Historical Perspectives on Flavivirus Research
Abstract
:1. Introduction
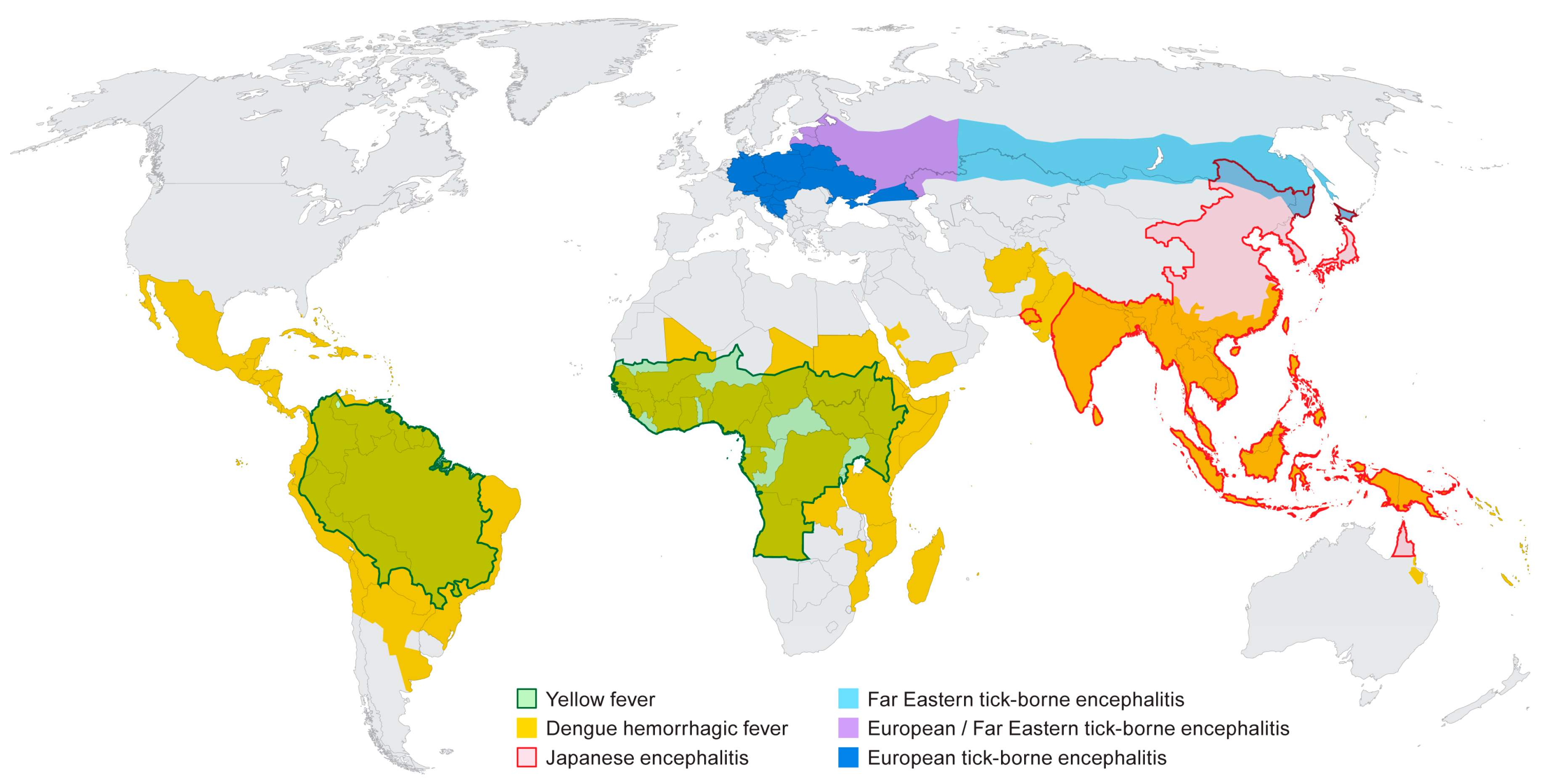
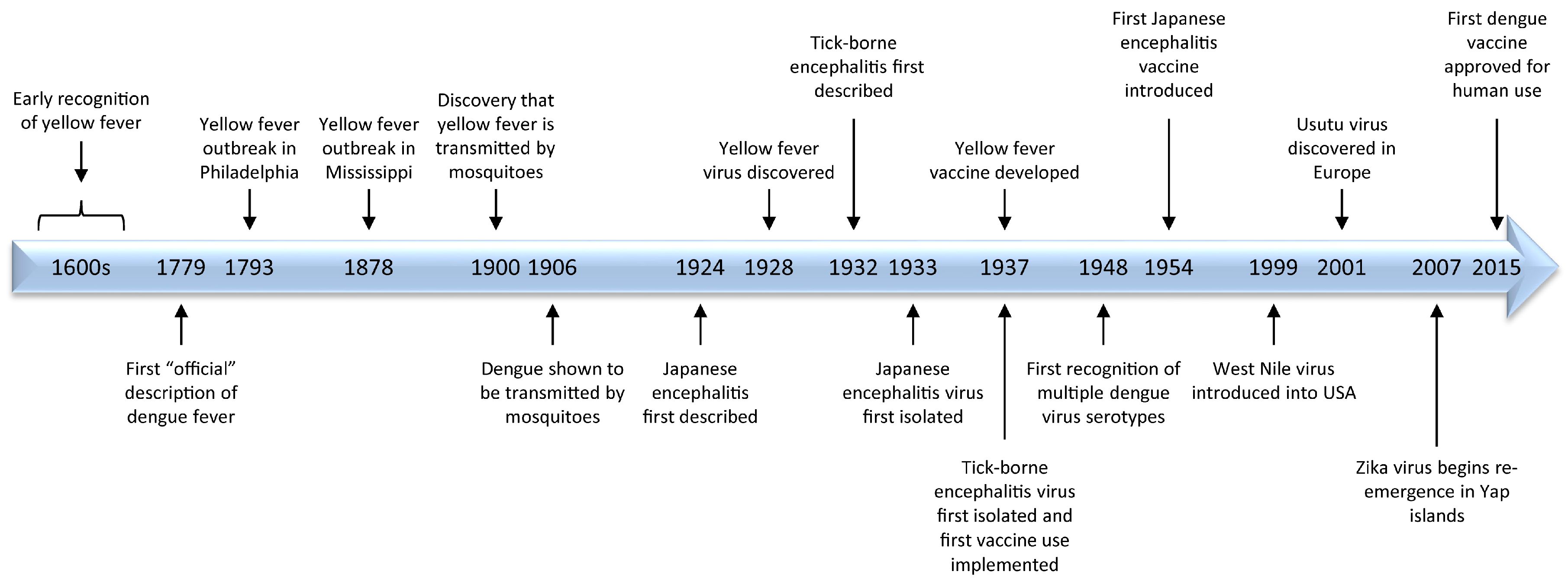
2. Origins of Flavivirus Research
2.1. Yellow Fever
2.2. Dengue
2.3. Japanese Encephalitis
2.4. Tick-Borne Encephalitis
3. Emergence/Re-Emergence
4. Vaccines
5. Gaps in Flavivirus Research
5.1. Identification of the Cognate Receptors for Flaviviruses
5.2. An Understanding of the Role of T Cell in Mediated Immunity in Flavivirus Infection
5.3. Animal Models that Faithfully Recapitulate Human Disease
5.4. The Role of Sexual Transmission in Flavivirus Infection
5.5. Mother to Child Transmission
5.6. No Therapeutic Options
6. Summary
Conflicts of Interest
References
- Pettersson, J.H.; Fiz-Palacios, O. Dating the origin of the genus Flavivirus in the light of Beringian biogeography. J. Gen. Virol. 2014, 95, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Westaway, E.G.; Brinton, M.A.; Gaidamovich, S.; Horzinek, M.C.; Igarashi, A.; Kaariainen, L.; Lvov, D.K.; Porterfield, J.S.; Russell, P.K.; Trent, D.W. Flaviviridae . Intervirolgy 1985, 24, 183–192. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.R. Yellow Fever, an Epidemiologicaland Historical Study of Its Place of Origin; William and Wilkins Company: Baltimore, MD, USA, 1931. [Google Scholar]
- Garrison, F.H. An Introduction to the History of Medicine, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1921. [Google Scholar]
- Rush, B. Medical Inquiries and Observations; Thomas Dobson: Philadelphia, PA, USA, 1796; Volume 4. [Google Scholar]
- Bloom, K.J. The Mississippi Valley's Great Yellow Fever Epidemic; Louisiana State University Press: Baton Rouge LA, USA, 1993; p. 296. [Google Scholar]
- Finlay, C.J. The mosquito hypotheitically considered as the transmission agent of yellow fever (In Spanish). Anales de la Real Academia de Ciencias Medicas Fisicas y Naturales de la Habana 1881, 18, 147–169. [Google Scholar]
- Finlay, C.J. The mosquito hypothetically considered as an agent in the transmission of yellow fever poison. New Orleans Med. Surg. J. 1881, 9, 601–616. [Google Scholar]
- Reed, W.; Carroll, J.; Agramonte, A.; Lazear, J.W. The etiology of yellow fever-A preliminary note. Public Health Pap. Rep. 1900, 26, 37–53. [Google Scholar] [PubMed]
- Stokes, A.; Bauer, J.H.; Hudson, N.P. Experimental transmission of yellow fever to laboratory animals. Am. J. Trop. Med. 1928, 8, 103–164. [Google Scholar] [CrossRef]
- Monath, T.P.; Barrett, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [PubMed]
- Quaresma, J.A.; Pagliari, C.; Medeiros, D.B.; Duarte, M.I.; Vasconcelos, P.F. Immunity and immune response, pathology and pathologic changes: Progress and challenges in the immunopathology of yellow fever. Rev. Med. Virol. 2013, 23, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.E.; Freiberg, A.N.; Holbrook, M.R. Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology 2011, 412, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.E.; Freiberg, A.N.; Holbrook, M.R. Coagulation factors, fibrinogen and plasminogen activator inhibitor-1, are differentially regulated by yellow fever virus infection of hepatocytes. Virus Res. 2013, 175, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.E.; Holbrook, M.R. Infection of hepatocytes with 17-D vaccine-strain yellow fever virus induces a strong pro-inflammatory host response. J. Gen. Virol. 2011, 92, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.H.; Reisberg, B.E.; Crosbie, J.; Crozier, D.; Conrad, M.E. The original haemorrhagic fever: Yellow fever. Br. J. Haematol. 1969, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Brinker, K.R.; Chandler, F.W.; Kemp, G.E.; Cropp, C.B. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 1981, 30, 431–443. [Google Scholar] [PubMed]
- Halstead, S.B. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Pepper, O.H.P. A note on David Bylon and dengue. Ann. Med. Hist. 1941, 3, 363–368. [Google Scholar]
- Chandler, A.C.; Rice, L.M. Observations on the etiology of dengue fever. Am. J. Trop. Med. Hyg. 1923, 3, 233–262. [Google Scholar] [CrossRef]
- Siler, J.F. Dengue Fever. In The Georgraphy of Disease; McKinley, E.B., Ed.; George Washington University Press: Washington, DC, USA, 1935; pp. 402–408. [Google Scholar]
- Smart, W.R. On Dengue or Dandy Fever. Br. Med. J. 1877, 1, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, T.L. On the etiology of dengue fever. Australas. Med. Gaz. 1906, 25, 17–18. [Google Scholar]
- Cleland, J.B.; Bradley, B.; Macdonald, W. Further Experiments in the Etiology of Dengue Fever. J. Hyg. 1919, 18, 217–254. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.B.; Bradley, B.; McDonald, W. On the transmission of Australian dengue by the mosquito Stegomyia faciata. Med. J. Aust. 1916, 11, 179–184. [Google Scholar]
- Siler, J.F.; Hall, M.W.; Hitchens, A.P. Dengue. Philipp. J. Sci. 1926, 29, 1–304. [Google Scholar]
- Ashburn, P.M.; Craig, C.F. Experimental investigations regarding the etiology of dengue fever. J. Infect. Dis. 1907, 4, 440–475. [Google Scholar] [CrossRef]
- Simmons, J.S.; St. John, J.H.; Reynolds, F.H.K. Experimental studies of dengue. Philipp. J. Sci. 1931, 44, 1–247. [Google Scholar]
- Sabin, A.B. Dengue. In Viral and Rickettsial Infections of Man, 1st ed.; Rivers, T.M., Ed.; J.B. Lippincott: Philadelphia, PA, USA, 1948; pp. 445–453. [Google Scholar]
- Carey, D.E. Use of a Combined Complement-Fixing Antigen to Detect Arthropod-Borne Viral Infection. Nature 1963, 200, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Casals, J.; Brown, L.V. Hemagglutination with arthropod-borne viruses. J. Exp. Med. 1954, 99, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B.; Young, I. A complement fixation test for dengue. Proc. Soc. Exp. Biol. Med. 1948, 69, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Sweet, B.H.; Sabin, A.B. Properties and antigenic relationships of hemagglutinins associated with the dengue viruses. J. Immunol. 1954, 73, 363–373. [Google Scholar] [PubMed]
- Vezza, A.C.; Rosen, L.; Repik, P.; Dalrymple, J.; Bishop, D.H. Characterization of the viral RNA species of prototype dengue viruses. Am. J. Trop. Med. Hyg. 1980, 29, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Dunham, E.J.; Holmes, E.C. Inferring the timescale of dengue virus evolution under realistic models of DNA substitution. J. Mol. Evol. 2007, 64, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Tolou, H.; Couissinier-Paris, P.; Mercier, V.; Pisano, M.R.; de Lamballerie, X.; de Micco, P.; Durand, J.P. Complete genomic sequence of a dengue type 2 virus from the French West Indies. Biochem. Biophys. Res. Commun. 2000, 277, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, P.M.; Gould, E.A.; Gao, G.F.; Harvey, P.H.; Holmes, E.C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc. Natl. Acad. Sci. USA 1996, 93, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 1990, 174, 479–493. [Google Scholar] [CrossRef]
- WHO, Dengue and severe dengue. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed on 9 February 2017).
- World Health Organization (WHO). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Sabin, A.B. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1952, 1, 30–50. [Google Scholar] [PubMed]
- World Health Organization (WHO). Dengue Haemorrhagic Fever: Diagnosis, Prevention, Treatment and Control; World Health Organization: Geneva, Switzerland, 1997; pp. 1–84. [Google Scholar]
- Jain, A.; Chaturvedi, U.C. Dengue in infants: An overview. FEMS Immunol. Med. Microbiol. 2010, 59, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Horstick, O.; Martinez, E.; Guzman, M.G.; Martin, J.L.; Ranzinger, S.R. WHO dengue case classification 2009 and its usefulness in practice: An expert consensus in the Americas. Pathog. Glob. Health 2015, 109, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Flipse, J.; Wilschut, J.; Smit, J.M. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 2013, 14, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.; Nascimento, E.J.; Cynthia, B.; Cordeiro, M.T.; de Carvalho, O.V.; de Mendonca, L.R.; Azevedo, E.A.; Franca, R.F.; Rafael, D.; Marques, E.T., Jr. Dengue virus (DENV)-specific antibodies enhance Brazilian Zika virus (ZIKV) infection. J. Infect. Dis. 2017, 215, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.M.; Carlin, E.R.; Jenkins, M.M.; Tan, A.L.; Barcellona, C.M.; Nicholson, C.O.; Michael, S.F.; Isern, S. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Jacobson, J. Japanese encephalitis. Adv. Virus Res. 2003, 61, 103–138. [Google Scholar] [PubMed]
- Rosen, L. The natural history of Japanese encephalitis virus. Annu. Rev. Microbiol. 1986, 40, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Kitaoka, M.; Miura, T. On the geographical distribution of Japanese B encephalitis in the Far East Asia. Jpn. Med. J. 1950, 3, 257–264. [Google Scholar] [CrossRef]
- Solomon, T.; Ni, H.; Beasley, D.W.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003, 77, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Fu, S.H.; Chen, W.X.; Wang, H.Y.; Guo, Y.H.; Liu, Q.Y.; Li, Y.X.; Luo, H.M.; Da, W.; Duo Ji, D.Z.; et al. Genotype V Japanese encephalitis virus is emerging. PLoS Negl. Trop. Dis. 2011, 5, e1231. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Galbraith, S.E.; Radford, A.D.; Dove, W.; Takasaki, T.; Kurane, I.; Solomon, T. Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype. Infect. Genet. Evol. 2011, 11, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Takhampunya, R.; Kim, H.C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.J.; Grieco, J.; Evans, B.P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol. J. 2011, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Japanese Encephalitis Vaccines: WHO position paper, February 2015—Recommendations. Vaccine 2016, 34, 302–303. [Google Scholar]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Turtle, L.; Solomon, T. Japanese encephalitis virus infection. Handb. Clin. Neurol. 2014, 123, 561–576. [Google Scholar] [PubMed]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccine Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Zilber, L.A.; Soloviev, V.D. Far eastern tick-borne spring-summer (spring) encephalitis. Am. Rev. Sov. Med. 1946, 3, 1–75. [Google Scholar]
- Smorodintsev, A.A. Tick-borne spring-summer encephalitis. Prog. Med. Virol. 1958, 1, 210–248. [Google Scholar] [CrossRef] [PubMed]
- Smorodintsev, A.A.; Kagan, N.W.; Levkovitsch, E.N.; Dankovskij, N.L. Experimenteller und epidemiologischer Beitrag zur activen Immunisierung gegen die Fruhling-Sommer-zecken-encephalitis. Arch. Ges. Virusforsch. 1941, 2, 1–25. [Google Scholar] [CrossRef]
- Calisher, C.H. Antigenic classification and taxonomy of flaviviruses (family Flaviviridae) emphasizing a universal system for the taxonomy of viruses causing tick-borne encephalitis. Acta Virol. 1988, 32, 469–478. [Google Scholar] [PubMed]
- Zanotto, P.M.; Gao, G.F.; Gritsun, T.; Marin, M.S.; Jiang, W.R.; Venugopal, K.; Reid, H.W.; Gould, E.A. An arbovirus cline across the northern hemisphere. Virology 1995, 210, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fauquet, C.M.; Mayo, M.A.; Maniloff, J.; Desselberger, U.; Ball, L.A. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses; Elsevier Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Slovak, M.; Kazimirova, M.; Siebenstichova, M.; Ustanikova, K.; Klempa, B.; Gritsun, T.; Gould, E.A.; Nuttall, P.A. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2014, 5, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda's enduring paradigm. Ticks Tick-Borne Dis. 2011, 2, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mlera, L.; Meade-White, K.; Saturday, G.; Scott, D.; Bloom, M.E. Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLoS Negl. Trop. Dis. 2017, 11, e0005346. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.H.; Casals, J. Arboviruses: Group B. In Viral and Rickettsial Infections of Man, 4th ed.; Horsfall, F.L., Jr., Tamm, I., Eds.; J.B. Lippincott: Philadelphia, PA, USA, 1965. [Google Scholar]
- Ruzek, D.; Dobler, G.; Donoso Mantke, O. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Bodemann, H.H.; Pausch, J.; Schmitz, H.; Hoppe-Seyler, G. Tick-born encephalitis (ESME) as laboratory infection. Die Med. Welt 1977, 28, 1779–1781. [Google Scholar]
- Gritsun, T.S.; Nuttall, P.A.; Gould, E.A. Tick-borne flaviviruses. Adv. Virus Res. 2003, 61, 317–371. [Google Scholar] [PubMed]
- Pogodina, V.V.; Frolova, M.P.; Malenko, G.V.; Fokina, G.I.; Levina, L.S.; Mamonenko, L.L.; Koreshkova, G.V.; Ralf, N.M. Persistence of tick-borne encephalitis virus in monkeys. I. Features of experimental infection. Acta Virol. 1981, 25, 337–343. [Google Scholar] [PubMed]
- Pogodina, V.V.; Levina, L.S.; Fokina, G.I.; Koreshkova, G.V.; Malenko, G.V.; Bochkova, N.G.; Rzhakhova, O.E. Persistence of tic-borne encephalitis virus in monkeys. III. Phenotypes of the persisting virus. Acta Virol. 1981, 25, 352–360. [Google Scholar] [PubMed]
- Pogodina, V.V.; Malenko, G.V.; Fokina, G.I.; Levina, L.S.; Koreshkova, G.V.; Rzhakhova, O.E.; Bochkova, N.G.; Mamonenko, L.L. Persistence of tick-borne encephalitis virus in monkeys. II. Effectiveness of methods used for virus detection. Acta Virol. 1981, 25, 344–351. [Google Scholar] [PubMed]
- Poponnikova, T.V. Specific clinical and epidemiological features of tick-borne encephalitis in Western Siberia. Int. J. Med. Microbiol. IJMM 2006, 296 (Suppl. 40), 59–62. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Yakimenko, V.V.; Karan, L.S.; Tkachev, S.E. Omsk haemorrhagic fever. Lancet 2010, 376, 2104–2113. [Google Scholar] [CrossRef]
- Sadanandane, C.; Elango, A.; Marja, N.; Sasidharan, P.V.; Raju, K.H.; Jambulingam, P. An outbreak of Kyasanur forest disease in the Wayanad and Malappuram districts of Kerala, India. Ticks Tick-Borne Dis. 2017, 8, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Fagbo, S.F.; Osman Ali, A.; AlHakeem, R.; Elnagi, F.M.; Bamgboye, E.A. Is the epidemiology of alkhurma hemorrhagic fever changing?: A three-year overview in Saudi Arabia. PLoS ONE 2014, 9, e85564. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Jia, X.Y.; Huang, C.; Grady, L.J.; Lipkin, W.I. Identification of a Kunjin/West Nile-like Flavivirus in brains of patients with New York encephalitis. Lancet 1999, 354, 1261–1262. [Google Scholar] [CrossRef]
- Murray, K.O.; Mertens, E.; Despres, P. West Nile virus and its emergence in the United States of America. Vet. Res. 2010, 41, 67. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M. Globalization, land use, and the invasion of West Nile virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Hinten, S.R.; Beckett, G.A.; Gensheimer, K.F.; Pritchard, E.; Courtney, T.M.; Sears, S.D.; Woytowicz, J.M.; Preston, D.G.; Smith, R.P., Jr.; Rand, P.W.; et al. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector-Borne Zoonotic Dis. 2008, 8, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.M.; Mohammed, H.; Zielinski-Gutierrez, E.; Hayden, M.H.; Lopez, J.L.; Fournier, M.; Trujillo, A.R.; Burton, R.; Brunkard, J.M.; Anaya-Lopez, L.; et al. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: Results of a household-based seroepidemiologic survey, December 2005. Am. J. Trop. Med. Hyg. 2008, 78, 364–369. [Google Scholar] [PubMed]
- Thomas, D.L.; Santiago, G.A.; Abeyta, R.; Hinojosa, S.; Torres-Velasquez, B.; Adam, J.K.; Evert, N.; Caraballo, E.; Hunsperger, E.; Munoz-Jordan, J.L.; et al. Reemergence of dengue in Southern Texas, 2013. Emerg. Infect. Dis. 2016, 22, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Adalja, A.A.; Sell, T.K.; Bouri, N.; Franco, C. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg. Infect. Dis. 2012, 18, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Effler, P.V.; Pang, L.; Kitsutani, P.; Vorndam, V.; Nakata, M.; Ayers, T.; Elm, J.; Tom, T.; Reiter, P.; Rigau-Perez, J.G.; et al. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 2005, 11, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Viray, M.; Ushiroda, J.; Whelen, A.C.; Sciulli, R.; Gose, R.; Lee, R.; Honda, E.; Park, S.Y.; Hawaii dengue response, T. Notes from the field: Outbreak of locally acquired cases of dengue fever—Hawaii, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Rodriguez, L.F.; Herrington, E.; Kharat, V.; Vasilakis, N.; Walker, C.; Turner, C.; Khuwaja, S.; Arafat, R.; Weaver, S.C.; et al. Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector-Borne Zoonotic Dis. 2013, 13, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Weissenbock, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne Flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Ye, J.; Ruan, X.; Wan, S.; Zhu, B.; Cao, S. Usutu virus: An emerging Flavivirus in Europe. Viruses 2015, 7, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Cadar, D.; Luhken, R.; van der Jeugd, H.; Garigliany, M.; Ziegler, U.; Keller, M.; Lahoreau, J.; Lachmann, L.; Becker, N.; Kik, M.; et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Woodall, J.P. The viruses isolated from arthropods at the East African virus research institute in the 26 years ending December 1963. Proc. E Afr. Acad. 1964, 2, 141–146. [Google Scholar]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.S.; Barrett, A.D. Current status and future prospects of yellow fever vaccines. Expert Rev. Vaccines 2015, 14, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Bocchini, J.A., Jr.; Rubin, L.; Fischer, M.; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine booster doses: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 647–650. [Google Scholar] [PubMed]
- Mason, R.A.; Tauraso, N.M.; Spertzel, R.O.; Ginn, R.K. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 1973, 25, 539–544. [Google Scholar] [PubMed]
- Seligman, S.J. Risk groups for yellow fever vaccine-associated viscerotropic disease (YEL-AVD). Vaccine 2014, 32, 5769–5775. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E. Yellow fever vaccine-associated viscerotropic disease: Current perspectives. Drug Des. Dev. Ther. 2016, 10, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.R.; Larsen, M.A.; Kongsgaard, M.; Rasmussen, M.; Buus, S.; Stryhn, A.; Thomsen, A.R.; Christensen, J.P. Vaccination with Replication Deficient Adenovectors Encoding YF-17D Antigens Induces Long-Lasting Protection from Severe Yellow Fever Virus Infection in Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004464. [Google Scholar] [CrossRef] [PubMed]
- Schafer, B.; Holzer, G.W.; Joachimsthaler, A.; Coulibaly, S.; Schwendinger, M.; Crowe, B.A.; Kreil, T.R.; Barrett, P.N.; Falkner, F.G. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PLoS ONE 2011, 6, e24505. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M., Jr.; Cruz Fda, S.; Cordeiro, M.T.; da Motta, M.A.; Cassemiro, K.M.; Maia Rde, C.; de Figueiredo, R.C.; Galler, R.; Freire Mda, S.; August, J.T.; et al. A DNA vaccine against yellow fever virus: Development and evaluation. PLoS Negl. Trop. Dis. 2015, 9, e0003693. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, I.; Nickols, B.; Hidajat, R.; Jokinen, J.; Lukashevich, I.S.; Pushko, P. Plasmid DNA initiates replication of yellow fever vaccine in vitro and elicits virus-specific immune response in mice. Virology 2014, 468–470, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Silva, A.N.; Souza, M.C.; Silva, M.V.; Neves, P.P.; Silva, A.A.; Matos, D.D.; Herrera, M.A.; Yamamura, A.M.; Freire, M.S.; et al. An inactivated yellow fever 17DD vaccine cultivated in Vero cell cultures. Vaccine 2015, 33, 4261–4268. [Google Scholar] [CrossRef] [PubMed]
- Hoke, C.H.; Nisalak, A.; Sangawhipa, N.; Jatanasen, S.; Laorakapongse, T.; Innis, B.L.; Kotchasenee, S.; Gingrich, J.B.; Latendresse, J.; Fukai, K.; et al. Protection against Japanese encephalitis by inactivated vaccines. N. Engl. J. Med. 1988, 319, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H. Control of Japanese encephalitis in Japan: Immunization of humans and animals, and vector control. In Japanese Encephalitis and West Nile Viruses; Mackenzie, J., Barrett, A.D.T., Deubel, V., Eds.; Springer: Berlin, Germany, 2002; pp. 139–152. [Google Scholar]
- Chumakov, M.P.; Gagarina, A.V.; Vilner, L.M.; Khanina, M.K.; Rodin, I.M.; Vasenovich, M.I.; Lakina, V.I.; Finogenova, E.V. Experience in the Experimental Production and Control of Tissue Culture Vaccine against Tick Encephalitis. Vopr. Virusol. 1963, 29, 415–420. [Google Scholar] [PubMed]
- Kunz, C. TBE vaccination and the Austrian experience. Vaccine 2003, 21, S50–S55. [Google Scholar] [CrossRef]
- Girgsdies, O.E.; Rosenkranz, G. Tick-borne encephalitis: Development of a paediatric vaccine. A controlled, randomized, double-blind and multicentre study. Vaccine 1996, 14, 1421–1428. [Google Scholar] [CrossRef]
- Lehrer, A.T.; Holbrook, M.R. Tick-borne encephalitis vaccines. In Vaccines for Biodefense and Emerging and Neglected Diseases; Barrett, A.D.T., Stanberry, L.R., Eds.; Academic Press: London, UK, 2009; pp. 713–718. [Google Scholar]
- Zent, O.; Broker, M. Tick-borne encephalitis vaccines: Past and present. Expert Rev. Vaccines 2005, 4, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Coudeville, L.; Baurin, N.; L’Azou, M.; Guy, B. Potential impact of dengue vaccination: Insights from two large-scale phase III trials with a tetravalent dengue vaccine. Vaccine 2016, 34, 6426–6435. [Google Scholar] [CrossRef] [PubMed]
- Coudeville, L.; Baurin, N.; Vergu, E. Estimation of parameters related to vaccine efficacy and dengue transmission from two large phase III studies. Vaccine 2016, 34, 6417–6425. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Barrere, B.; Malinowski, C.; Saville, M.; Teyssou, R.; Lang, J. From research to phase III: Preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011, 29, 7229–7241. [Google Scholar] [CrossRef] [PubMed]
- McArthur, M.A.; Sztein, M.B.; Edelman, R. Dengue vaccines: Recent developments, ongoing challenges and current candidates. Expert Rev. Vaccines 2013, 12, 933–953. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease. Drugs 2016, 76, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Dengue vaccine: WHO position paper—July 2016. Wkly. Epidemiol. Rec. 2016, 91, 349–364. [Google Scholar]
- Halstead, S.B.; Russell, P.K. Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 2016, 34, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Butrapet, S.; Tsuchiya, K.R.; Bhamarapravati, N.; Gubler, D.J.; Kinney, R.M. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J. Virol. 2003, 77, 11436–11447. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Huang, C.Y.; Kinney, R.M.; Stinchcomb, D.T. Development of DENVax: A chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine 2011, 29, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Velez, I.D.; Thomson, C.; Lopez, L.; Jimenez, A.; Haller, A.A.; Silengo, S.; Scott, J.; Boroughs, K.L.; Stovall, J.L.; et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: A randomised, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2014, 14, 830–838. [Google Scholar] [CrossRef]
- Osorio, J.E.; Partidos, C.D.; Wallace, D.; Stinchcomb, D.T. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015, 33, 7112–7120. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lindow, J.C.; Durbin, A.P.; Whitehead, S.S.; Pierce, K.K.; Carmolli, M.P.; Kirkpatrick, B.D. Vaccination of volunteers with low-dose, live-attenuated, dengue viruses leads to serotype-specific immunologic and virologic profiles. Vaccine 2013, 31, 3347–3352. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330ra36. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.D.; Durbin, A.P.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Diehl, S.A.; Elwood, D.; Jarvis, A.P.; et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J. Infect. Dis. 2015, 212, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Rothman, A.L. Trials and Tribulations on the Path to Developing a Dengue Vaccine. Am. J. Prev. Med. 2015, 49 (Suppl. 4), S334–S344. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J. Developing a dengue vaccine: Progress and future challenges. Ann. N. Y. Acad. Sci. 2014, 1323, 140–159. [Google Scholar] [CrossRef] [PubMed]
- Kroschewski, H.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 2003, 308, 92–100. [Google Scholar] [CrossRef]
- Liu, P.; Ridilla, M.; Patel, P.; Betts, L.; Gallichotte, E.; Shahidi, L.; Thompson, N.L.; Jacobson, K. Beyond attachment: Roles of DC-SIGN in dengue virus infection. Traffic 2017, 18, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, M.D.; Meertens, L.; Chazal, M.; Hafirassou, M.L.; Dejarnac, O.; Zamborlini, A.; Despres, P.; Sauvonnet, N.; Arenzana-Seisdedos, F.; Jouvenet, N.; et al. Vaccine and wild-type strains of yellow fever virus engage distinct entry mechanisms and differentially stimulate antiviral immune responses. mBio 2016, 7, e01956-15. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Bevan, M.J. CD8 and CD4 T cells in west nile virus immunity and pathogenesis. Viruses 2013, 5, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Welte, T. Role of natural killer and Gamma-delta T cells in West Nile virus infection. Viruses 2013, 5, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Aberle, J.H.; Schwaiger, J.; Aberle, S.W.; Stiasny, K.; Scheinost, O.; Kundi, M.; Chmelik, V.; Heinz, F.X. Human CD4+ T Helper Cell Responses after Tick-Borne Encephalitis Vaccination and Infection. PLoS ONE 2015, 10, e0140545. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Braun, M.; Pakalniene, J.; Dailidyte, L.; Beziat, V.; Lampen, M.H.; Klingstrom, J.; Lagerqvist, N.; Kjerstadius, T.; Michaelsson, J.; et al. Specificity and dynamics of effector and memory CD8 T cell responses in human tick-borne encephalitis virus infection. PLoS Pathog. 2015, 11, e1004622. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Salat, J.; Palus, M.; Gritsun, T.S.; Gould, E.A.; Dykova, I.; Skallova, A.; Jelinek, J.; Kopecky, J.; Grubhoffer, L. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 2009, 384, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Akondy, R.S. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol. Cell Biol. 2011, 89, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Wieten, R.W.; Goorhuis, A.; Jonker, E.F.; de Bree, G.J.; de Visser, A.W.; van Genderen, P.J.; Remmerswaal, E.B.; Ten Berge, I.J.; Visser, L.G.; Grobusch, M.P.; et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J. Infect. 2016, 72, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Wieten, R.W.; Jonker, E.F.; van Leeuwen, E.M.; Remmerswaal, E.B.; Ten Berge, I.J.; de Visser, A.W.; van Genderen, P.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS ONE 2016, 11, e0149871. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, M.R.; Gowen, B.B. Animal models of highly pathogenic RNA viral infections: Encephalitis viruses. Antivir. Res. 2008, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, R.H.; Rippy, M.K.; McKee, K.T., Jr.; Zack, P.M.; Peters, C.J. Infection of Macaca radiata with viruses of the tick-borne encephalitis group. Microb. Pathog. 1992, 13, 399–409. [Google Scholar] [CrossRef]
- McArthur, M.A.; Suderman, M.T.; Mutebi, J.P.; Xiao, S.Y.; Barrett, A.D. Molecular characterization of a hamster viscerotropic strain of yellow fever virus. J. Virol. 2003, 77, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Guzman, H.; da Rosa, A.P.; Vasconcelos, P.F.; Dias, L.B.; Bunnell, J.E.; Zhang, H.; Xiao, S.Y. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 2001, 183, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.C.; Gardner, C.L.; Khoretonenko, M.V.; Klimstra, W.B.; Ryman, K.D. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009, 5, e1000614. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M.; Lam, L.K.; Klimstra, W.B.; Ryman, K.D. The 17D-204 vaccine strain-induced protection against virulent yellow fever virus is mediated by humoral immunity and CD4+ but not CD8+ T cells. PLoS Pathog. 2016, 12, e1005786. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, V.V.; Milligan, G.N.; Bourne, N.; Barrett, A.D. Mouse models of dengue virus infection for vaccine testing. Vaccine 2015, 33, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Watanabe, S.; Kavishna, R.; Alonso, S.; Vasudevan, S.G. Animal models for studying dengue pathogenesis and therapy. Antivir. Res. 2015, 123, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Peixoto, T.M.; Machado de Siqueira, A.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Alpert, S.G.; Fergerson, J.; Noel, L.P. Intrauterine West Nile virus: Ocular and systemic findings. Am. J. Ophthalmol. 2003, 136, 733–735. [Google Scholar] [CrossRef]
- O’Leary, D.R.; Kuhn, S.; Kniss, K.L.; Hinckley, A.F.; Rasmussen, S.A.; Pape, W.J.; Kightlinger, L.K.; Beecham, B.D.; Miller, T.K.; Neitzel, D.F.; et al. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003–2004. Pediatrics 2006, 117, e537–e545. [Google Scholar] [CrossRef] [PubMed]
- Colt, S.; Garcia-Casal, M.N.; Pena-Rosas, J.P.; Finkelstein, J.L.; Rayco-Solon, P.; Weise Prinzo, Z.C.; Mehta, S. Transmission of Zika virus through breast milk and other breastfeeding-related bodily-fluids: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005528. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. From the Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. JAMA 2002, 288, 1976–1977. [Google Scholar]
- Barthel, A.; Gourinat, A.C.; Cazorla, C.; Joubert, C.; Dupont-Rouzeyrol, M.; Descloux, E. Breast milk as a possible route of vertical transmission of dengue virus? Clin. Infect. Dis. 2013, 57, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Twele-Montecinos, L.; MacDonald, J.; Webster, P.; Law, B. Case report: Probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. CMAJ 2011, 183, E243–E245. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holbrook, M.R. Historical Perspectives on Flavivirus Research. Viruses 2017, 9, 97. https://doi.org/10.3390/v9050097
Holbrook MR. Historical Perspectives on Flavivirus Research. Viruses. 2017; 9(5):97. https://doi.org/10.3390/v9050097
Chicago/Turabian StyleHolbrook, Michael R. 2017. "Historical Perspectives on Flavivirus Research" Viruses 9, no. 5: 97. https://doi.org/10.3390/v9050097




