UK Pigs at the Time of Slaughter: Investigation into the Correlation of Infection with PRRSV and HEV
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Detection of Active PRRSV Infection at Slaughter Age
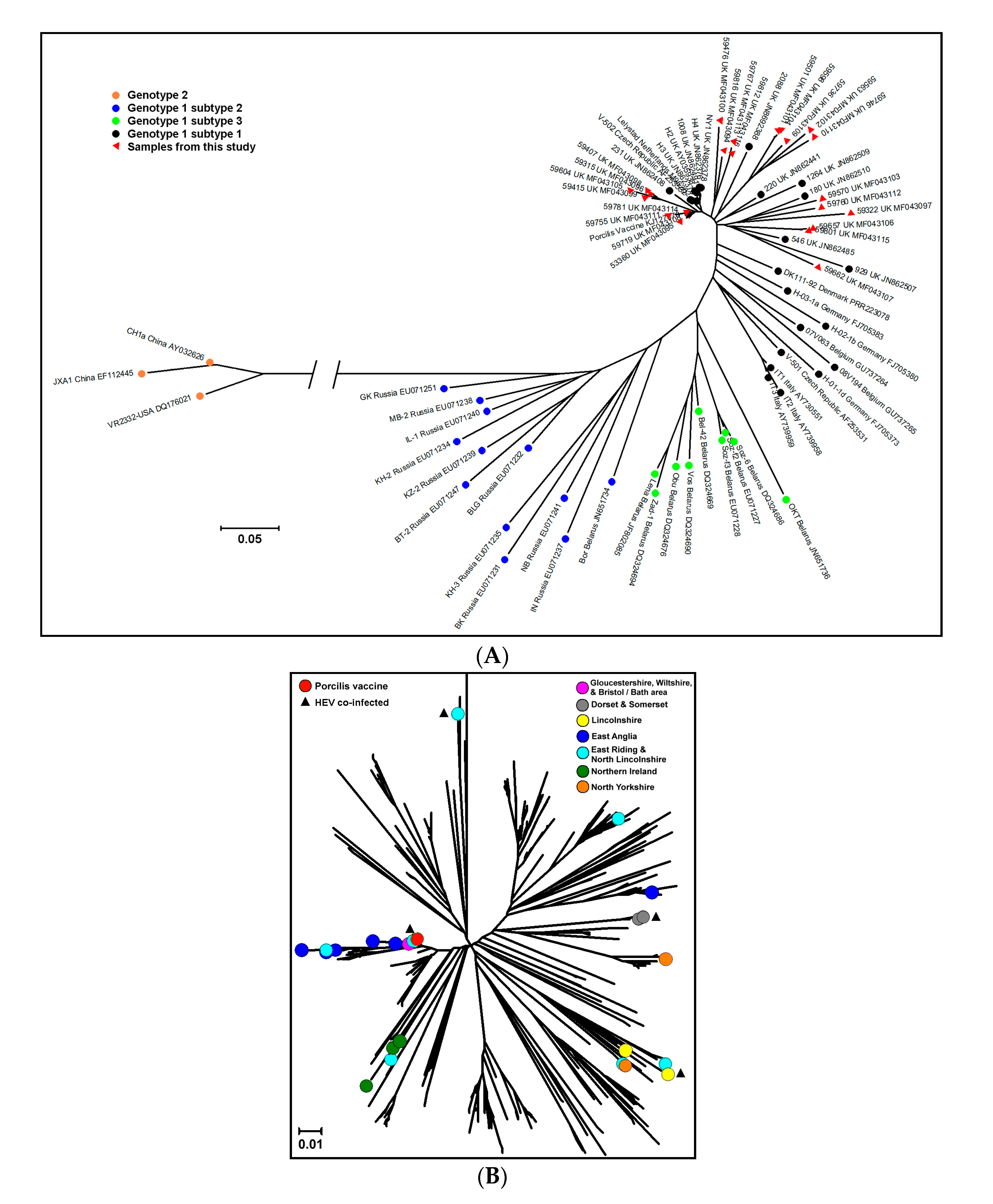
3.2. PRRSV Genetic Characterization
3.3. PRRSV and HEV Co-Infections
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dalton, H.R.; Bendall, R.; Ijaz, S.; Banks, M. Hepatitis E: An emerging infection in developed countries. Lancet Infect. Dis. 2008, 8, 698–709. [Google Scholar] [CrossRef]
- Pavio, N.; Meng, X.J.; Doceul, V. Zoonotic origin of hepatitis E. Curr. Opin. Virol. 2015, 10, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Chand, M.A.; Kafatos, G.; Tedder, R.; Morgan, D. Hepatitis E virus in England and Wales: Indigenous infection is associated with the consumption of processed pork products. Epidemiol. Infect. 2014, 142, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Robertson, I.; Wilesmith, J.; Ryan, J.; Kilner, C.; Paton, D.; Drew, T.; Brown, I; Sands, J. PRRS (Blue Eared Pig Disease) in Great Britain. Am. Assoc. Swine Pract. Newsl. 1992, 4, 32–36. [Google Scholar]
- Richardson, J.S. The cost of endemic disease in pig production. Pig J. 2011, 65, 10–17. [Google Scholar]
- Frossard, J.P.; Hughes, G.J.; Westcott, D.G.; Naidu, B.; Williamson, S.; Woodger, N.G.A.; Steinbach, F.; Drew, T.W. Porcine reproductive and respiratory syndrome virus: Genetic diversity of recent British isolates. Vet. Microbiol. 2013, 162, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Riber, U.; Nielsen, J.; Lind, P. In utero infection with PRRS virus modulates cellular functions of blood monocytes and alveolar lung macrophages in piglets. Vet. Immunol. Immunopathol. 2004, 99, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Thanawongnuwech, R.; Brown, G.B.; Halbur, P.G.; Roth, J.A.; Royer, R.L.; Thacker, B.J. Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility in Streptococcus suis infection. Vet. Pathol. 2000, 37, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H. Infection of porcine reproductive and respiratory syndrome virus suppresses the antibody response to classical swine fever virus vaccination. Vet. Microbiol. 2003, 95, 295–301. [Google Scholar] [CrossRef]
- Beloeil, P.A.; Fravalo, P.; Fablet, C.; Jolly, J.P.; Eveno, E.; Hascoet, Y.; Chauvin, C.; Salvat, G.; Madec, F. Risk factors for Salmonella enterica subsp enterica shedding by market-age pigs in French farrow-to-finish herds. Prev. Vet. Med. 2004, 63, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Salines, M.; Barnaud, E.; Andraud, M.; Eono, F.; Renson, P.; Bourry, O.; Pavio, N.; Rose, N. Hepatitis E virus chronic infection of swine co-infected with porcine reproductive and respiratory syndrome virus. Vet. Res. 2015, 46, 55. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Frossard, J.P.; Rebel, J.M.; Stadejek, T.; Morgan, S.B.; Graham, S.P.; Steinbach, F. Host-pathogen interactions during porcine reproductive and respiratory syndrome virus 1 infection of piglets. Virus Res. 2015, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.B.; Graham, S.P.; Salguero, F.J.; Sánchez Cordón, P.J.; Mokhtar, H.; Rebel, J.M.J.; Weesendorp, E.; Bodman-Smith, K.B.; Steinbach, F.; Frossard, J.P. Increased pathogenicity of European porcine reproductive and respiratory syndrome virus is associated with enhanced adaptive responses and viral clearance. Vet. Microbiol. 2013, 163, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.F.; Cheney, T.E.A.; Williamson, S.; Guy, E.; Smith, R.P.; Davies, R.H. A prevalence study of Salmonella spp., Yersinia spp., Toxoplasma gondii and porcine reproductive and respiratory syndrome virus in UK pigs at slaughter. Epidemiol. Infect. 2016, 144, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of hepatitis E virus infection in pigs at the time of slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Zoonoses Report UK 2012; Defra: London, UK, 2013. Available online: https://www.gov.uk/government/publications/zoonoses-report-uk-2012 (accessed on 10 May 2017).
- Frossard, J.P.; Fearnley, C.; Naidu, B.; Errington, J.; Westcott, D.G.; Drew, T.W. Porcine reproductive and respiratory syndrome virus: Antigenic and molecular diversity of British isolates and implications for diagnosis. Vet. Microbiol. 2012, 158, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids. Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.H.; Laster, S.M.; Tompkins, M.; Brown, T.; Xu, J.S.; Altier, C.; Gomez, W.; Benfield, D.; McCaw, M.B. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis type II. J. Virol. 2001, 75, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Renukaradhya, G.J.; Alekseev, K.P.; Fang, Y.; Tang, Y.; Saif, L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: Implications for respiratory viral co-infections. J. Gen. Virol. 2009, 90, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Velasova, M.; Alarcon, P.; Williamson, S.; Wieland, B. Risk factors for porcine reproductive and respiratory syndrome virus infection and resulting challenges for effective disease surveillance. BMC Vet. Res. 2012, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.W.; Doster, A.R.; Galeota, J.A.; Sur, J.H.; Osorio, F.A. Duration of infection and proportion of pigs persistently infected with porcine reproductive and respiratory syndrome virus. J. Clin. Microbiol. 2003, 41, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Deen, J.; Otake, S.; Pijoan, C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can. J. Vet. Res. 2004, 68, 128–133. [Google Scholar] [PubMed]
- Mao, J.; Zhao, Y.; She, R.; Xiao, P.; Tian, J.; Chen, J. One case of swine hepatitis E virus and porcine reproductive and respiratory syndrome virus co-infection in weaned pigs. Vir. J. 2013, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Toma, S.; Di Bartolo, I.; Caprioli, A.; Ruggeri, F.M.; Lelli, D.; Bonci, M.; Ostanello, F. Detection of hepatitis E virus (HEV) in Italian pigs displaying different pathological lesions. Res. Vet. Sci. 2010, 88, 492–496. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.G.; Samsom, J.N.; Voermans, J.J.; Van Rooij, E.M.; De Visser, Y.E.; Bianchi, A.T. Effects of a porcine reproductive and respiratory syndrome virus infection on the development of the immune response against pseudorabies virus. Vet. Immunol. Immunopathol. 2000, 76, 125–135. [Google Scholar] [CrossRef]
- De Deus, N.; Casas, M.; Peralta, B.; Nofrarías, M.; Pina, S.; Martín, M.; Segalés, J. Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow-to-finish farm. Vet. Microbiol. 2008, 132, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, R.; She, R.; Mao, J.; Zhao, Y.; Du, F.; Liu, C.; Liu, J.; Cheng, M.; Zhu, R.; et al. Fatal disease associated with swine hepatitis E virus and porcine circovirus 2 co-infection in four weaned pigs in China. BMC Vet. Res. 2015, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodríguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, D.; Poitras, E.; Gagné, M.J.; Ward, P.; Houde, A. Hepatitis E virus load in swine organs and tissues at slaughterhouse determined by real-time RT-PCR. Int. J. Food Microbiol. 2010, 139, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.R.; de Paula, V.S.; de Oliveira, J.M.; Marchevsky, R.S.; Pinto, M.A. Hepatitis E virus in swine and effluent samples from slaughterhouses in Brazil. Vet. Microbiol. 2011, 149, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhao, Q.; Jiang, F.L.; Liu, B.Y.; Zhao, J.N.; Dang, L.; Sun, Y.N.; Mu, Y.; Xiao, S.Q.; Wang, C.B.; et al. Genetic characterization and serological prevalence of swine hepatitis E virus in Shandong province, China. Vet. Microbiol. 2014, 172, 415–424. [Google Scholar] [CrossRef] [PubMed]
| Age | Number Tested | Number Positive | % Positive |
|---|---|---|---|
| <6 months | 34 | 4 | 11.8% |
| 6–12 months | 312 | 25 | 8.0% |
| >12 months | 19 | 2 | 10.5% |
| Not known | 7 | 0 | 0% |
| Pathogen | Serology Status | HEV | |||
|---|---|---|---|---|---|
| Seropositive n (%) | RNA + Plasma and/or Cecum n (%) | RNA + Plasma n (%) | RNA + Cecum n (%) | ||
| PRRSV | Seronegative (n = 256) | 235 (91.8) | 62 (24.2) | 20 (7.8) | 50 (19.5) |
| Seropositive (n = 354) | 333 (94.1) | 60 (17.0) | 14 (4.0) | 52 (14.7) | |
| p = 0.31 | p = 0.03 | p = 0.05 | p = 0.13 | ||
| Pathogen | RNA Status | HEV | |||
|---|---|---|---|---|---|
| Seropositive n (%) | RNA + Plasma and/or Cecum n (%) | RNA + Plasma n (%) | RNA + Cecum n (%) | ||
| PRRSV | PCR negative (n = 327) | 311 (95.1) | 55 (16.8) | 12 (3.7) | 48 (14.7) |
| PCR positive (n = 31) | 26 (83.9) | 6 (19.4) | 2 (6.5) | 5 (16.1) | |
| p = 0.01 | p = 0.73 | p = 0.45 | p = 0.83 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frossard, J.-P.; Grierson, S.; Cheney, T.; Steinbach, F.; Choudhury, B.; Williamson, S. UK Pigs at the Time of Slaughter: Investigation into the Correlation of Infection with PRRSV and HEV. Viruses 2017, 9, 110. https://doi.org/10.3390/v9060110
Frossard J-P, Grierson S, Cheney T, Steinbach F, Choudhury B, Williamson S. UK Pigs at the Time of Slaughter: Investigation into the Correlation of Infection with PRRSV and HEV. Viruses. 2017; 9(6):110. https://doi.org/10.3390/v9060110
Chicago/Turabian StyleFrossard, Jean-Pierre, Sylvia Grierson, Tanya Cheney, Falko Steinbach, Bhudipa Choudhury, and Susanna Williamson. 2017. "UK Pigs at the Time of Slaughter: Investigation into the Correlation of Infection with PRRSV and HEV" Viruses 9, no. 6: 110. https://doi.org/10.3390/v9060110






