ATP-Driven Contraction of Phage T3 Capsids with DNA Incompletely Packaged In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propagation of Phages and Preparation of Phage-Infected Cell Lysates
2.2. Fractionation of ipDNA-Capsids
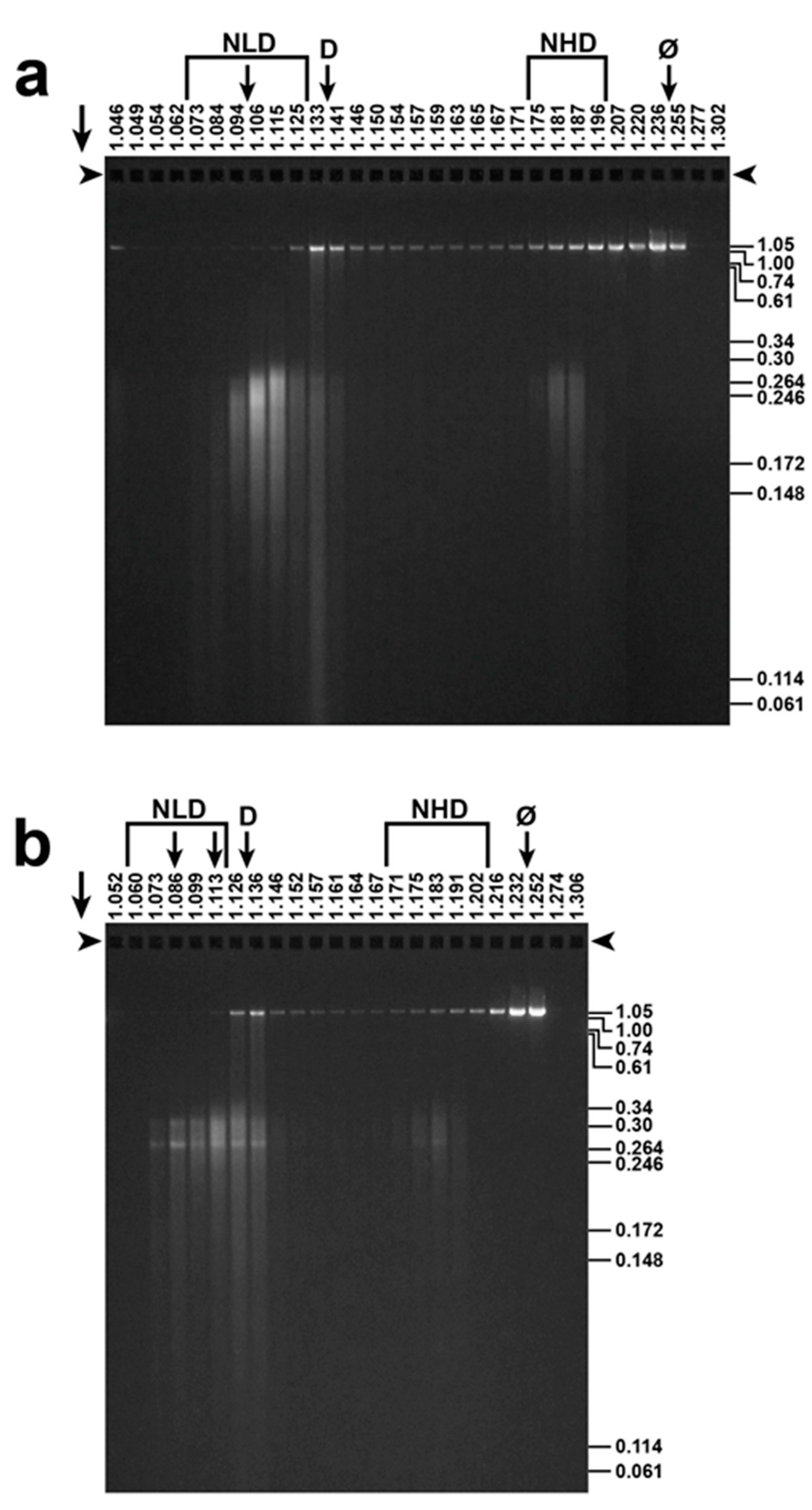
2.3. Gel Electrophoresis of DNA: Detection of ipDNA-Capsids
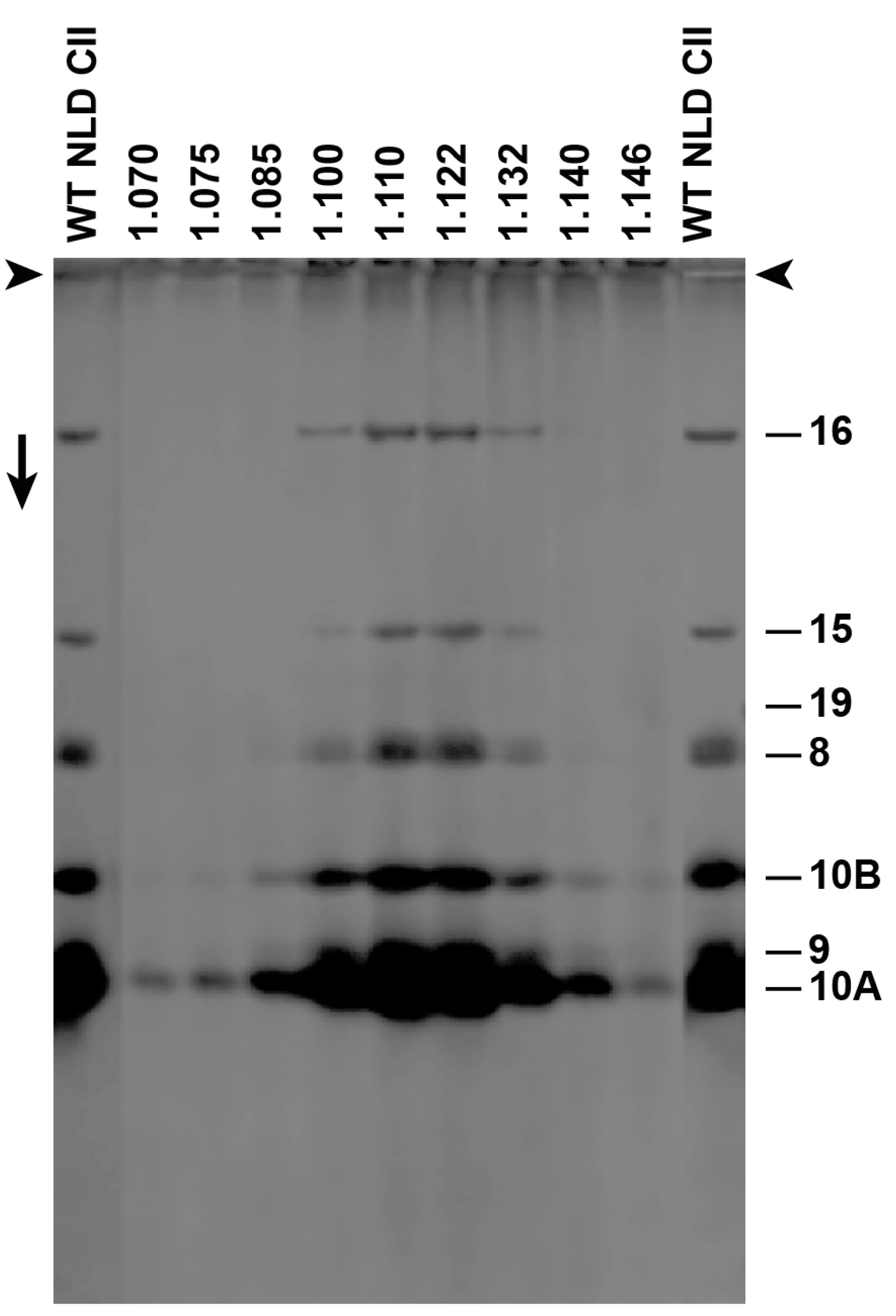
2.4. SDSPAGE
2.5. Electron Microscopy: Incubation with ATP and ADP
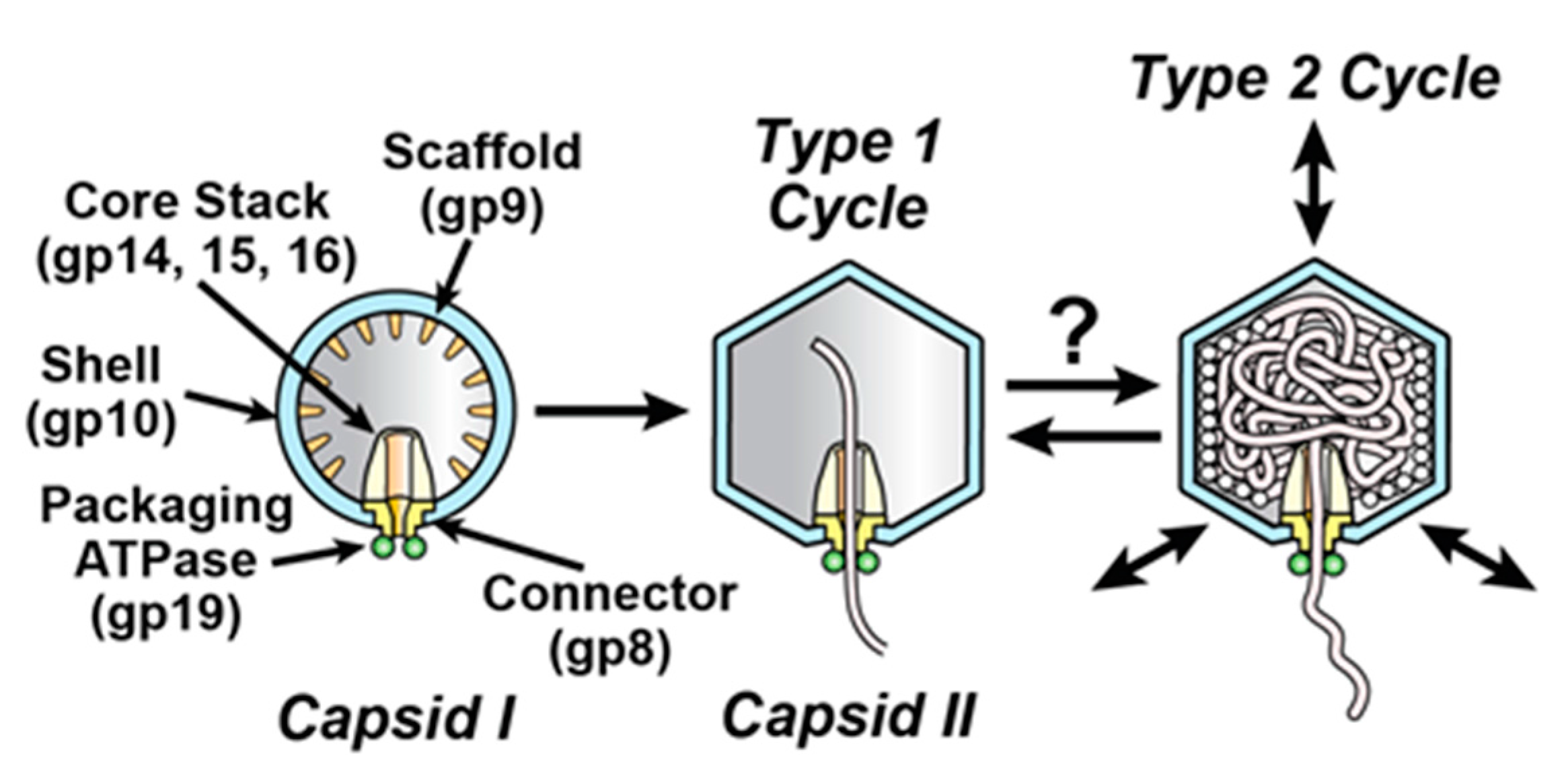
3. Results
3.1. Fractionation of ipDNA-Capsids
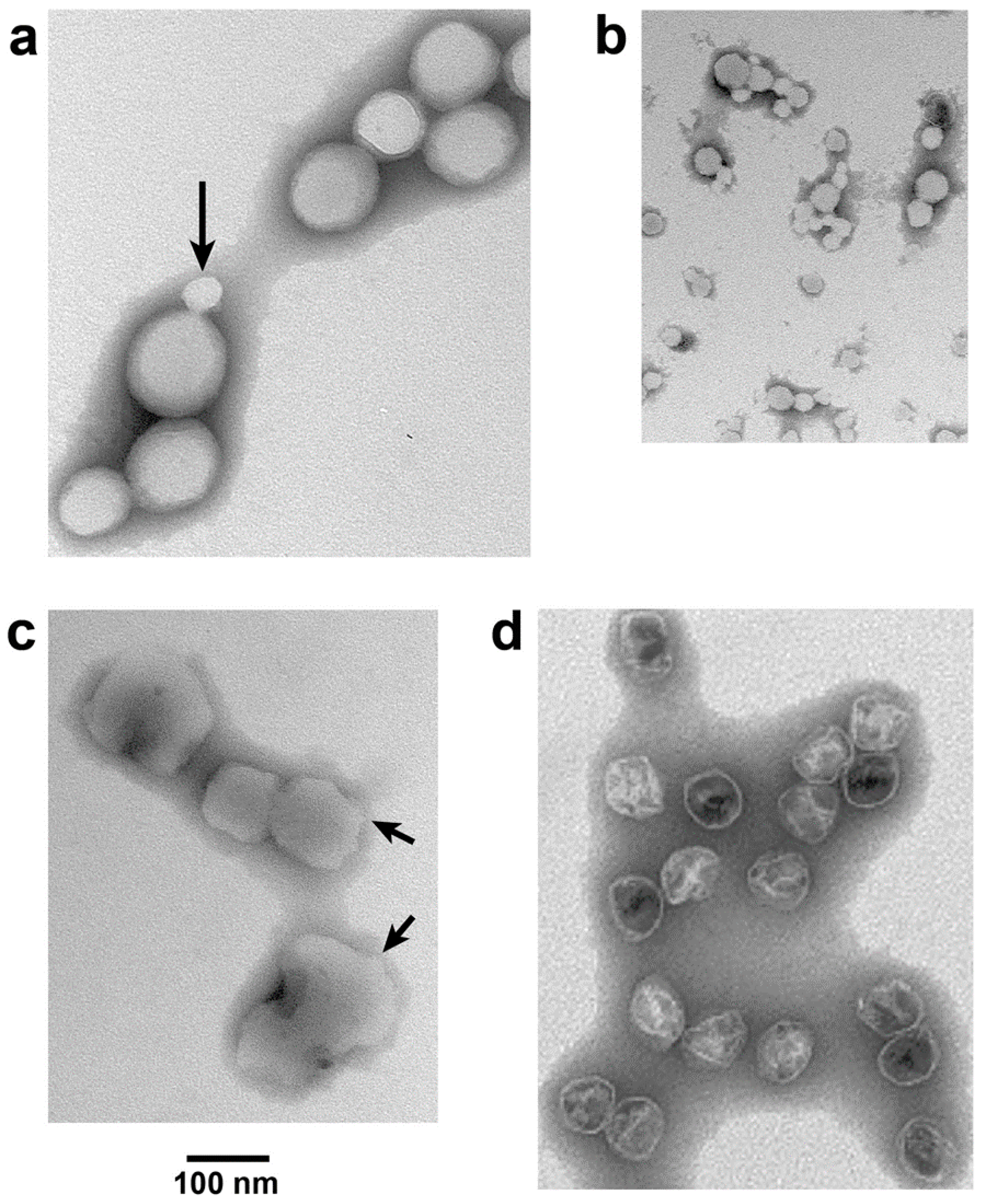
3.2. Electron Microscopy (EM)
3.3. Effects of ATP on NLD ipDNA-Capsid II
3.4. Reversal of the ATP-Induced Contraction
4. Discussion
4.1. Electron Microscopy (EM)
4.2. DNA Packaging
4.3. Strategy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koonin, E.V.; Krupovic, M.; Yutin, N. Evolution of double-stranded DNA viruses of eukaryotes: From bacteriophages to transposons to giant viruses. Ann. N. Y. Acad. Sci. 2015, 1341, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. Proposed ancestors of phage nucleic acid packaging motors (and cells). Viruses 2011, 3, 1249–1280. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P.; Wright, E.T.; Chang, J.T.; Liu, X. Enhancing and initiating phage-based therapies. Bacteriophage 2014, 4, e961869. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chistol, G.; Bustamante, C. Mechanical operation and intersubunit coordination of ring-shaped molecular motors: Insights from single-molecule studies. Biophys. J. 2014, 25, 1844–1858. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ha, T. Single molecule nanometry for biological physics. Rep. Prog. Phys. 2013, 76, 016601. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Morita, M. Phage DNA packaging. Genes Cells 1997, 2, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R. The DNA-packaging nanomotor of tailed bacteriophages. Nat. Rev. Microbiol. 2011, 9, 647–657. [Google Scholar] [PubMed]
- Rao, V.B.; Feiss, M. Mechanisms of DNA packaging by large double-stranded DNA viruses. Annu. Rev. Virol. 2015, 2, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Black, L.W. Old, new, and widely true: The bacteriophage T4 DNA packaging mechanism. Virology 2015, 479–480, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Fujisawa, H.; Minagawa, T. Characterization of ATPase activity of a defined in vitro system for packaging of bacteriophage T3 DNA. Virology 1987, 159, 244–249. [Google Scholar] [CrossRef]
- Rao, V.B.; Black, L.W. Evidence that a T4 DNA packaging enzyme is a processed form of the major capsid gene product. Cell 1985, 42, 967–977. [Google Scholar] [CrossRef]
- Liu, S.; Chistol, G.; Hetherington, C.L.; Tafoya, S.; Aathavan, K.; Schnitzbauer, J.; Grimes, S.; Jardine, P.; Bustamante, C. A viral packaging motor varies its DNA rotation and step size to preserve subunit coordination as the capsid fills. Cell 2014, 157, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Saha, M.; Zhao, W.; Jardine, P.J.; Zhang, W.; Grimes, S.; Morais, M.C. Insights into the structure and assembly of the bacteriophage 29 double-stranded DNA packaging motor. J. Virol. 2014, 88, 3986–3996. [Google Scholar] [CrossRef]
- Serwer, P.; Wright, E. Testing a proposed paradigm shift in analysis of phage DNA packaging. Bacteriophage 2016, 6, e1268664. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P.; Wright, E.T.; Liu, Z.; Jiang, W. Length quantization of DNA partially expelled from heads of a bacteriophage T3 mutant. Virology 2014, 456–457, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P.; Wright, E.T.; Hakala, K.; Weintraub, S.T.; Su, M.; Jiang, W. DNA packaging-associated hyper-capsid expansion of bacteriophage T3. J. Mol. Biol. 2010, 397, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Rickwood, D.; Ford, T.; Graham, J. Nycodenz: A new nonionic iodinated gradient medium. Anal. Biochem. 1982, 123, 23–31. [Google Scholar] [CrossRef]
- Serwer, P. Internal proteins of bacteriophage T7. J. Mol. Biol. 1976, 107, 271–291. [Google Scholar] [CrossRef]
- Pajunen, M.I.; Elizondo, M.R.; Skurnik, M.; Kieleczawa, J.; Molineux, I.J. Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J. Mol. Biol. 2002, 319, 1115–1132. [Google Scholar] [CrossRef]
- Serwer, P.; Wright, E.T.; Hakala, K.W.; Weintraub, S.T. Evidence for phage T7 tail extension during DNA injection. BMC Res. Notes 2008, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J. Mol. Biol. 1980, 138, 65–91. [Google Scholar] [CrossRef]
- Serwer, P.; Watson, R.H.; Hayes, S.J.; Allen, J.L. Comparison of the physical properties and assembly pathways of the related bacteriophages T7, T3 and phi II. J. Mol. Biol. 1983, 170, 447–469. [Google Scholar] [CrossRef]
- Hearst, J.E.; Vinograd, J. The net hydration of T-4 bacteriophage deoxyribonucleic acid and the effect of hydration on buoyant behavior in a density gradient at equilibrium in the ultracentrifuge. Proc. Natl. Acad. Sci. USA 1961, 47, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J. Mol. Biol. 1975, 92, 433–448. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Z.; Fang, P.A.; Zhang, Q.; Wright, E.T.; Wu, W.; Zhang, C.; Vago, F.; Ren, Y.; Jakana, J.; et al. Capsid expansion mechanism of bacteriophage T7 revealed by multistate atomic models derived from cryo-EM reconstructions. Proc. Natl. Acad. Sci. USA 2014, 111, E4606–E4614. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. Flattening and shrinkage of bacteriophage T7 after preparation for electron microscopy by negative staining. J. Ultrastruct. Res. 1977, 58, 235–243. [Google Scholar] [CrossRef]
- Yaginuma, H.; Kawai, S.; Tabata, K.V.; Tomiyama, K.; Kakizuka, A.; Komatsuzaki, T.; Noji, H.; Imamura, H. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep. 2014, 4, 6522. [Google Scholar] [CrossRef] [PubMed]
- Mbah, A.N. Application of hybrid functional groups to predict ATP binding proteins. ISRN Comput. Biol. 2014, 2014, 581245. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwer, P.; Wright, E.T. ATP-Driven Contraction of Phage T3 Capsids with DNA Incompletely Packaged In Vivo. Viruses 2017, 9, 119. https://doi.org/10.3390/v9050119
Serwer P, Wright ET. ATP-Driven Contraction of Phage T3 Capsids with DNA Incompletely Packaged In Vivo. Viruses. 2017; 9(5):119. https://doi.org/10.3390/v9050119
Chicago/Turabian StyleSerwer, Philip, and Elena T. Wright. 2017. "ATP-Driven Contraction of Phage T3 Capsids with DNA Incompletely Packaged In Vivo" Viruses 9, no. 5: 119. https://doi.org/10.3390/v9050119






