skuja. Arch. Microbiol. 1973, 92, 91–103. [Google Scholar] [CrossRef]
- Markey, D.R. A possivle virus infection in the brown alga Phlaiella littoralis. Protoplasma 1974, 80, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, O.; Thomsen, H.A. An ultrastructural study of the flagellate Phyramimonas orientalis with particular emphasis on golgi apparatus activity and the flagellar apparatus. Protoplasma 1974, 81, 247–269. [Google Scholar] [CrossRef]
- Gibbs, A.; Skotnicki, A.H.; Gardiner, J.E.; Walker, E.S.; Hollings, M. A tabamovirus of a green alga. Virology 1975, 64, 571–574. [Google Scholar] [CrossRef]
- Oliveira, L.; Bisalputra, T. A virus infection in the brown alga Sorocarpus uvaeformis (Lyngbye) pringsheim (Phaeophyta, Ecocarpales). Ann. Bot. 1978, 42, 439–445. [Google Scholar] [CrossRef]
- Sicko-Goad, L.; Walker, G. Viroplasm and large virus-like particles in the dinoflagellate Gymnodiunium uberrimum. Protoplasma 1979, 99, 203–210. [Google Scholar] [CrossRef]
- Dodds, J.A.; Cole, A. Microscopy and biology of Uronema gigas, a filamentous eucaryotic green alga, and its associated tailed virus-like particle. Virology 1980, 100, 156–165. [Google Scholar] [CrossRef]
- Preisig, H.R.; Hibberd, D.J. Virus-like particles and endophytic bacteria in Paraphysomonas and Chromophysomonas (Chrysophyceae). Nord. J. Bot. 1984, 4, 279–285. [Google Scholar] [CrossRef]
- Gowing, M.M. Large virus-like particles from vacuoles of phaeodarian radiolarians and from other marine samples. Mar. Ecol. Prog. Ser. 1993, 101, 33–43. [Google Scholar] [CrossRef]
- Smayda, T.J. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the important of grazing. Harmful Algae 2008, 8, 140–151. [Google Scholar] [CrossRef]
- Filee, J.; Pouget, N.; Chandler, M. Phylogenetic evidence for extensive lateral acquisition of cellular genes by nucleocytoplasmic large DNA viruses. BMC Evol. Biol. 2008, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Monier, A.; Pagarete, A.; de Vargas, C.; Allen, M.J.; Read, B.; Claverie, J.-M.; Ogata, H. Horizontal gene transfer of an entire metabolic pathway between a eukaryotic alga and its DNA virus. Genome Res. 2009, 19, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, S.; Mausz, M.A.; Schatz, D.; Sheyn, U.; Malitsky, S.; Aharoni, A.; Weinstock, E.; Tzfadia, O.; Ben-Dor, S.; Feldmesser, E.; et al. Rewiring host lipid metabolism by large viruses determines the fate of Emiliania huxleyi, a bloom-forming alga in the ocean. Plant Cell Online 2014, 26, 2689–2707. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Van Mooy, B.A.S.; Fredricks, H.F.; Popendorf, K.J.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Filee, J.; Siguier, P.; Chandler, M. I am what I eat and I eat what I am: Acquisition of bacterial genes by giant viruses. Trends Genet. 2007, 23, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Filee, J. Multiple occurrences of giant virus core genes acquired by eukaryotic genomes: The visible part of the iceberg? Virology 2014, 466–467, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Gallot-Lavallee, L.; Maumus, F. Provirophages in the bigelowiella genome bear testimony to past encounters with giant viruses. Proc. Natl. Acad. Sci. USA 2015, 112, E5318–5326. [Google Scholar] [CrossRef] [PubMed]
- Read, B.A.; Kegel, J.; Klute, M.J.; Kuo, A.; Lefebvre, S.C.; Maumus, F.; Mayer, C.; Miller, J.; Monier, A.; Salamov, A.; et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 2013, 499, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumus, F.; Epert, A.; Nogué, F.; Blanc, G. Plant genomes enclose footprints of past infections by giant virus relatives. Nat. Commun. 2014, 5, 4268. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M. Marine viruses: Truth or dare. Ann Rev Mar Sci 2012, 4, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Frada, M.; Probert, I.; Allen, M.J.; Wilson, W.H.; de Vargas, C. The “cheshire cat” escape strategy of the coccolithophore Emiliania huxleyi in response to viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 15944–15949. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.; Lauro, F.M.; DeMaere, M.Z.; Brown, M.V.; Thomas, T.; Raftery, M.J.; Andrews-Pfannkoch, C.; Lewis, M.; Hoffman, J.M.; Gibson, J.A.; et al. Virophage control of antarctic algal host-virus dynamics. Proc. Natl. Acad. Sci. USA 2011, 108, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Boratto, P.V.; Assis, F.L.; Aguiar, E.R.; Silva, L.C.; Albarnaz, J.D.; Dornas, F.P.; Trindade, G.S.; Ferreira, P.P.; Marques, J.T.; et al. Samba virus: A novel mimivirus from a giant rain forest, the Brazilian amazon. Virol. J. 2014, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.G.; Suttle, C.A. A virophage at the origin of large DNA transposons. Science 2011, 332, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Gaia, M.; Benamar, S.; Boughalmi, M.; Pagnier, I.; Croce, O.; Colson, P.; Raoult, D.; La Scola, B. Zamilon, a novel virophage with Mimiviridae host specificity. PLoS ONE 2014, 9, e94923. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, A.; Bekliz, M.; Chabrière, E.; Pontarotti, P.; La Scola, B.; Raoult, D. Mimivire is a defence system in mimivirus that confers resistance to virophage. Nature 2016, 531, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; Jeudy, S.; Bartoli, J.; Poirot, O.; Lescot, M.; Abergel, C.; Barbe, V.; Wommack, K.E.; Noordeloos, A.A.M.; Brussaard, C.P.D.; et al. Genome of Phaeocystis globosa virus PgV-16t highlights the common ancestry of the largest known DNA viruses infecting eukaryotes. Proc. Natl. Acad. Sci. USA 2013, 110, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Claverie, J.M.; Abergel, C. CRISPR-CAS-like system in giant viruses: Why mimivire is not likely to be an adaptive immune system. Virol. Sin. 2016, 31, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.B.; Weitz, J.S.; Wilhelm, S.W. Viral ecology comes of age. Environ. Microbiol. Repo. 2017, 9, 33–35. [Google Scholar] [CrossRef] [PubMed]
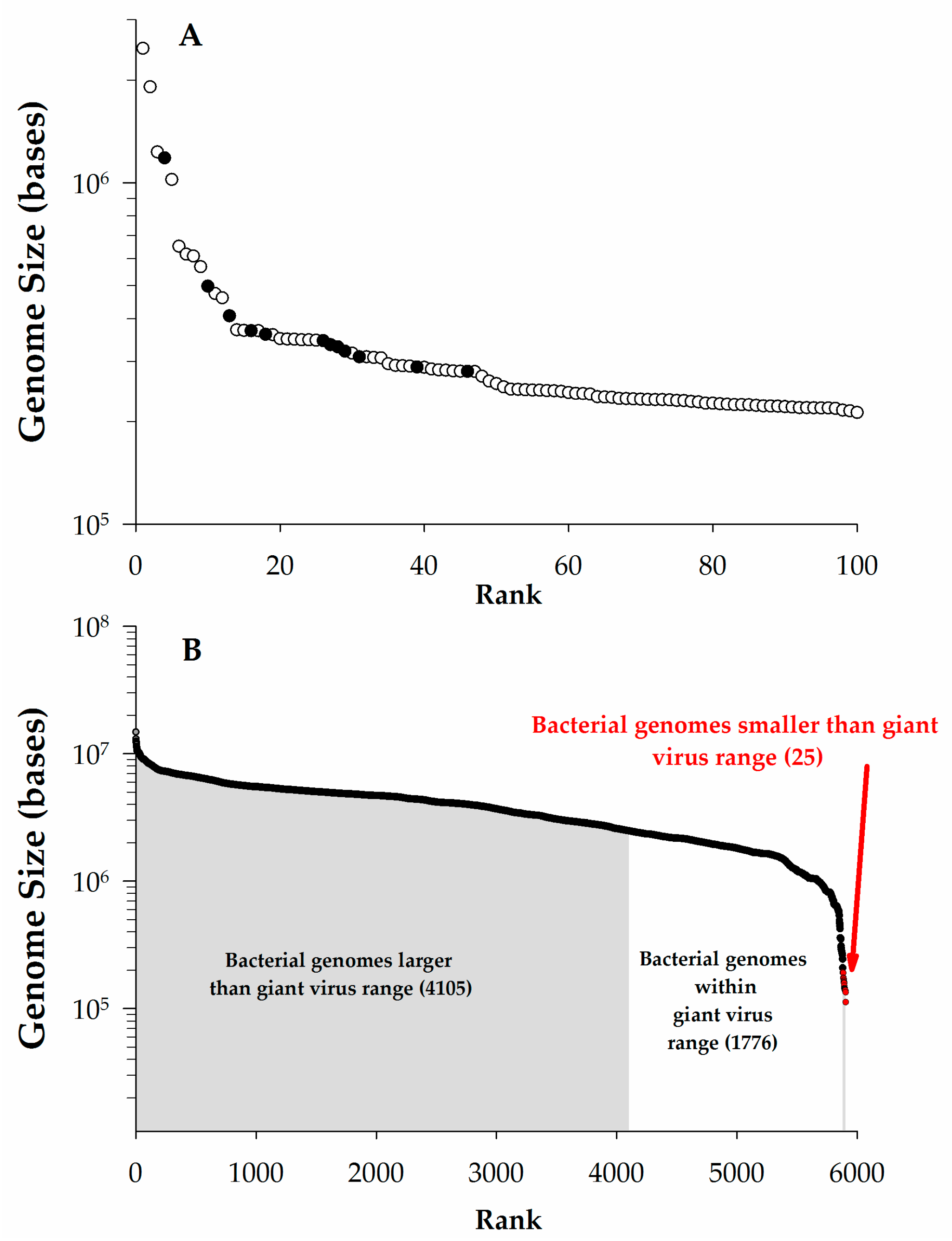
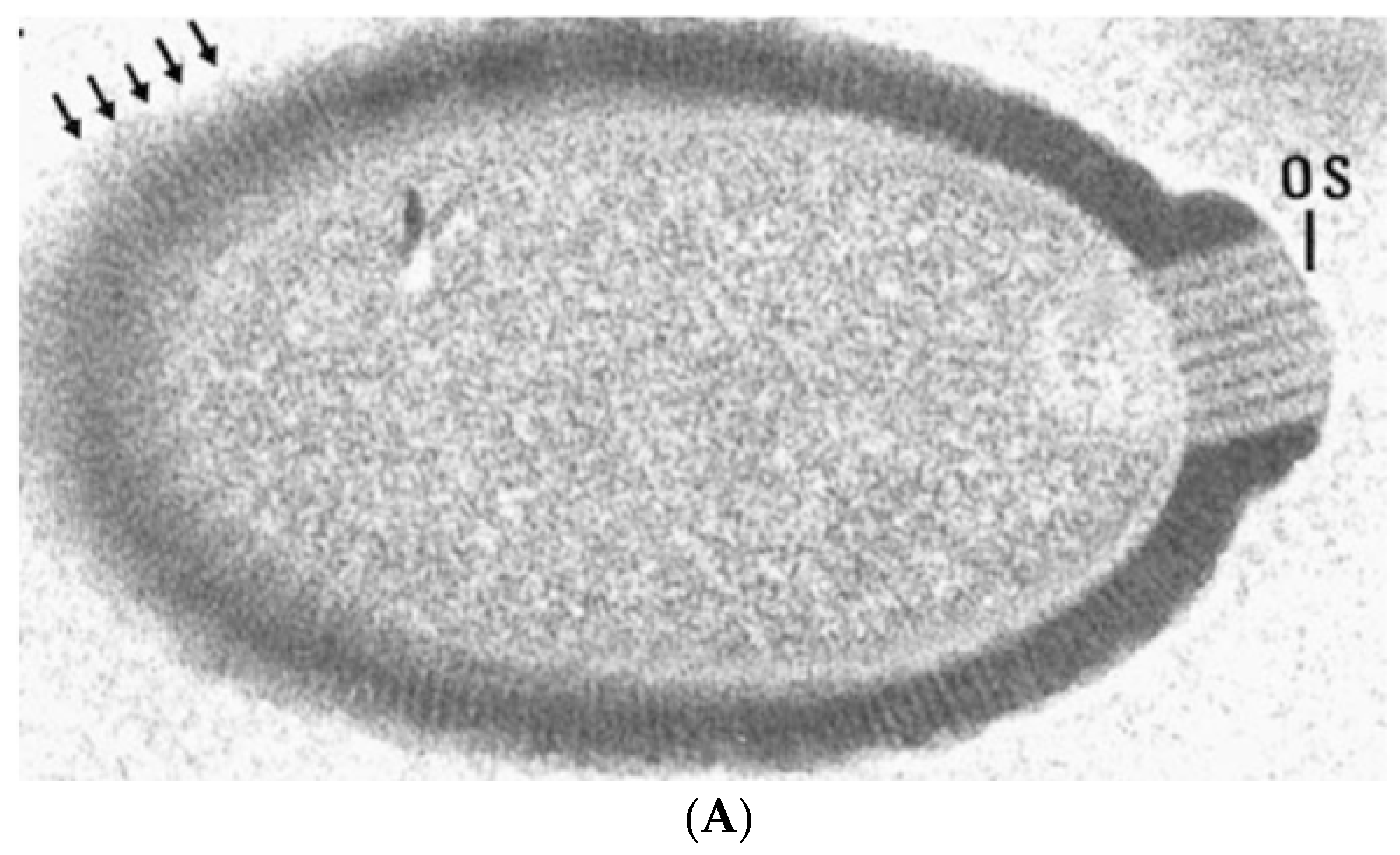
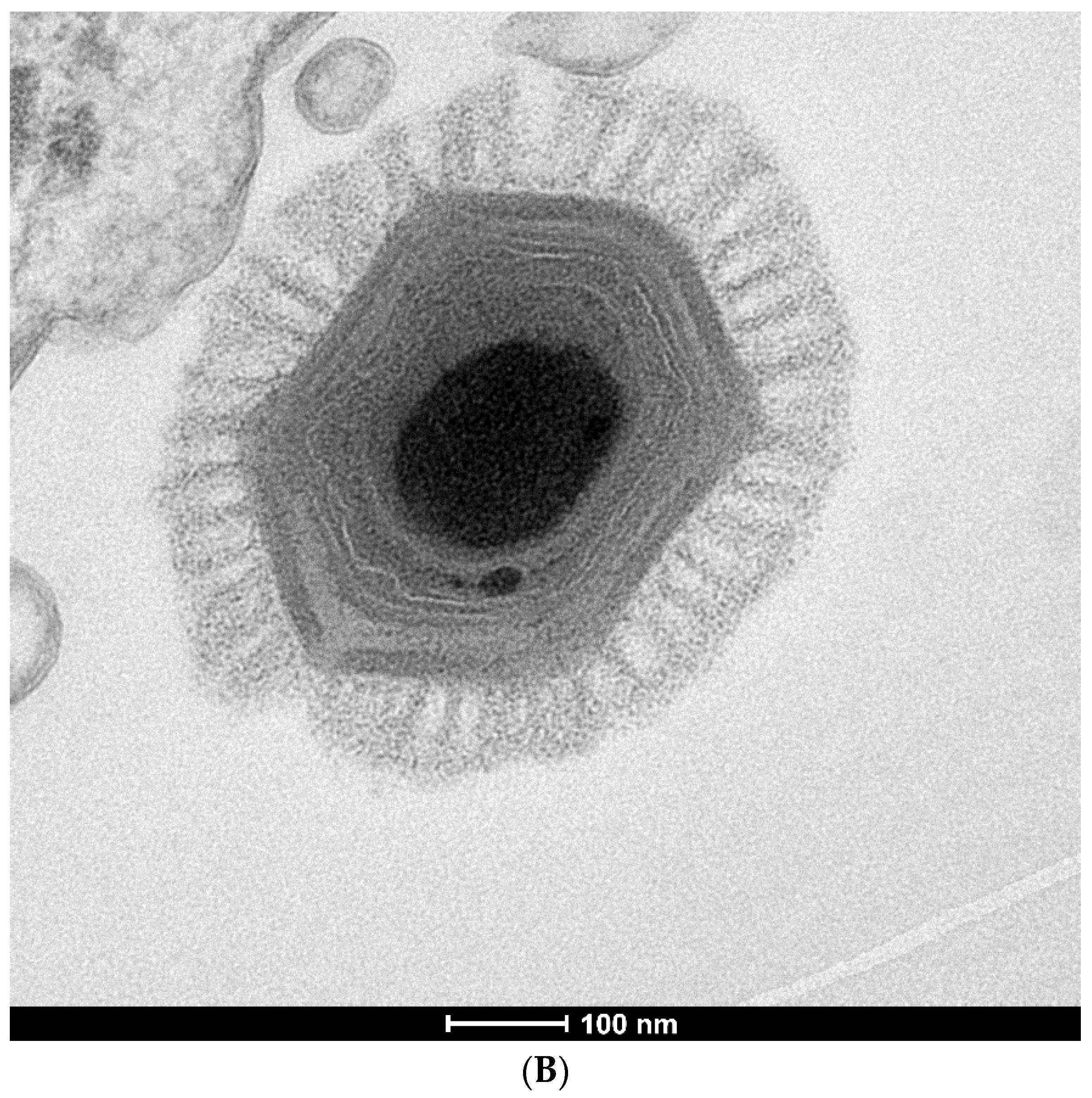
| Giant Virus | Size Virus (Mb) | Virus GC (%) | ORFs * | Accession | Host | Size Host (Mb) | Host GC (%) | Host-Virus Genome Size | Host-Virus GC | Accession |
|---|---|---|---|---|---|---|---|---|---|---|
| Pandoravirus salinus | 2.5 | 61.7 | 2541 | NC_022098.1 | Acanthamoeba castellanii | 46.7 | 58.3 | 18.9 | −3.4 | AHJI00000000.1 |
| Pandoravirus dulcis | 1.9 | 63.7 | 1487 | NC_021858.1 | A. castellanii | 46.7 | 58.3 | 24.5 | −5.4 | AHJI00000000.1 |
| Acanthamoeba polyphaga mimivirus | 1.2 | 28.0 | 1018 | NC_014649.1 | A. polyphaga | 120.4 | 59.3 | 102.0 | 31.3 | CDFK00000000.1 |
| Acanthamoeba polyphaga moumouvirus | 1.0 | 24.6 | 915 | NC_020104.1 | A. polyphaga | 120.4 | 59.3 | 118.1 | 34.7 | CDFK00000000.1 |
| Mollivirus sibericum | 0.7 | 60.1 | 523 | NC_027867.1 | A. castellanii | 42.0 | 58.4 | 64.6 | −1.7 | AHJI00000000.1 |
| Pithovirus sibericum | 0.6 | 35.8 | 467 | NC_023423.1 | A. castellanii | 42.0 | 58.4 | 68.9 | 22.6 | AHJI00000000.1 |
| Emiliania huxleyi virus 86 | 0.4 | 40.2 | 478 | NC_007346.1 | Emiliania huxleyi | 167.7 | 65.7 | 409.0 | 25.5 | AHAL00000000.1 |
| Marseillevirus marseillevirus | 0.4 | 44.7 | 457 | NC_013756.1 | A. polyphaga | 120.4 | 59.3 | 325.5 | 14.6 | CDFK00000000.1 |
| Aureococcus anophagefferens virus | 0.4 | 28.7 | 384 | NC_024697.1 | A. anophagefferens | 56.7 | 69.5 | 153.1 | 40.8 | NZ_ACJI00000000.1 |
| Melbournevirus | 0.4 | 44.7 | 403 | NC_025412.1 | A. castellanii | 42.0 | 58.4 | 113.6 | 13.7 | AHJI00000000.1 |
| Paramecium bursaria Chlorella virus NY2A | 0.4 | 40.7 | 411 | NC_009898.1 | Chlorella variabilis NC64A | 46.2 | 67.1 | 124.8 | 26.4 | ADIC00000000.1 |
| Brazilian marseillevirus | 0.4 | 43.3 | 491 | NC_029692.1 | A. castellanii | 42.0 | 58.4 | 116.7 | 15.1 | AHJI00000000.1 |
| Lausannevirus | 0.4 | 42.9 | 444 | NC_015326.1 | A. castellanii | 42.0 | 58.4 | 120.1 | 15.5 | AHJI00000000.1 |
| Ectocarpus siliculosus virus 1 | 0.3 | 51.7 | 240 | NC_002687.1 | Ectocarpus siliculosus | 195.8 | 53.5 | 575.9 | 1.8 | CABU00000000.1 |
| Paramecium bursaria Chlorella virus AR158 | 0.3 | 40.8 | 366 | NC_009899.1 | C. variabilis NC64A | 46.2 | 67.1 | 135.8 | 26.3 | ADIC00000000.1 |
| Paramecium bursaria Chlorella virus 1 | 0.3 | 40.0 | 376 | NC_000852.5 | C. variabilis NC64A | 46.2 | 67.1 | 139.9 | 27.1 | ADIC00000000.1 |
| Micromonas pusilla virus 12T | 0.2 | 39.8 | 265 | NC_020864.1 | Micromonas pusilla | 22.0 | 65.9 | 104.6 | 26.1 | NZ_ACCP00000000.1 |
| Sample Bacteriophage | ||||||||||
| Bacillus phage G | 0.5 | 29.9 | 694 | NC_023719.1 | Bacillus megaterium | 5.3 | 38.1 | 10.7 | 8.2 | NZ_CP009920.1 |
| Prochlorococcus phage P-SSM2 | 0.3 | 35.5 | 335 | NC_006883.2 | Prochlorococcus marinus | 1.8 | 36.4 | 7.0 | 0.9 | NC_005042.1 |
| Ralstonia phage RSL1 | 0.2 | 58.0 | 345 | NC_010811.2 | Ralstonia solanacearum | 5.6 | 66.5 | 24.3 | 8.5 | NC_003295.1 |
| Sinorhizobium phage phiN3 | 0.2 | 49.1 | 408 | NC_028945.1 | Sinorhizobium meliloti | 3.7 | 62.7 | 17.4 | 13.6 | NC_003047.1 |
| Pseudomonas phage EL | 0.2 | 49.3 | 201 | NC_007623.1 | Pseudomonas aeruginosa | 6.3 | 66.6 | 29.8 | 17.3 | NC_002516.2 |
| Environment | Location | Abundance | Total Reads | Most Common Virus Families Present | Source |
|---|---|---|---|---|---|
| Marine | Indian Ocean | 0.3%–1.4% | N/A | Mimiviridae, Phycodnaviridae | [91] |
| Antarctic soil | Antarctica | 2.82%–7.71% | 123/1595-177/6264 | Mimiviridae, Phycodnaviridae | [94] |
| Coral | USA | 1.2% | 744/60485 | Mimiviridae, Phycodnaviridae | [95] |
| Human (respiratory system) | Sweden | 0.00002% | 2/111931 | Mimiviridae | [96] |
| Year | Organism | Particle Size | References |
|---|---|---|---|
| 1970 | Aphelidium sp. (fungal parasite of algae) | 190–210 nm | [113] |
| 1972 | Oedogonium spp. “L” (Chlorophyceae) | 240 nm | [114] |
| Chorda tomentosa (Phaeophyceae) | 170 nm | [115] | |
| 1973 | Ectocarpus sp.; Ectocarpus fasciculatus (Phaeophyceae) | 150 nm, 170 nm | [116,117] |
| Aulacomonas submarina (Chlorophyceae) | 200–230 nm | [118] | |
| 1974 | Pylaiella littoralis (Phaeophyceae) | 130–170 nm | [119] |
| Pyramimonas orientalis (Prasinophyceae) | 200 nm | [120] | |
| 1975 † | Chara corallina (Charophyceae) | 18 nm × 532 nm | [121] |
| 1978 | Sorocarpus uvaeformis (Phaeophyceae) | 170 nm | [122] |
| 1979 | Gymnodinium uberrimum (Dinophyceae) | 385 nm | [123] |
| Mallomonas sp. (Synurophyceae) | 175 nm | [123] | |
| 1980 | Uronema gigas (Chlorophyceae) | 390 nm | [124] |
| 1984 | Paraphysomonas corynephora (Chrysophyceae) | 150–180 nm, 270–300 nm | [125] |
| 1993 | Various Phaeodarian food vacuoles | 300–750 nm | [126] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelm, S.W.; Bird, J.T.; Bonifer, K.S.; Calfee, B.C.; Chen, T.; Coy, S.R.; Gainer, P.J.; Gann, E.R.; Heatherly, H.T.; Lee, J.; et al. A Student’s Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae. Viruses 2017, 9, 46. https://doi.org/10.3390/v9030046
Wilhelm SW, Bird JT, Bonifer KS, Calfee BC, Chen T, Coy SR, Gainer PJ, Gann ER, Heatherly HT, Lee J, et al. A Student’s Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae. Viruses. 2017; 9(3):46. https://doi.org/10.3390/v9030046
Chicago/Turabian StyleWilhelm, Steven W., Jordan T. Bird, Kyle S. Bonifer, Benjamin C. Calfee, Tian Chen, Samantha R. Coy, P. Jackson Gainer, Eric R. Gann, Huston T. Heatherly, Jasper Lee, and et al. 2017. "A Student’s Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae" Viruses 9, no. 3: 46. https://doi.org/10.3390/v9030046






