Simultaneous Detection of Both RNA and DNA Viruses Infecting Dry Bean and Occurrence of Mixed Infections by BGYMV, BCMV and BCMNV in the Central-West Region of Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. BCMV, BCMNV and BGYMV Virus Strains
2.2. Simultaneous Extraction of Both Total RNA and Total DNA
2.3. Reverse Transcription
2.4. Design of New Primers for BGYMV Detection
2.5. Specificity of the Primers Designed for BGYMV Detection
2.6. Optimization of Uniplex, Duplex and Multiplex PCR for BCMV, BCMNV and BGYMV Detection
2.7. Composite Sampling
2.8. Multiplex Detection of BCMV, BCMNV and BGYMV in Samples Collected in Crop Fields
3. Results
3.1. Specificity of the Primers Designed for BGYMV Detection
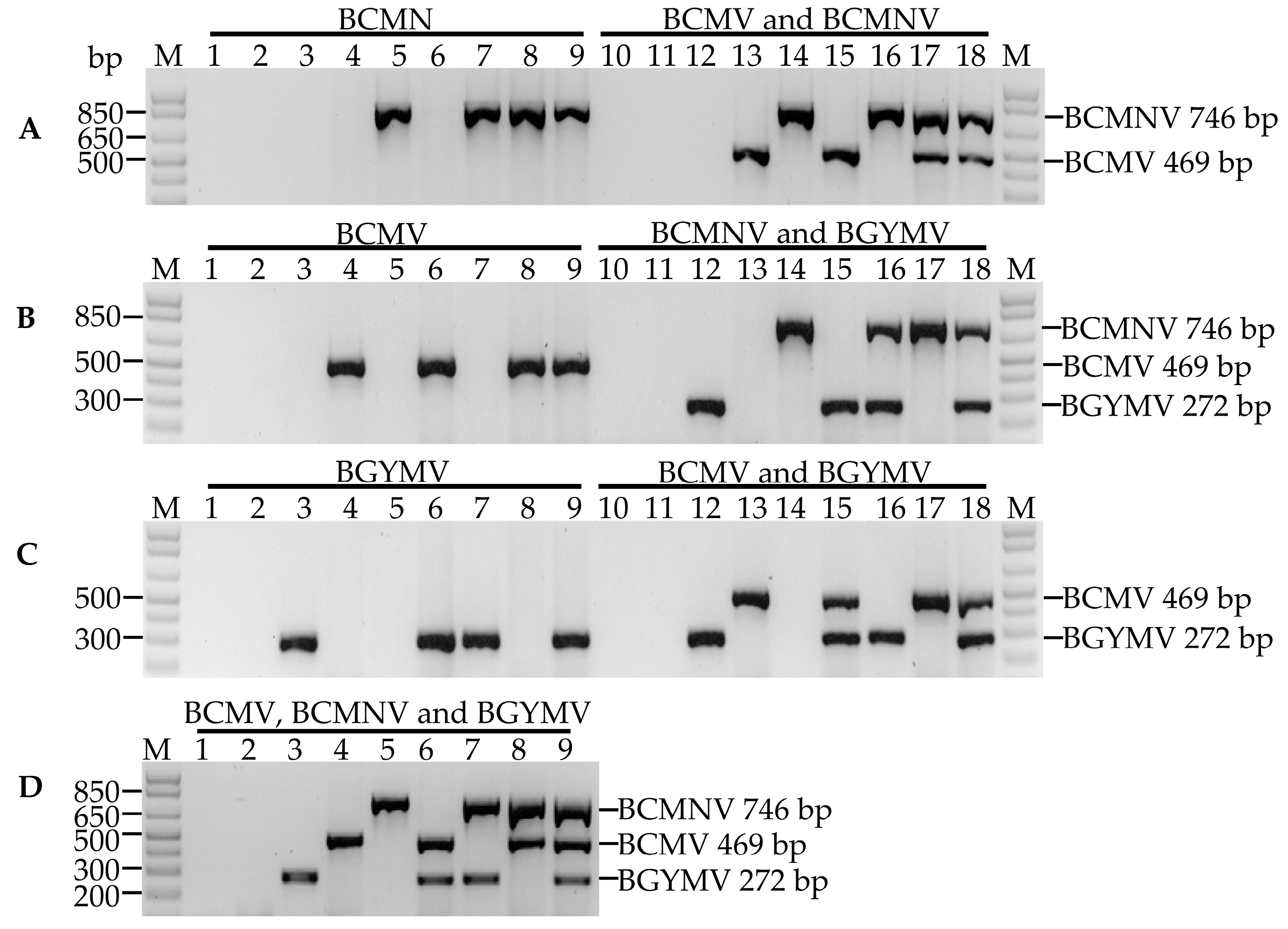
3.2. Optimization of Uniplex, Duplex and Multiplex PCR for Detection of BCMV, BCMNV and BGYMV
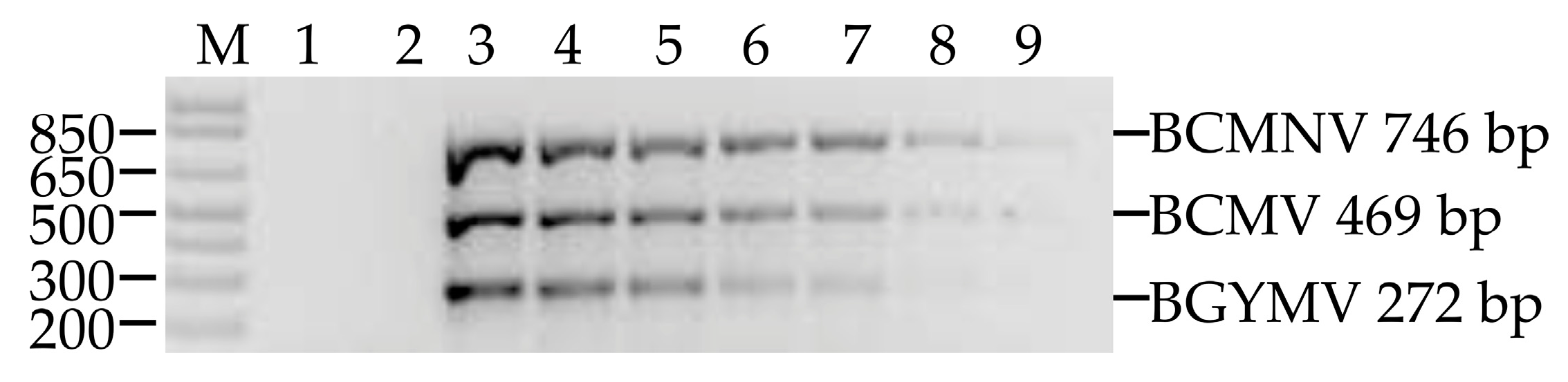
3.3. Composite Sampling
3.4. Multiplex Detection of BCMV, BCMNV and BGYMV in Samples Collected In Crop Fields
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loebenstein, G.; Thottappilly, G. Virus and Virus-like Diseases of Major Crops in Developing Countries, 1st ed.; Springer: Dordrecht, The Netherlands, 2003; Volume 1, pp. 1–440. [Google Scholar]
- Sastry, K.S.; Zitter, T.A. Plant Virus and Viroid Diseases in the Tropics: Epidemiology and Management, 1st ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 1–111. [Google Scholar]
- Morales, J.F. Common beans. In Natural Resistance Mechanisms of Plants to Viruses, 1st ed.; Loebenstein, G., Carr, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 367–382. [Google Scholar]
- FAOSTAT (Food and Agriculture Organization of the United Nations). Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 28 November 2016).
- Boland, G.J.; Melzer, M.S.; Hopkin, A.; Higgins, V.; Nassuth, A. Climate change and plant diseases in Ontario. Can. J. Plant Pathol. 2004, 26, 335–350. [Google Scholar] [CrossRef]
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, E.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic improvement of common beans and the challenges of climate change. In Crop Adaptation to Climate Change, 1st ed.; Yadav, S.S., Redden, R., Hatfield, J.L., Lotze-Campen, H., Hall, A.J.W., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 356–369. [Google Scholar]
- Morales, F.J.; Castaño, M. Seed transmission characteristics of selected bean common mosaic virus strains in differential bean cultivars. J. Plant Dis. Prot. 1987, 71, 51–53. [Google Scholar] [CrossRef]
- Morales, F.J.; Castaño, M. Enfermedades Virales Del Frijol Común en América Latina, 1st ed.; CIAT: Cali, Colombia, 2008; pp. 9–17. [Google Scholar]
- Sastry, K.S. Seed-Borne Plant Virus Diseases, 1st ed.; Springer: New Delhi, India, 2013; pp. 1–145. [Google Scholar]
- Morales, F.J.; Bos, L. Bean Common Mosaic Virus. Available online: http://www.dpvweb.net/dpv/showdpv.php?dpvno=337 (accessed on 28 November 2016).
- Sastry, K.S. Plant Virus and Viroid Diseases in the Tropics: Introduction of Plant Viruses and Sub-Viral Agents, Classification, Assessment of Loss, Transmission and Diagnosis; Springer: Dordrecht, The Netherlands, 2013; Volume 1, pp. 109–189. [Google Scholar]
- Morales, F.J.; Anderson, P.K. The emergence and dissemination of whitefly-transmitted geminiviruses in Latin America. Arch. Virol. 2001, 146, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Chiquito-Almanza, E.; Acosta-Gallegos, J.A.; García-Álvarez, N.C.; Cuellar, W.; Martínez-Martínez, T.O.; Anaya-López, J.L. Detection of virus damaging the dry bean crop in Nayarit, Mexico. J. Chem. Biol. Phys. Sci. 2014, 4, 48–55. [Google Scholar]
- Morales, F.J. Common bean. In Virus and Virus-Like Diseases of Major Crops in Developing Countries, 1st ed.; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 1, pp. 425–445. [Google Scholar]
- Londoño, M.A.; Harmon, C.L.; Polston, J.E. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol. J. 2016, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.I.; Siju, S. Development of a single-tube multiplex RT-PCR for the simultaneous detection of Cucumber mosaic virus and Piper yellow mottle virus associated with stunt disease of black pepper. Curr. Sci. 2007, 93, 973–976. [Google Scholar]
- Chang, L.; Zhang, Z.; Yang, H.; Li, H.; Dai, H. Detection of strawberry RNA and DNA viruses by RT-PCR using total nucleic acid as a template. J. Phytopathol. 2007, 155, 431–436. [Google Scholar] [CrossRef]
- Abarshi, M.M.; Mohammed, I.U.; Jeremiah, S.C.; Legg, J.P.; Kumar, P.L.; Hillocks, R.J.; Maruthi, M.N. Multiplex RT-PCR assays for the simultaneous detection of both RNA and DNA viruses infecting cassava and the common occurrence of mixed infections by two cassava brown streak viruses in East Africa. J. Virol. Methods 2012, 179, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramírez, E.R.; Sudarshana, M.R.; Gilbertson, R.L. Bean golden yellow mosaic virus from Chiapas, Mexico: Characterization, pseudorecombination with other bean-infecting geminiviruses and germ plasm screening. J. Phytopathol. 2000, 90, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Abarshi, M.M.; Mohammed, I.U.; Wasswa, P.; Hillocks, R.J.; Holt, J.; Legg, J.P.; Seal, S.E.; Maruthi, M.N. Optimization of diagnostic RT-PCR protocols and sampling procedures for the reliable and cost-effective detection of Cassava brown streak virus. J. Virol. Methods 2010, 163, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.L.; Nakhla, M.K.; Mejía, L.; Maxwell, D.P. PCR and DNA hybridization methods for specific detection of bean-infecting begomoviruses in the Americas and Caribbean. Plant Dis. 2003, 87, 1205–1212. [Google Scholar] [CrossRef]
- Owczarzy, R.; Tataurov, A.V.; Wu, Y.; Manthey, J.A.; McQuisten, K.A.; Almabrazi, H.G.; Pedersen, K.F.; Lin, Y.; Garretson, J.; McEntaggart, N.O.; et al. IDT SciTools: A suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 2008, 36, W163–W169. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Rojas, M.R.; Gilbertson, R.L.; Maxwell, D.P. Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Dis. 1993, 77, 340–347. [Google Scholar] [CrossRef]
- Torres-Pacheco, I.; Garzón-Tiznado, J.A.; Brown, J.K.; Becerra-Flora, A.; Rivera-Bustamante, R.F. Detection and distribution of geminiviruses in Mexico and Southern United States. J. Phytopathol. 1996, 86, 1186–1192. [Google Scholar] [CrossRef]
- Bonilla-Ramírez, G.M.; Guevara-González, R.G.; Garzon-Tiznado, J.A.; Ascencio-Ibañez, J.T.; Torres-Pacheco, I.; Rivera-Bustamante, R.F. Analysis of the infectivity of monomeric clones of Pepper huasteco virus. J. Gen. Virol. 1997, 78, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Nieto, J.; Aguirre-Mancilla, C.; Acosta-Gallegos, J.; Raya-Pérez, J.; Piedra-Ibarra, E.; Vázquez-Medrano, J.; Montero-Tavera, V. Photosynthesis and chloroplast genes are involved in water-use efficiency in common bean. Plant Physiol. Biochem. 2015, 86, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gemeinholzer, B.; Rey, I.; Weising, K.; Grundmann, M.; Muellner, A.N.; Zetzsche, H.; Droege, G.; Seberg, O.; Petersen, G.; Rawson, D.; et al. Organizing specimen and tissue preservation in the field for subsequent molecular analyses. In Manual of Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories, 1st ed.; Eymann, J., Degreef, J., Häuser, C., Monje, J.C., Samyn, Y., VandenSpiegel, D., Eds.; The Belgian Development Cooperation: Brussels, Belgium, 2010; Volume 8, pp. 129–157. [Google Scholar]
- Guo, X.; Wang, X.; Su, W.; Zhang, G.; Zhou, R. DNA barcodes for discriminating the medicinal plant Scutellaria baicalensis (Lamiaceae) and its adulterants. Biol. Pharm. Bull. 2011, 34, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.W.; Webb, C.O. Plant DNA barcodes, taxonomic management, and species discovery in tropical forests. Methods Mol. Biol. 2012, 858, 379–393. [Google Scholar] [PubMed]
- Semagn, K. Leaf tissue sampling and DNA extraction protocols. Methods Mol. Biol. 2014, 1115, 53–67. [Google Scholar] [PubMed]
- Narzary, D.; Verma, S.; Mahar, K.S.; Rana, T.S. A rapid and effective method for isolation of genomic DNA from small amount of silica-dried leaf tissues. Proc. Natl. Acad. Sci. Lett. 2015, 38, 441–444. [Google Scholar] [CrossRef]
- Saponari, M.; Manjunath, K.; Yokomi, R.K. Quantitative detection of Citrus tristeza virus in citrus and aphids by real-time reverse transcription-PCR (TaqMan®). J. Virol. Methods 2008, 147, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bernad, L.; Duran-Vila, N. A novel RT-PCR approach for detection and characterization of viroids. Mol. Cell. Probes 2006, 20, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Zerbini, F.M.; French, R.; Rabenstein, F.; Stenger, D.C.; Valkonen, J.P.T. Family Potyviridae. In Virus Taxonomy, 1st ed.; Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2012; pp. 1069–1089. [Google Scholar]
- Brown, J.K.; Fauquet, C.M.; Briddon, R.W.; Zerbini, M.; Moriones, E.; Navas-Castillo, J. Family Geminiviridae. In Virus Taxonomy, 1st ed.; Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2012; pp. 351–373. [Google Scholar]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
- García-Arenal, F.; Fraile, A.; Malpica, J.M. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 2001, 39, 157–186. [Google Scholar] [CrossRef] [PubMed]
- De Wispelaere, M.; Gaubert, S.; Trouilloud, S.; Belin, C.; Tepfer, M. A map of the diversity of RNA3 recombinants appearing in plants infected with Cucumber mosaic virus and Tomato aspermy virus. Virology 2005, 331, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Froissart, R.; Wilke, C.O.; Montville, R.; Remold, S.K.; Chao, L.; Turner, P.E. Co-infection weakens selection against epistatic mutations in RNA viruses. Genetics 2004, 168, 9–19. [Google Scholar] [CrossRef] [PubMed]
- García-Arenal, F.; McDonald, B.A. An analysis of the durability of resistance to plant viruses. J. Phytopathol. 2003, 93, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Monci, F.; Sánchez-Campos, S.; Navas-Castillo, J.; Moriones, E. A natural recombinant between the geminiviruses Tomato yellow leaf curl sardinia virus and Tomato yellow leaf curl virus exhibits a novel pathogenic phenotype and is becoming prevalent in Spanish populations. Virology 2002, 303, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.G.; Ambrozevicius, L.P.; Avila, A.C.; Bezerra, I.C.; Calegario, R.F.; Fernández, J.J.; Lima, M.F.; De Mello, R.N.; Rocha, H.; Zerbini, F.M. Distribution and genetic diversity of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2003, 148, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Kvarnheden, A.; Marcenaro, D.; Valkonen, J.P.T. Sequence characterization of Tomato leaf curl Sinaloa virus and Tomato severe leaf curl virus: Phylogeny of New World begomoviruses and detection of recombination. Arch. Virol. 2005, 150, 1281–1299. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, M.J.; Mink, G.I.; Zhao, R.L.; Zheng, G.Y. Phenotypic recombination between bean common mosaic and bean common mosaic necrosis potyviruses in vivo. Arch. Virol. 2001, 146, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.C.; Miklas, P.N.; Druffel, K.L.; Wyatt, S.D. NL-3 K strain is a stable and naturally occurring interspecific recombinant derived from Bean common mosaic necrosis virus and Bean common mosaic virus. J. Phytopathol. 2005, 95, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Poplawsky, A.R.; Nikolaeva, O.V.; Myers, J.R.; Karasev, A.V. Recombinants of Bean common mosaic virus (BCMV) and genetic determinants of BCMV involved in overcoming resistance in common bean. J. Phytopathol. 2014, 104, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Poplawsky, A.R.; Karasev, A. A recombinant of Bean common mosaic virus induces temperature insensitive necrosis in an I gene bearing line of common bean. J. Phytopathol. 2014, 104, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Myers, J.R.; Karasev, A.V. Bean common mosaic virus isolate exhibits a novel pathogenicity profile in common bean, overcoming the bc-3 resistance allele coding for the mutated eIF4E translation initiation factor. J. Phytopathol. 2015, 105, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, P. Microbial Plants Pathogens-Detection and Disease Diagnosis, 1st ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 2–12. [Google Scholar]
- Schwartz, H.F.; Brick, M.A. Dry Bean Pest Management and Production, 3rd ed.; Colorado State University: Fort Collins, CO, USA, 2015; pp. 75–94. [Google Scholar]
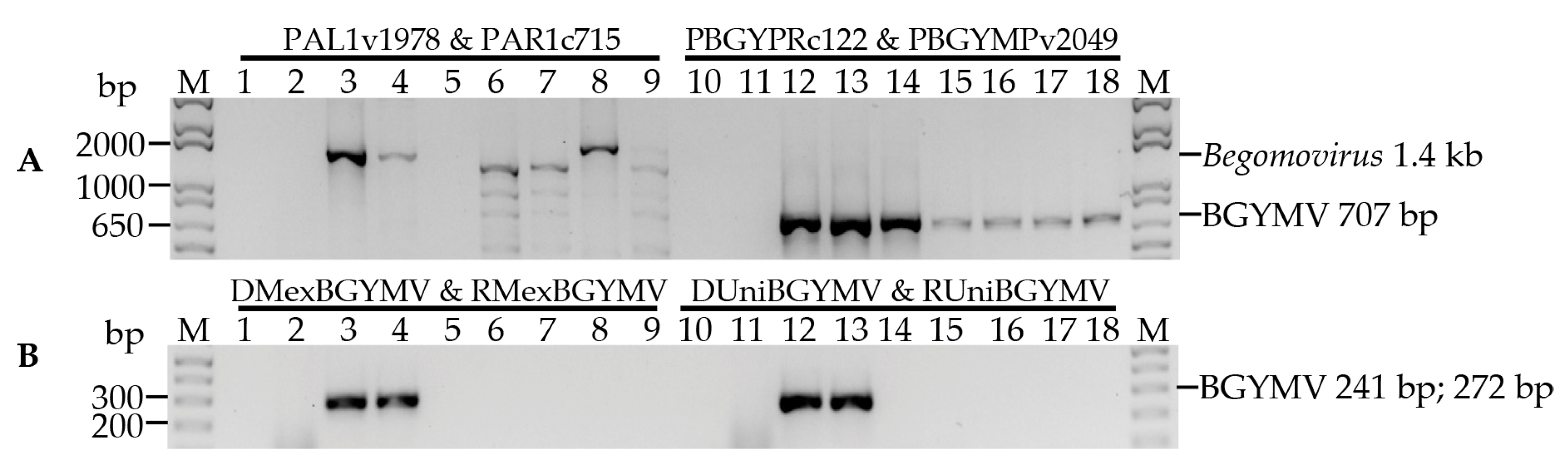
| Target | Primer | Location | ||
|---|---|---|---|---|
| Virus | Gene | Name | Sequence (5′–3′) | (nt) |
| Begomovirus | AC1 | PAL1v1978 a | GCATCTGCAGGCCCACATYGTCTTYCCNGT | 1956–1975 e |
| AV1 | PAR1c715 a | GATTTCTGCAGTTDATRTTYTCRTCCATCCA | 712–731 e | |
| BGYMV | CR | PBGYPRc122 b | CGTGAGTGAATCTGATAATTCAMGAG | 121–146 f |
| BC1 | PBGYMPv2049 b | CTGCGACTGAATCTYGCAGATARTT | 2049–2073 f | |
| AC1 | DUnivBGYMV c | GAATGATGACAACGGAAATGGAGG | 2084–2107 e | |
| AC1 | RUnivBGYMV c | CACAATCGAATGGGGACAATTCC | 2302–2324 e | |
| AC1 | DMexBGYMV c | GATGAATGATGACAACGGAAATGG | 2081–2104 e | |
| AC1 | RMexBGYMV c | TCAAGGCATACATCGACAAAGGTG | 2329–2352 e | |
| BCMV | CP | D1BCMV d | AAATGTGGTACAATGCTGTGAAGG | 9267–9290 g |
| CP | RU d | TCAGTATTCTCGCTGGTTGTTGC | 9713–9735 g | |
| BCMNV | CP | D1BCMNV d | GAGGTGTATGAATCCGTGTCAACA | 8558–8581 h |
| CP | RU d | TCAGTATTCTCGCTGGTTGTTGC | 9281–9303 h | |
| State | No. of Samples | Virus Detected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BGYMV | BCMV | BCMNV | BCMV/BCMNV | BCMV/BGYMV | BCMNV/BGYMV | BCMV/BCMNV/BGYMV | None Detected | ||
| Guanajuato | 76 | 4 (5.3%) | 16 (21.1%) | 7 (9.2%) | – | 3 (3.9%) | – | 2 (2.6%) | 44 (57.9%) |
| Jalisco | 38 | 2 (5.3%) | 15 (39.5%) | 4 (10.5%) | 8 (21.1%) | 1 (2.6%) | – | – | 8 (21.1%) |
| Nayarit | 73 | 8 (11.0%) | 10 (13.7%) | 24 (32.9%) | 5 (6.8%) | 8 (11.0%) | 4 (5.5%) | 8 (11.0%) | 6 (8.2%) |
| Total | 187 | 14 (7.5%) | 41 (21.9%) | 35 (18.7%) | 13 (7.0%) | 12 (6.4%) | 4 (2.1%) | 10 (5.3%) | 58 (31.0%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiquito-Almanza, E.; Acosta-Gallegos, J.A.; García-Álvarez, N.C.; Garrido-Ramírez, E.R.; Montero-Tavera, V.; Guevara-Olvera, L.; Anaya-López, J.L. Simultaneous Detection of Both RNA and DNA Viruses Infecting Dry Bean and Occurrence of Mixed Infections by BGYMV, BCMV and BCMNV in the Central-West Region of Mexico. Viruses 2017, 9, 63. https://doi.org/10.3390/v9040063
Chiquito-Almanza E, Acosta-Gallegos JA, García-Álvarez NC, Garrido-Ramírez ER, Montero-Tavera V, Guevara-Olvera L, Anaya-López JL. Simultaneous Detection of Both RNA and DNA Viruses Infecting Dry Bean and Occurrence of Mixed Infections by BGYMV, BCMV and BCMNV in the Central-West Region of Mexico. Viruses. 2017; 9(4):63. https://doi.org/10.3390/v9040063
Chicago/Turabian StyleChiquito-Almanza, Elizabeth, Jorge A. Acosta-Gallegos, Nadia C. García-Álvarez, Eduardo R. Garrido-Ramírez, Victor Montero-Tavera, Lorenzo Guevara-Olvera, and José L. Anaya-López. 2017. "Simultaneous Detection of Both RNA and DNA Viruses Infecting Dry Bean and Occurrence of Mixed Infections by BGYMV, BCMV and BCMNV in the Central-West Region of Mexico" Viruses 9, no. 4: 63. https://doi.org/10.3390/v9040063





