Co-Infection with Marek’s Disease Virus and Reticuloendotheliosis Virus Increases Illness Severity and Reduces Marek’s Disease Vaccine Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and MD Vaccines
2.2. PCR-Based Detection of MDV and REV
2.3. Design of Animal Experiments
2.4. Histopathological Examination
2.5. Real-Time Quantitative PCR and Reverse Transcription qPCR
2.6. Statistical Analysis
3. Results
3.1. MDV Strain ZW/15 and REV Strain JLR1501
3.2. PCR Identification and Purity of the Viruses and MD Vaccines
3.3. Animal Experiments of REV
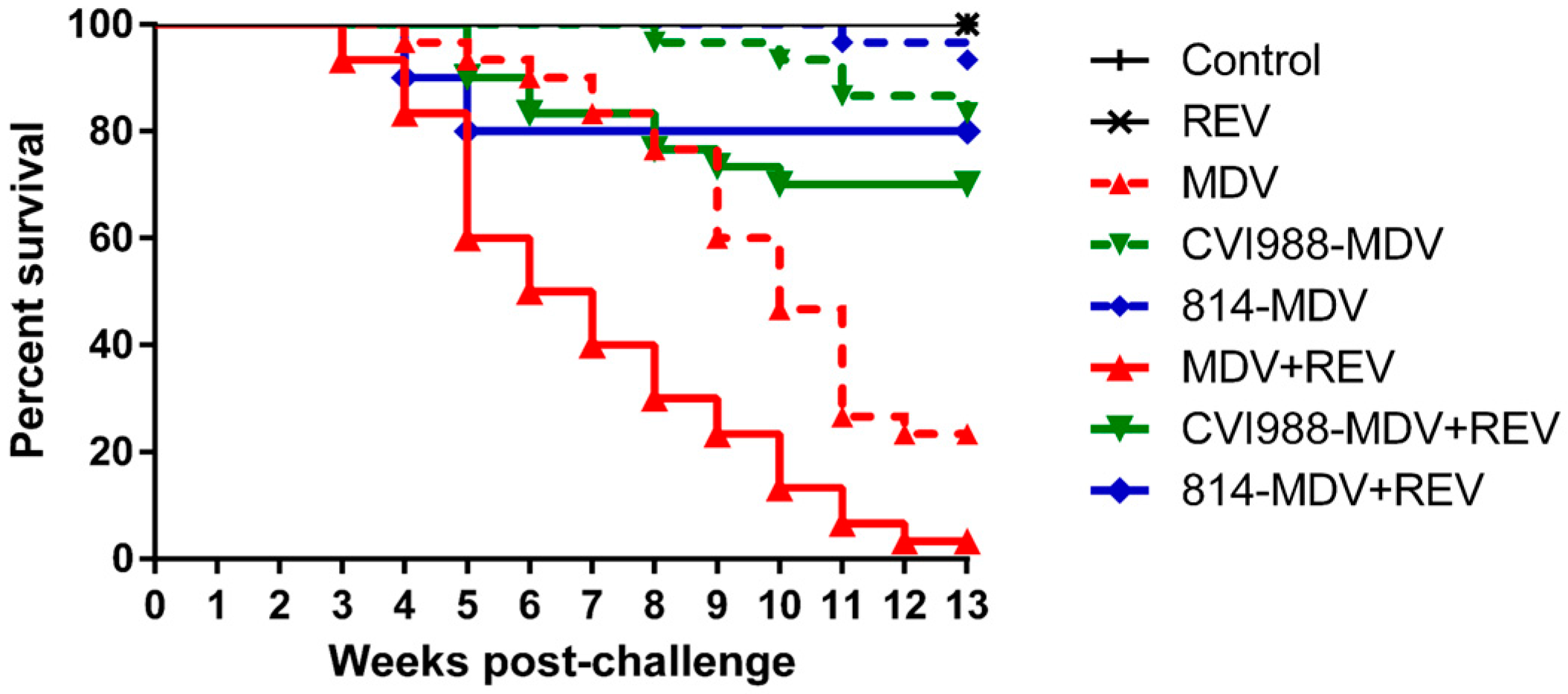
3.4. MD Incidence and Mortality
3.5. Distribution of Visible Tumors
3.6. Histological Lesions in Collected Tissues
3.7. Efficacy of MD Vaccines
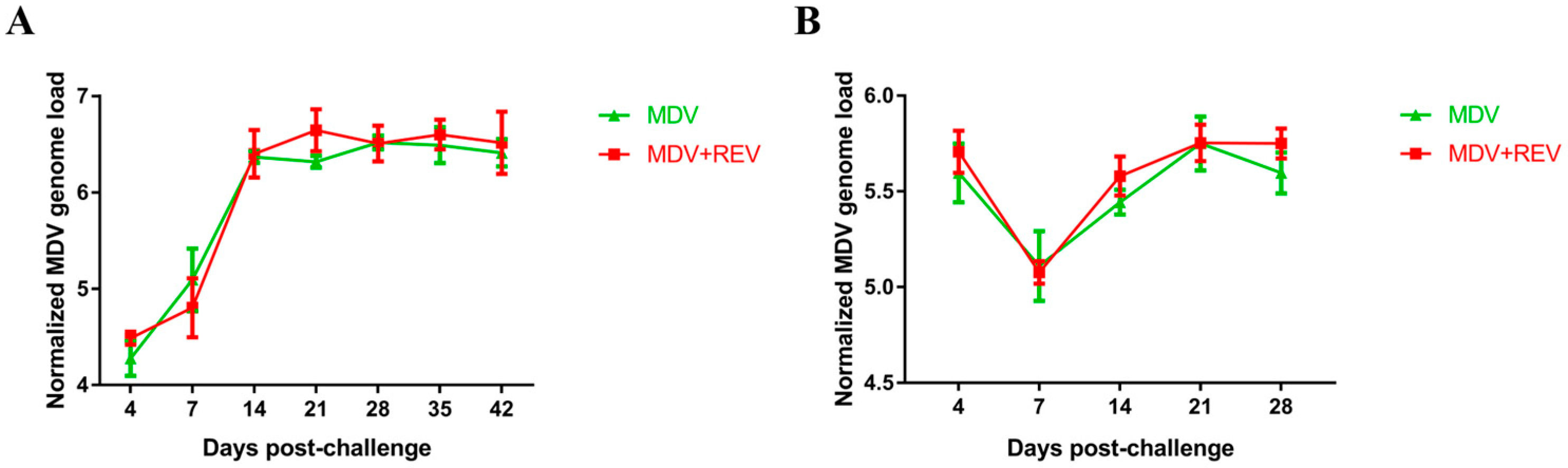
3.8. MDV Genome Load in Chicken Feather Pulps and the Spleen
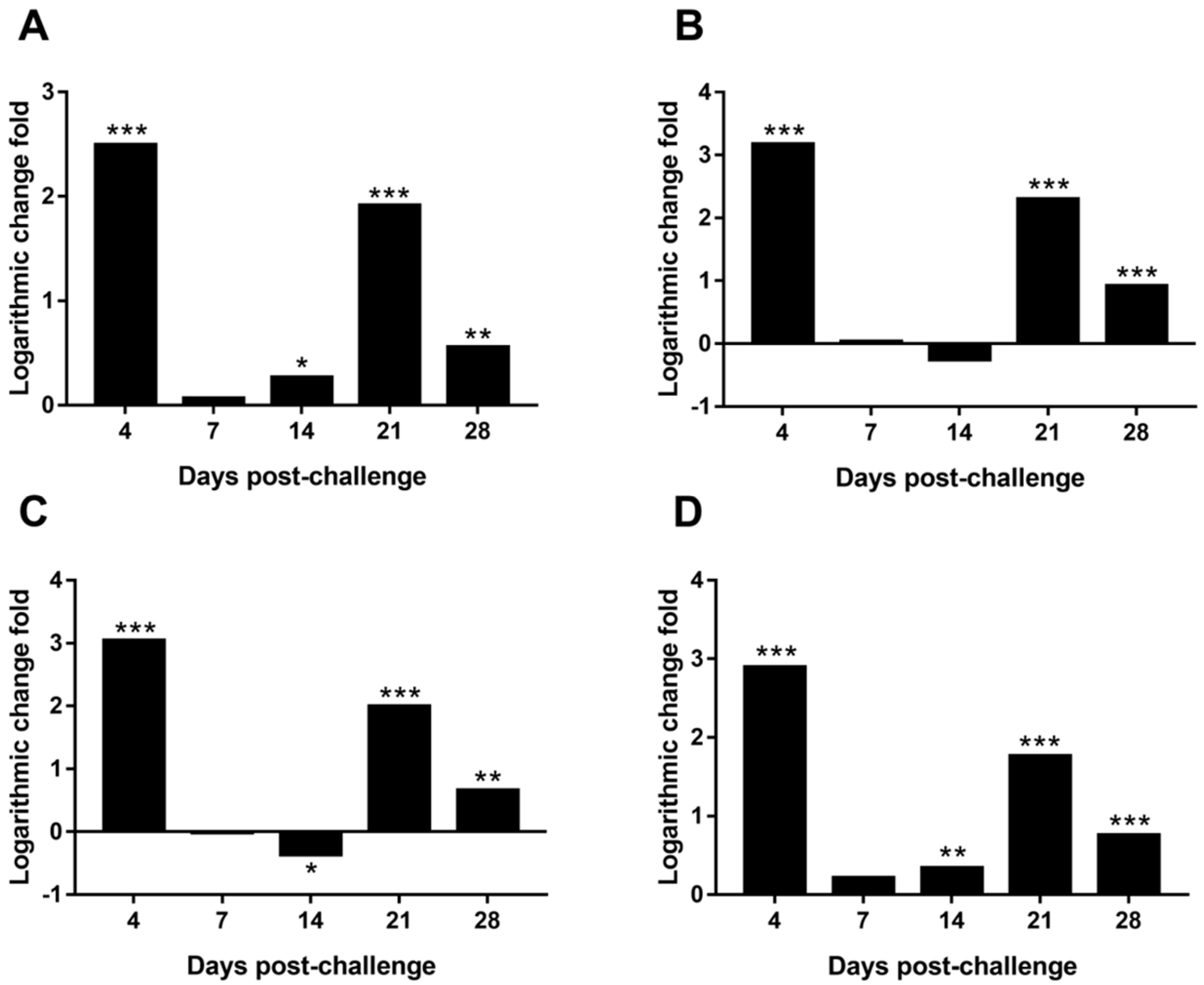
3.9. Differential Expression of MDV Genes in the Spleen
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s disease virus: From miasma to model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, I.M.; Witter, R.L.; Reed, W.M. Four distinct neurologic syndromes in Marek’s disease: Effect of viral strain and pathotype. Avian Dis. 1999, 43, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W.; Harris, R.W.; Buscaglia, C.; Schat, K.A.; Lucio, B. Relationship between the immunosuppressive potential and the pathotype of Marek’s disease virus isolates. Avian Dis. 1998, 42, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Baigent, S.J.; Petherbridge, L.J.; Howes, K.; Smith, L.P.; Currie, R.J.; Nair, V.K. Absolute quantitation of Marek’s disease virus genome copy number in chicken feather and lymphocyte samples using real-time PCR. J. Virol. Methods 2005, 123, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Cheetham, B.F.; Mahony, T.J.; Young, P.L.; Walkden-Brown, S.W. Absolute quantification of Marek’s disease virus and herpesvirus of turkeys in chicken lymohocyte, feather tip and dust samples using real-time PCR. J. Virol. Methods 2006, 132, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Smith, L.P.; Kgosana, L.; Baigent, S.J.; Nair, V.; Allday, M.J. Homodimerization of the Meq viral oncoprotein is necessary for induction of t-cell lymphoma by Marek’s disease virus. J. Virol. 2009, 83, 11142–11151. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Baigent, S.J.; Smith, L.P.; Chattoo, J.P.; Petherbridge, L.J.; Hawes, P.; Allday, M.J.; Nair, V. Interaction of Meq protein and C-terminal-binding protein is critical for induction of lymphomas by Marek’s disease virus. Proc. Natl. Acad. Sci. USA 2006, 103, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Lupiani, B.; Lee, L.F.; Cui, X.P.; Gimeno, I.; Anderson, A.; Morgan, R.W.; Silva, R.F.; Witter, R.L.; Kung, H.J.; Reddy, S.M. Marek’s disease virus-encoded meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl. Acad. Sci. USA 2004, 101, 11815–11820. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Z.; Lee, L.F.; Liu, J.L.; Kung, H.J. Structural analysis and transcriptional mapping of the Marek’s disease virus gene encoding pp38, an antigen associated with transformed cells. J. Virol. 1991, 65, 6509–6515. [Google Scholar] [PubMed]
- Reddy, S.M.; Lupiani, B.; Gimeno, I.M.; Silva, R.F.; Lee, L.F.; Witter, R.L. Rescue of a pathogenic marek’s disease virus with overlapping cosmid dnas: Use of a pp38 mutant to validate the technology for the study of gene function. Proc. Natl. Acad. Sci. USA 2002, 99, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.P.; Lee, L.F.; Reed, W.M.; Kung, H.J.; Reddy, S.M. Marek’s disease virus-encoded vIL-8 gene is involved in early cytolytic infection but dispensable for establishment of latency. J. Virol. 2004, 78, 4753–4760. [Google Scholar] [CrossRef] [PubMed]
- Parcells, M.S.; Lin, S.F.; Dienglewicz, R.L.; Majerciak, V.; Robinson, D.R.; Chen, H.C.; Wu, Z.; Dubyak, G.R.; Brunovskis, P.; Hunt, H.D.; et al. Marek’s disease virus (MDV) encodes an interleukin-8 homolog (vIL-8): Characterization of the vIL-8 protein and a vIL-8 deletion mutant MDV. J. Virol. 2001, 75, 5159–5173. [Google Scholar] [CrossRef] [PubMed]
- Strassheim, S.; Stik, G.; Rasschaert, D.; Laurent, S. mdv1-mir-m7–5p, located in the newly identified first intron of the latency-associated transcript of Marek’s disease virus, targets the immediate-early genes ICP4 and ICP27. J. Gen. Virol. 2012, 93, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.M.; Izumiya, Y.; Brunovskis, P.; Xia, L.; Parcells, M.S.; Reddy, S.M.; Lee, L.; Chen, H.W.; Kung, H.J. Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek’s disease virus-transformed t cells. J. Virol. 2003, 77, 12841–12851. [Google Scholar] [CrossRef] [PubMed]
- Gennart, I.; Coupeau, D.; Pejakovic, S.; Laurent, S.; Rasschaert, D.; Muylkens, B. Marek’s disease: Genetic regulation of gallid herpesvirus 2 infection and latency. Vet. J. 2015, 205, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.; Schat, K.A.; Sharif, S. Immunity to Marek’s disease: Where are we now? Dev. Comp. Immunol. 2013, 41, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s disease in chickens: A review with focus on immunology. Vet. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [PubMed]
- Nair, V. Successful control of Marek’s disease by vaccination. Dev. Biol. (Basel) 2004, 119, 147–154. [Google Scholar] [PubMed]
- Witter, R.L. Protective efficacy of Marek’s disease vaccines. Avian Dis. 1993, 37, 57–90. [Google Scholar]
- Walkden-Brown, S.W.; Islam, A.; Islam, A.F.; Burgess, S.K.; Groves, P.J.; Cooke, J. Pathotyping of Australian isolates of Marek’s disease virus in commercial broiler chickens vaccinated with herpesvirus of turkeys (HVT) or bivalent (HVT/SB1) vaccine and association with viral load in the spleen and feather dander. Aust. Vet. J. 2013, 91, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Li, Z.J.; Bao, K.Y.; Lv, H.C.; Gao, Y.L.; Gao, H.L.; Qi, X.L.; Cui, H.Y.; Wang, Y.Q.; Ren, X.G.; et al. Pathogenic characteristics of Marek’s disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Vet. Microbiol. 2015, 177, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.; Venugopal, K. Molecular characteristics of very virulent european MDV isolates. Acta Virol. 1999, 43, 90–93. [Google Scholar] [PubMed]
- Witter, R.L. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 1997, 41, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-R.; Zhang, Y.-P.; Lv, H.-C.; Zhou, L.-Y.; Cui, H.-Y.; Gao, Y.-L.; Qi, X.-L.; Wang, Y.-Q.; Li, K.; Gao, L.; et al. A Chinese variant Marek’s disease virus strain with divergence between virulence and vaccine resistance. Viruses 2017, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Lv, H.C.; Bao, K.Y.; Gao, Y.L.; Gao, H.L.; le Qi, X.; Cui, H.Y.; Wang, Y.Q.; Li, K.; Gao, L.; et al. Molecular and pathogenicity characterization of gallid herpesvirus 2 newly isolated in China from 2009 to 2013. Virus Genes 2016, 52, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gao, H.; Cui, X.; Zhao, Y.; Shi, X.; Li, Q.; Yan, S.; Gao, M.; Wang, M.; Liu, C.; et al. Avirulent Marek’s disease virus type 1 strain 814 vectored vaccine expressing avian influenza (AI) virus h5 haemagglutinin induced better protection than turkey herpesvirus vectored ai vaccine. PLoS ONE 2013, 8, e53340. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Cui, H.Y.; Qiao-Ling, L.I.; Zhao, Y.; Shi, X.M.; Zhao, X.Y.; Shun-Lei, H.U.; Wang, M.; Gao, M.; Yue, L.I. Construction and identification of recombinant Marek’s disease virus vaccine strain 814 expressing the F protein of NDV. Chin. J. Prev. Vet. Med. 2012, 34, 423–427. [Google Scholar]
- Witter, R.L.; Kreager, K.S. Serotype 1 viruses modified by backpassage or insertional mutagenesis: Approaching the threshold of vaccine efficacy in Marek’s disease. Avian Dis. 2004, 48, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.M.; Gifford, R.J. The extraordinary evolutionary history of the reticuloendotheliosis viruses. PLoS Biol. 2013, 11, e1001642. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.H.; Rup, B.J.; Rubin, A.S.; Bose, H.R., Jr. Specificity in the immunosuppression induced by avian reticuloendotheliosis virus. Infect. Immun. 1983, 40, 225–235. [Google Scholar] [PubMed]
- Purchase, H.G.; Ludford, C.; Nazerian, K.; Cox, H.W. A new group of oncogenic viruses: Reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J. Natl. Cancer Inst. 1973, 51, 489–499. [Google Scholar] [PubMed]
- Bohls, R.L.; Linares, J.A.; Gross, S.L.; Ferro, P.J.; Silvy, N.J.; Collisson, E.W. Phylogenetic analyses indicate little variation among reticuloendotheliosis viruses infecting avian species, including the endangered attwater’s prairie chicken. Virus Res. 2006, 119, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shi, Y.; Zhang, L.; Zhu, G.; Diao, X.; Cui, Z. Occurrence of reticuloendotheliosis in Chinese partridge. J. Vet. Med. Sci. 2007, 69, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.; Cheng, S.; Barbosa, T.; Haefele, H. Enzootic reticuloendotheliosis in the endangered Attwater’s and greater prairie chickens. Avian Dis. 2006, 50, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Trampel, D.W.; Pepper, T.M.; Witter, R.L. Reticuloendotheliosis in hungarian partridge. J. Wildl. Dis. 2009, 38, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.; Zavala, G.; Cheng, S.; Villegas, P. Full genome sequence and some biological properties of reticuloendotheliosis virus strain APC-566 isolated from endangered Attwater’s prairie chickens. Virus Res. 2007, 124, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Deng, X.; Gao, Y.; Li, K.; Chai, H.; Fan, Z.; Ren, X.; Wang, Q.; Zhang, L.; Yun, B.; et al. First isolation of reticuloendotheliosis virus from mallards in China. Arch Virol. 2014, 159, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.L.; Chen, S.N.; Lin, T.; Wen, X.H.; Wei, W.K.; Lv, D.H.; Chen, R.A. Emergence of reticuloendotheliosis virus in pigeons in Guangdong province, Southern China. Arch Virol. 2016, 161, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qi, X.; Gao, Y.; Hua, Y.; Li, K.; Deng, X.; Wang, Q.; Zhang, L.; Chai, H.; Chen, Y.; et al. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet. Microbiol. 2013, 166, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bulow, V.V. Immunological effects of reticuloendotheliosis virus as potential contaminant of Marek’s disease vaccines. Avian Pathol. 1977, 6, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Lee, L.F.; Bacon, L.D.; Smith, E.J. Depression of vaccinal immunity to marek’s disease by infection with reticuloendotheliosis virus. Infect. Immun. 1979, 26, 90–98. [Google Scholar] [PubMed]
- Yang, L.; Shuai, C.; Cui, Z.; Shuang, C.; Peng, Z. Genome analysis and pathogenicity of reticuloendotheliosis virus isolated from a contaminated vaccine seed against infectious bursal disease virus, first report in China. J. Gen. Virol. 2016, 97, 2809–2815. [Google Scholar]
- Fadly, A.; Garcia, M.C. Detection of reticuloendotheliosis virus in live virus vaccines of poultry. Dev. Biol. (Basel) 2006, 126, 301–305; discussion 327. [Google Scholar] [PubMed]
- Garcia, M.; Narang, N.; Reed, W.M.; Fadly, A.M. Molecular characterization of reticuloendotheliosis virus insertions in the genome of field and vaccine strains of fowl poxvirus. Avian Dis. 2003, 47, 343–354. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, J.; Su, J.; Pu, J.; Zhang, G.; Liu, J. Full genome sequences of two reticuloendotheliosis viruses contaminating commercial vaccines. Avian Dis. 2009, 53, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tadese, T.; Fitzgerald, S.; Reed, W.M. Detection and differentiation of re-emerging fowlpox virus (FWPV) strains carrying integrated reticuloendotheliosis virus (FWPV-REV) by real-time PCR. Vet. Microbiol. 2008, 127, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Li, Q.; Li, H.; Xia, Y.; Liu, D.; Yu, K.; Yang, H. Complete genome sequence of reticuloendotheliosis virus strain MD-2, isolated from a contaminated turkey herpesvirus vaccine. Genome Announc. 2013, 1, e00785-13. [Google Scholar] [CrossRef] [PubMed]
- Wozniakowski, G.; Mamczur, A.; Samorek-Salamonowicz, E. Common occurrence of gallid herpesvirus-2 with reticuloendotheliosis virus in chickens caused by possible contamination of vaccine stocks. J. Appl. Microbiol. 2015, 118, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Yang, C.; Li, Q.; Cui, Z.; Chang, S.; Zhao, P.; Yu, K.; Yang, H. Isolation, identification, and whole genome sequencing of reticuloendotheliosis virus from a vaccine against Marek’s disease. Poult. Sci. 2015, 94, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Peterson, I.L.; Smith, E.J.; Johnson, D.C. Serologic evidence in commercial chicken and turkey flocks of infection with reticuloendotheliosis virus. Avian Dis. 1982, 26, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.T.; Gao, Y.L.; Pan, W.; Deng, X.Y.; Sun, F.F.; Kai, L.I.; Xiao-Le, Q.I.; Gao, H.L.; Liu, C.N.; Wang, X.M. Investigation of co-infection of ALV-J with REV, MDV, CAV in layer chicken flocks in some regions of China. Chin. J. Prev. Vet. Med. 2010, 32, 90–93. [Google Scholar]
- Deng, X.Y.; Qi, X.L.; Gao, Y.L.; Qin, L.T.; Gao, L.; Wu, G.; Wang, Y.Q.; Gao, H.L.; Wang, X.M.; XiaoYun, D.; et al. Molecular characteristics of gp90 gene of 14 reticuloendotheliosis viruses isolated in China. Agric. Sci. Technol. 2011, 12, 1954–1957. [Google Scholar]
- Peng, Z.; Ma, C.T.; Yan, D.; Wu, Z.C.; Cui, Z.Z. Serological survey of the reticuloendotheliosis virus infection in China native chicken flocks. Pak. Vet. J. 2012, 32, 621–623. [Google Scholar]
- Bao, K.Y.; Zhang, Y.P.; Zheng, H.W.; Lv, H.C.; Gao, Y.L.; Wang, J.F.; Gao, H.L.; Qi, X.L.; Cui, H.Y.; Wang, Y.Q.; et al. Isolation and full-genome sequence of two reticuloendotheliosis virus strains from mixed infections with Marek’s disease virus in China. Virus Genes 2015, 50, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Bao, K.Y.; Sun, G.R.; Lv, H.C.; Cui, H.Y.; Gao, Y.L.; Wang, X.M.; Liu, C.J. Characterization of a gallid herpesvirus 2 strain with novel reticuloendotheliosis virus long terminal repeat inserts. Virus Genes 2017, 53, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cui, N.; Cui, Z.; Zhao, P.; Li, Y.; Ding, J.; Dong, X. Complete genome sequence of a recombinant Marek’s disease virus field strain with one reticuloendotheliosis virus long terminal repeat insert. J. Virol. 2012, 86, 13818–13819. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhuang, G.; Xu, X.; Sun, A.; Su, S. Molecular and biological characterization of a Marek’s disease virus field strain with reticuloendotheliosis virus LTR insert. Virus Genes 2010, 40, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Cui, H.Y.; Niu, J.W.; Yan, S.; Gao, M.; Yang, H.Y.; Zhao, Y.; Wang, Y.F. Preparation and identification of monoclonal antibody against glycoprotein e of Marek’s disease virus. Chin. Vet. Sci. 2013, 43, 510–514. [Google Scholar]
- Shi, X.M.; Zhang, J.; Zhao, Y.; Wang, M.; Wei, X.Y.; Hu, S.-L.; Quan, Y.M.; Yan, S.; Cui, H.Y.; Wang, Y.F. Development and identification of the monoclonal antibodies against recombinant reticuloendotheliosis virus gp90 protein. Chin. J. Anim. Vet. Sci. 2011, 42, 1289–1294. [Google Scholar]
- Eidson, C.S.; Richey, D.J.; Schmittle, S.C. Studies on acute marek’s disease. Xi. Propagation of the GA isolate of Marek’s disease in tissue culture. Avian Dis. 1969, 13, 636–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.J.; Qin, Y.A.; Zhang, Y.P.; Zhang, X.W.; Hao, Y.Q. Application of duplex fluorescent quantitative polymerase-chain-reaction for detecting Marek’s disease virus serotype 1. Chin. J. Prev. Vet. Med. 2007, 29, 46–51. [Google Scholar]
- Sharma, J.M.; Burmester, B.R. Resistance to Marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. 1982, 26, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Liu, Q.; Qiu, B.; Liu, G.Z.; Cheng, Z.Q. Mixed infection of ALV-J and MDV in a flock of shandong free range chickens. Chin. J. Anim. Vet. Sci. 2009, 40, 1215–1221. [Google Scholar]
- Zhang, K.; Gong, Z.; Liu, K.; Wang, L.; Liu, G.; Li, L.; Zhang, L.; Hou, G.; Zhang, X.; Shan, H. Epidemiological investigation on infectious tumor diseases of poultry in north part of east China. China Anim. Health Insp. 2013, 30, 55–57. [Google Scholar]
- Wei, K.; Sun, Z.; Zhu, S.; Guo, W.; Sheng, P.; Wang, Z.; Zhao, C.; Zhao, Q.; Zhu, R. Probable congenital transmission of reticuloendotheliosis virus caused by vaccination with contaminated vaccines. PLoS ONE 2012, 7, e43422. [Google Scholar] [CrossRef] [PubMed]
- Fadly, A.M.; Witter, R.L.; Smith, E.J.; Silva, R.F.; Reed, W.M.; Hoerr, F.J.; Putnam, M.R. An outbreak of lymphomas in commercial broiler breeder chickens vaccinated with a fowlpox vaccine contaminated with reticuloendotheliosis virus. Avian Pathol. 1996, 25, 35–47. [Google Scholar] [CrossRef] [PubMed]

| Vaccine | Challenge | MD Incidence Diseased/Total (%) | PI | Mortality Deaths/Total (%) | Time (dpc) a |
|---|---|---|---|---|---|
| - | - | 0/15 (0%) | - | 0/15 (0%) | - |
| - | REV | 0/15 (0%) | - | 0/15 (0%) | - |
| - | MDV | 30/30 (100%) | - | 23/30 (76.7%) | 27 |
| CVI988 | MDV | 6/30 (20.0%) | 80.0 | 5/30 (16.7%) | 51 |
| 814 | MDV | 3/30 (10.0%) | 90.0 | 2/30 (6.7%) | 72 |
| - | MDV + REV | 30/30 (100%) | - | 29/30 (96.7%) | 17 |
| CVI988 | MDV + REV | 26/30 (53.3%) | 46.7 | 9/30 (30.0%) | 30 |
| 814 | MDV + REV | 7/30 (23.3%) | 76.7 | 6/30 (20.0%) | 28 |
| Vaccine | Challenge | Total (%) a | Multiple Tumors (%) b | Proventriculus (%) c | Heart (%) c | Liver (%) c | Spleen (%) c | Kidney (%) c | Gonad (%) c |
|---|---|---|---|---|---|---|---|---|---|
| - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| - | REV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| - | MDV | 53.3 | 23.3 | 30.0 | 6.7 | 36.7 | 16.7 | 6.7 | 0 |
| CVI988 | MDV | 10.0 | 10.0 | 0 | 0 | 10.0 | 10.0 | 0 | 0 |
| 814 | MDV | 6.7 | 0 | 3.3 | 0 | 3.3 | 0 | 0 | 0 |
| - | MDV + REV | 80.0 | 56.6 | 56.6 | 0 | 73.3 | 43.3 | 60.0 | 6.7 |
| CVI988 | MDV + REV | 6.7 | 6.7 | 6.7 | 0 | 6.7 | 6.7 | 0 | 0 |
| 814 | MDV + REV | 16.7 | 16.7 | 16.7 | 0 | 16.7 | 16.7 | 16.7 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.-R.; Zhang, Y.-P.; Zhou, L.-Y.; Lv, H.-C.; Zhang, F.; Li, K.; Gao, Y.-L.; Qi, X.-L.; Cui, H.-Y.; Wang, Y.-Q.; et al. Co-Infection with Marek’s Disease Virus and Reticuloendotheliosis Virus Increases Illness Severity and Reduces Marek’s Disease Vaccine Efficacy. Viruses 2017, 9, 158. https://doi.org/10.3390/v9060158
Sun G-R, Zhang Y-P, Zhou L-Y, Lv H-C, Zhang F, Li K, Gao Y-L, Qi X-L, Cui H-Y, Wang Y-Q, et al. Co-Infection with Marek’s Disease Virus and Reticuloendotheliosis Virus Increases Illness Severity and Reduces Marek’s Disease Vaccine Efficacy. Viruses. 2017; 9(6):158. https://doi.org/10.3390/v9060158
Chicago/Turabian StyleSun, Guo-Rong, Yan-Ping Zhang, Lin-Yi Zhou, Hong-Chao Lv, Feng Zhang, Kai Li, Yu-Long Gao, Xiao-Le Qi, Hong-Yu Cui, Yong-Qiang Wang, and et al. 2017. "Co-Infection with Marek’s Disease Virus and Reticuloendotheliosis Virus Increases Illness Severity and Reduces Marek’s Disease Vaccine Efficacy" Viruses 9, no. 6: 158. https://doi.org/10.3390/v9060158





