Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Quantitative Reverse Transcription (qRT)-PCR
2.3. ELISA
2.4. Dengue Virus
2.5. Garlic Treatment
2.6. Lipid Peroxidation Assay
2.7. Western Blot
3. Results
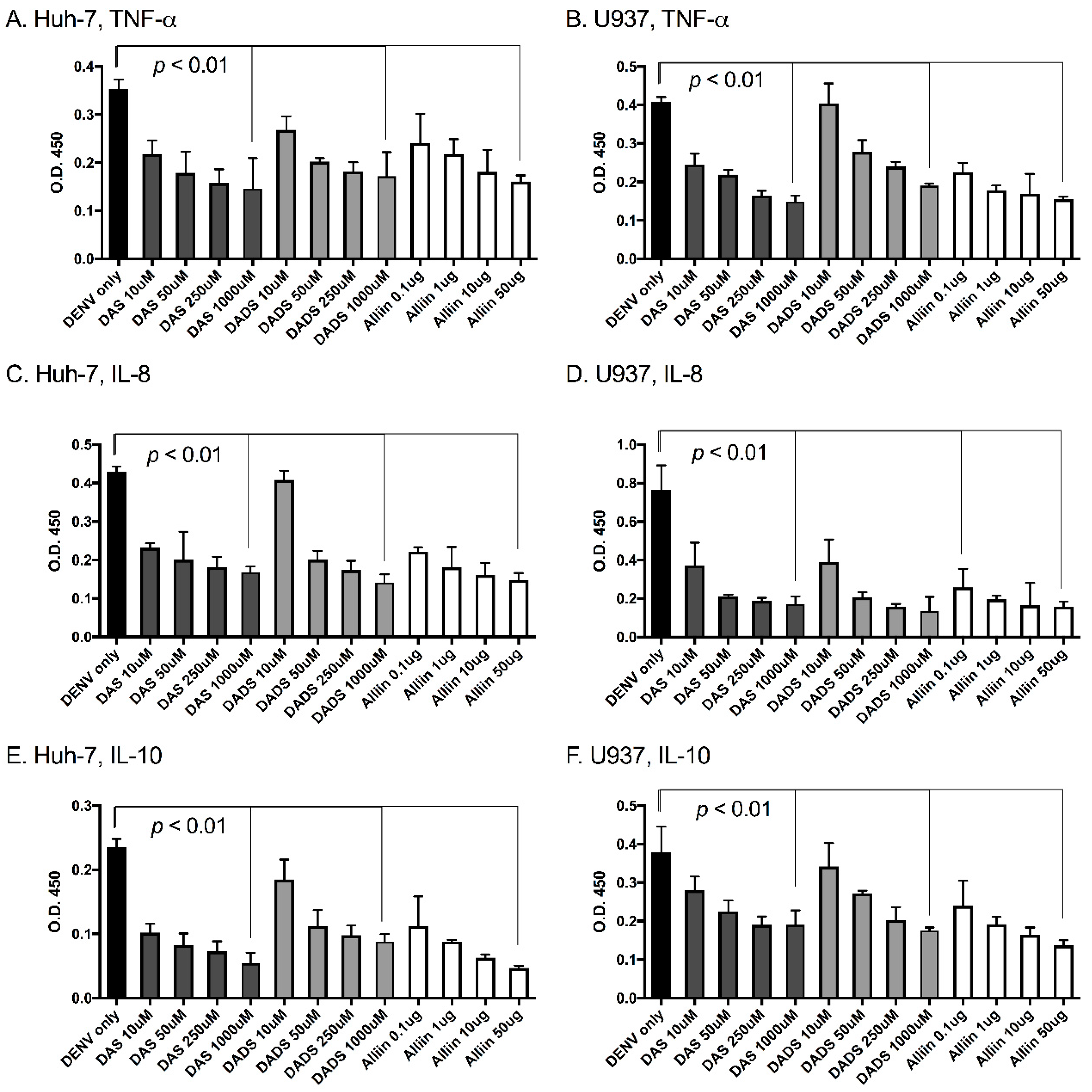
3.1. Garlic Organosulfur Compounds Reduce Inflammation during Dengue Virus Infection
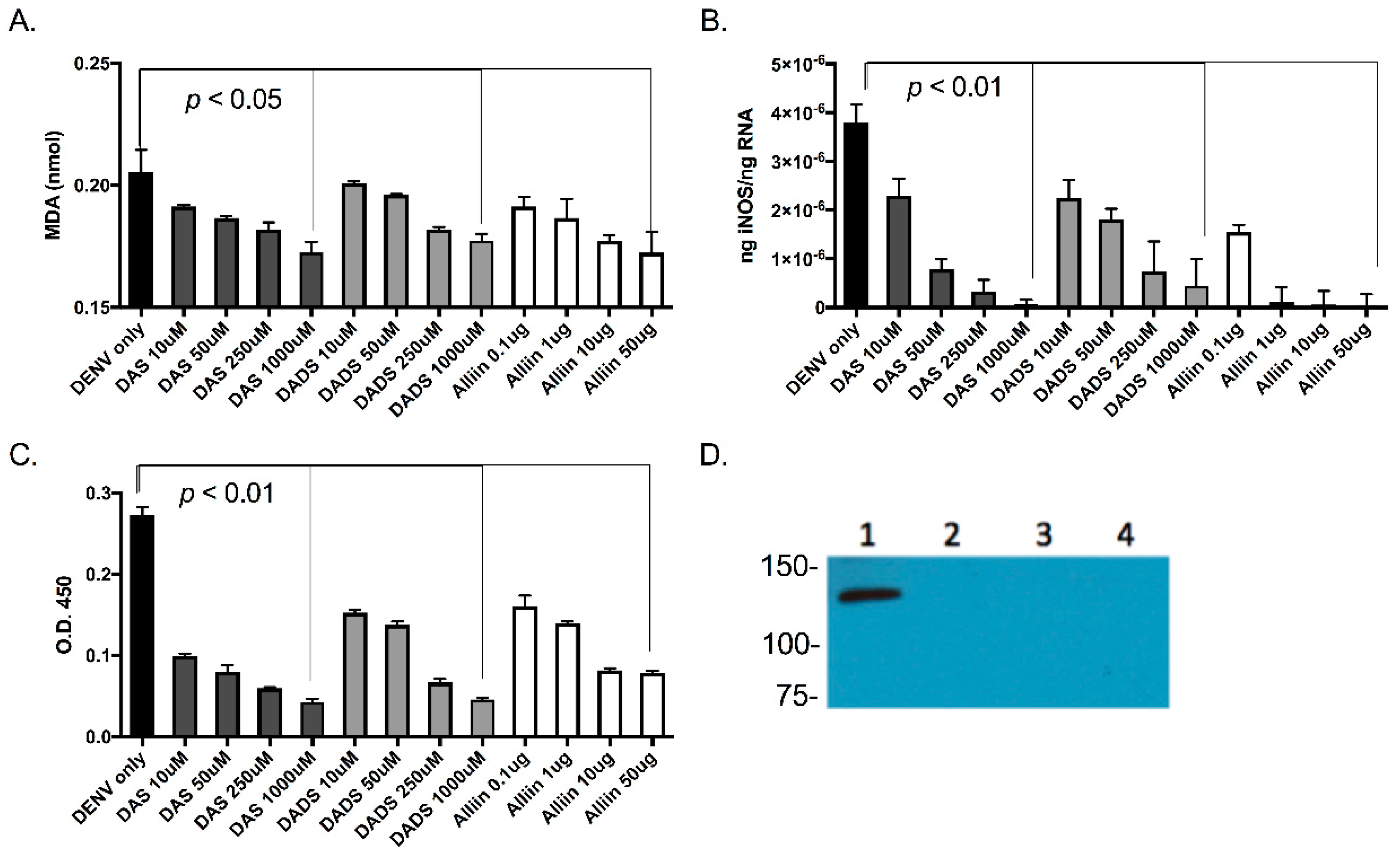
3.2. Garlic Organosulfur Compounds Reduce Oxidative Stress during Dengue Virus Infection
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schafer, G.; Kaschula, C.H. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Arab, L. Garlic and cancer: A critical review of the epidemiologic literature. J. Nutr. 2001, 131, 1032S–1040S. [Google Scholar] [PubMed]
- Ide, N.; Lau, B.H.; Ryu, K.; Matsuura, H.; Itakura, Y. Antioxidant effects of fructosyl arginine, a Maillard reaction product in aged garlic extract. J. Nutr. Biochem. 1999, 10, 372–376. [Google Scholar] [CrossRef]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis 2007, 12, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Won Yang, J.; Kim, T.H.; Lee, D.Y.; Kang, B.; Ryu, J.H.; Jeon, R.; Kim, S.G. Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J. Nutr. 2010, 140, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Keophiphath, M.; Priem, F.; Jacquemond-Collet, I.; Clement, K.; Lacasa, D. 1,2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J. Nutr. 2009, 139, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Lee da, Y.; Li, H.; Lim, H.J.; Lee, H.J.; Jeon, R.; Ryu, J.H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food 2012, 15, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.E.; Stone, A.; Fitzsimons, D.W.; Hanna, J.N.; Lam, S.K.; Vong, S.; Guzman, M.G.; Mendez-Galvan, J.F.; Halstead, S.B.; Letson, G.W.; et al. Best practices in dengue surveillance: A report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl. Trop. Dis. 2010, 4, e890. [Google Scholar] [CrossRef] [PubMed]
- Mangold, K.A.; Reynolds, S.L. A review of Dengue fever: A resurging tropical disease. Pediatr. Emerg. Care 2013, 29, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.V.; Vaughn, D.W. Dengue: An escalating problem. BMJ 2002, 324, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Flasche, S.; Jit, M.; Rodriguez-Barraquer, I.; Coudeville, L.; Recker, M.; Koelle, K.; Milne, G.; Hladish, T.J.; Perkins, T.A.; Cummings, D.A.; et al. The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study. PLoS Med. 2016, 13, e1002181. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.T.; Costa, V.V.; Cisalpino, D.; Amaral, F.A.; Souza, P.R.; Souza, R.S.; Ryffel, B.; Vieira, L.Q.; Silva, T.A.; Atrasheuskaya, A.; et al. IFN-γ production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl. Trop. Dis. 2011, 5, e1449. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; Fagundes, C.T.; Valadao, D.F.; Cisalpino, D.; Dias, A.C.; Silveira, K.D.; Kangussu, L.M.; Avila, T.V.; Bonfim, M.R.; Bonaventura, D.; et al. A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-γ in host resistance to infection. PLoS Negl. Trop. Dis. 2012, 6, e1663. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Endy, T.P.; Nisalak, A.; Chunsuttiwat, S.; Vaughn, D.W.; Libraty, D.H.; Green, S.; Ennis, F.A.; Rothman, A.L. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J. Infect. Dis. 2002, 185, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Chaturvedi, U.C.; Al-Sayer, H.; Elbishbishi, E.A.; Agarwal, R.; Nagar, R.; Kapoor, S.; Misra, A.; Mathur, A.; Nusrat, H.; et al. Elevated levels of IL-8 in dengue hemorrhagic fever. J. Med. Virol. 1998, 56, 280–285. [Google Scholar] [CrossRef]
- Tauseef, A.; Umar, N.; Sabir, S.; Akmal, A.; Sajjad, S.; Zulfiqar, S. Interleukin-10 as a Marker of Disease Progression in Dengue Hemorrhagic Fever. J. Coll. Phys. Surg. Pak. 2016, 26, 187–190. [Google Scholar]
- Nielsen, D.G. The relationship of interacting immunological components in dengue pathogenesis. Virol. J. 2009, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 2009, 102, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, E.; Despres, P.; Cedillo-Barron, L. Innate immune responses to dengue virus. Arch. Med. Res. 2005, 36, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Kalra, N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007, 247, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.C.T.; Haar, C.P.; Vandergrift, W.A., 3rd; Giglio, P.; Dixon-Mah, Y.N.; Varma, A.K.; Ray, S.K.; Patel, S.J.; Banik, N.L.; Das, A. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J. Neuro-Oncol. 2013, 114, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 2007, 110, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Soundravally, R.; Sankar, P.; Bobby, Z.; Hoti, S.L. Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidation. Platelets 2008, 19, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Quek, A.M.; Yeo, L.L.; Huang, S.H.; Halliwell, B. Oxidative damage in dengue fever. Free Radic. Biol. Med. 2009, 47, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Nagar, R. Dengue and dengue haemorrhagic fever: Indian perspective. J. Biosci. 2008, 33, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Soundravally, R.; Hoti, S.L.; Patil, S.A.; Cleetus, C.C.; Zachariah, B.; Kadhiravan, T.; Narayanan, P.; Kumar, B.A. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int. J. Infect. Dis. 2014, 18, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Park, H.J.; Jeong, Y.Y.; Han, S.; Shin, J.H.; Lee, S.J.; Kang, M.J.; Sung, N.J.; Kang, D. Aged Red Garlic Extract Suppresses Nitric Oxide Production in Lipopolysaccharide-Treated RAW 264.7 Macrophages Through Inhibition of NF-κB. J. Med. Food 2015, 18, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Ki, S.H.; Kim, Y.W.; Noh, K.; Lee da, Y.; Kang, B.; Ryu, J.H.; Jeon, R.; Kim, E.H.; Hwang, S.J.; et al. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid. Redox Signal. 2011, 14, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Chanas, A.C.; Hubalek, Z.; Johnson, B.K.; Simpson, D.I. A comparative study of O’nyong nyong virus with Chikungunya virus and plaque variants. Arch. Virol. 1979, 59, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Prithiviraj, B.; Sarma, B.K.; Singh, M.; Ray, A.B. Role of garlic (Allium sativum L.) in human and plant diseases. Indian J. Exp. Biol. 2001, 39, 310–322. [Google Scholar] [PubMed]
- Ho, C.Y.; Weng, C.J.; Jhang, J.J.; Cheng, Y.T.; Huang, S.M.; Yen, G.C. Diallyl sulfide as a potential dietary agent to reduce TNF-α- and histamine-induced proinflammatory responses in A7r5 cells. Mol. Nutr. Food Res. 2014, 58, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Gow, A.J.; Francis, M.; Abramova, E.V.; Laskin, J.D.; Laskin, D.L. Radiation-induced lung injury and inflammation in mice: Role of inducible nitric oxide synthase and surfactant protein D. Toxicol. Sci. 2015, 144, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Thein, T.L.; Wong, J.; Leo, Y.S.; Ooi, E.E.; Lye, D.; Yeo, T.W. Association Between Increased Vascular Nitric Oxide Bioavailability and Progression to Dengue Hemorrhagic Fever in Adults. J. Infect. Dis. 2015, 212, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Clark, G.G. Dengue/dengue hemorrhagic fever: The emergence of a global health problem. Emerg. Infect. Dis. 1995, 1, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.S.; Kliks, S. Antibody-dependent enhancement in dengue virus infections. J. Infect. Dis. 2006, 193, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Rodriguez, L.F.; Herrington, E.; Kharat, V.; Vasilakis, N.; Walker, C.; Turner, C.; Khuwaja, S.; Arafat, R.; Weaver, S.C.; et al. Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis. 2013, 13, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L.; Santiago, G.A.; Margolis, H.; Stark, L. Genetic relatedness of dengue viruses in Key West, Florida, USA, 2009–2010. Emerg. Infect. Dis. 2013, 19, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [PubMed]
- Atrasheuskaya, A.; Petzelbauer, P.; Fredeking, T.M.; Ignatyev, G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 2003, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Clyde, K.; Kyle, J.L.; Harris, E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J. Virol. 2006, 80, 11418–11431. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Fu, T.F.; Yeh, T.M. Macrophage migration inhibitory factor induced by dengue virus infection increases vascular permeability. Cytokine 2011, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Assuncao-Miranda, I.; Amaral, F.A.; Bozza, F.A.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J. 2010, 24, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.; Azeredo, E.L.; Nogueira, R.M.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex cytokine profile from dengue patients: MIP-1β and IFN-γ as predictive factors for severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Marques, R.E.; Besnard, A.G.; Fagundes, C.T.; Souza, D.G.; Ryffel, B.; Teixeira, M.M. Role of the chemokine receptors CCR1, CCR2 and CCR4 in the pathogenesis of experimental dengue infection in mice. PLoS ONE 2010, 5, e15680. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, A.; Troupin, A.; Londono-Renteria, B.; Colpitts, T.M. Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection. Viruses 2017, 9, 159. https://doi.org/10.3390/v9070159
Hall A, Troupin A, Londono-Renteria B, Colpitts TM. Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection. Viruses. 2017; 9(7):159. https://doi.org/10.3390/v9070159
Chicago/Turabian StyleHall, Alex, Andrea Troupin, Berlin Londono-Renteria, and Tonya M. Colpitts. 2017. "Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection" Viruses 9, no. 7: 159. https://doi.org/10.3390/v9070159






