Update on Senecavirus Infection in Pigs
Abstract
:1. Introduction
2. History, Classification, and Molecular Features
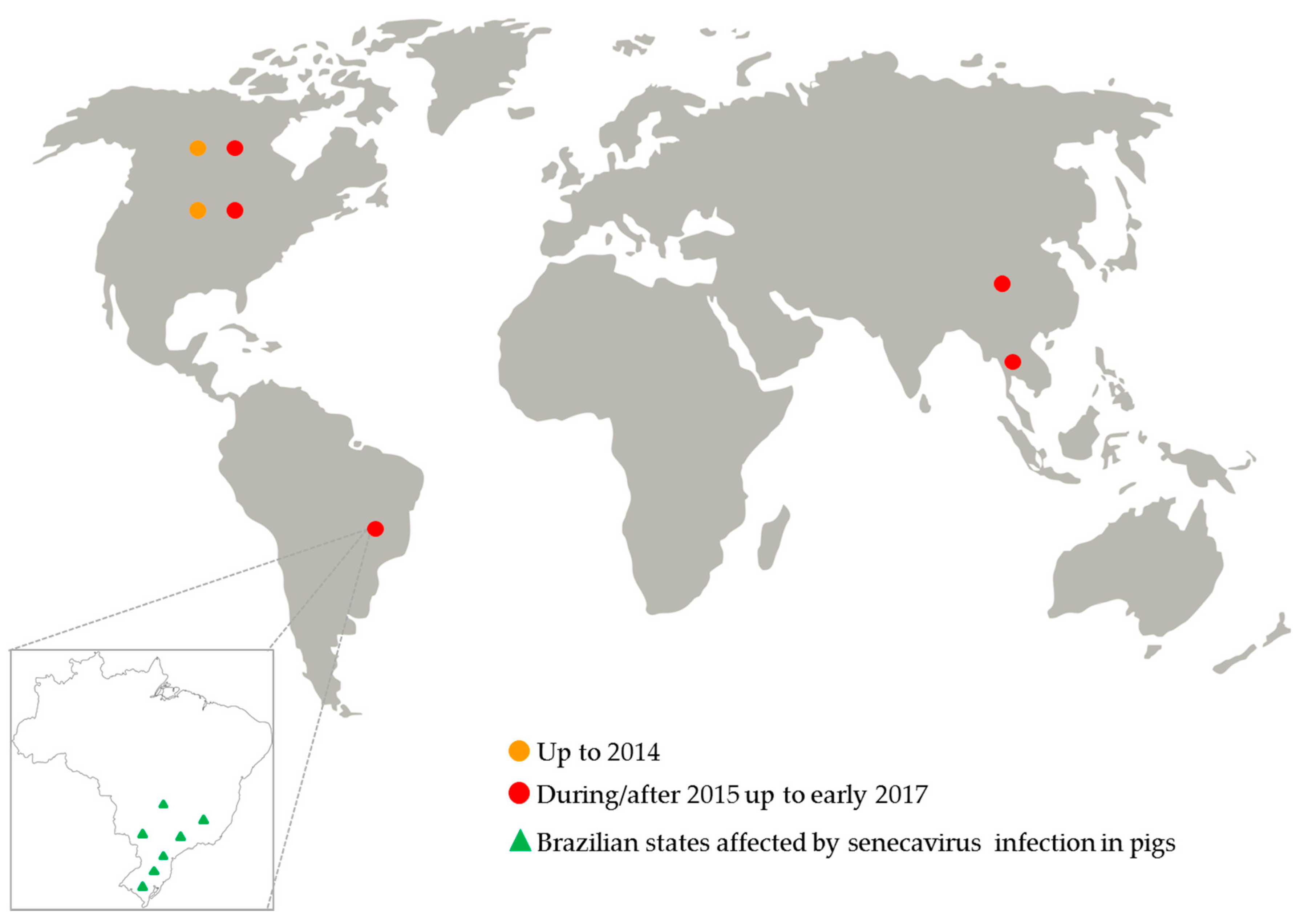
3. Senecavirus Infection in Swine Until 2014
4. Senecavirus Infection in Swine during/after 2015
4.1. Epidemiology of Senecavirus Infection
4.2. Pathogenesis Evidence of Senecavirus
4.3. Immunological Response against Senecavirus
4.4. Senecavirus Diagnosis
4.5. Prophylaxis and Control Management
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knowles, N.J.; Hallenbeck, P.L. A new picornavirus is most closely related to cardioviruses. In Proceedings of the EUROPIC 2005: XIIIth Meeting of the European Study Group on the Molecular Biology of Picornaviruses, Lunteren, The Netherlands, 23–29 May 2005; p. A14. [Google Scholar]
- Segales, J.; Barcellos, D.; Alfieri, A.; Burrough, E.; Marthaler, D. Senecavirus A: An Emerging Pathogen Causing Vesicular Disease and Mortality in Pigs? Vet. Pathol. 2017, 54, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hales, L.M.; Knowles, N.J.; Reddy, P.S.; Xu, L.; Hay, C.; Hallenbeck, P.L. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J. Gen. Virol. 2008, 89, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Reddy, S.P.; Loo, J.; Idamakanti, N.; Hallenbeck, P.L.; Reddy, V.S. Structure of Seneca Valley virus-001: An oncolytic picornavirus representing a new genus. Structure 2008, 16, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Willcocks, M.M.; Locker, N.; Gomwalk, Z.; Royall, E.; Bakhshesh, M.; Belsham, G.J.; Idamakanti, N.; Burroughs, K.D.; Reddy, P.S.; Hallenbeck, P.L.; et al. Structural features of the Seneca Valley virus internal ribosome entry site (IRES) element: A picornavirus with a pestivirus-like IRES. J. Virol. 2011, 85, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses. Virus Taxonomy: 2015 Release. Available online: http://www.ictvonline.org/virustaxonomy.asp (accessed on 10 January 2017).
- Flather, D.; Semler, B.L. Picornaviruses and nuclear functions: Targeting a cellular compartment distinct from the replication site of a positive-strand RNA virus. Front. Microbiol. 2015, 6, 594. [Google Scholar] [CrossRef] [PubMed]
- Hellen, C.U.; de Breyne, S. A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: Evidence for modular exchange of functional noncoding RNA elements by recombination. J. Virol. 2007, 81, 5850–5863. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salas, E.; Francisco-Velilla, R.; Fernandez-Chamorro, J.; Lozano, G.; Diaz-Toledano, R. Picornavirus IRES elements: RNA structure and host protein interactions. Virus Res. 2015, 206, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Burroughs, K.D.; Hales, L.M.; Ganesh, S.; Jones, B.H.; Idamakanti, N.; Hay, C.; Li, S.S.; Skele, K.L.; Vasko, A.J.; et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl. Cancer Inst. 2007, 99, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Pasma, T.; Davidson, S.; Shaw, S.L. Idiopathic vesicular disease in swine in Manitoba. Can. Vet. J. 2008, 49, 84–85. [Google Scholar] [PubMed]
- Singh, K.; Corner, S.; Clark, S.G.; Scherba, G.; Fredrickson, R. Seneca Valley virus and vesicular lesions in a pig with idiopathic vesicular disease. J. Vet. Sci. Technol. 2012, 3, 1–3. [Google Scholar]
- United States Animal Health Association. Committee on Transmissible Diseases of Swine—Research on Seneca Valley Virus. Available online: http://www.usaha.org/Portals/6/Resolutions/2012/resolution14-2012.pdf (accessed on 21 March 2015).
- Montgomery, J.F.; Oliver, R.E.; Poole, W.S. A vesiculo-bullous disease in pigs resembling foot and mouth disease. I. Field cases. N. Z. Vet. J. 1987, 35, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.F.; Oliver, R.E.; Poole, W.S.; Julian, A.F. A vesiculo-bullous disease in pigs resembling foot and mouth disease. II. Experimental reproduction of the lesion. N. Z. Vet. J. 1987, 35, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Munday, B.L.; Ryan, F.B. Vesicular lesions in swine—Possible association with the feeding of marine products. Aust. Vet. J. 1982, 59, 193. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.P.J.; Stoddard, H.L.; Yedloutchnig, R.J.; House, J.A.; Legge, M. A vesicular disease of pigs in Florida of unknown etiology. Fla. Vet. J. 1983, 12, 25–27. [Google Scholar]
- International Society for Infectious Diseases. Vesicular Disease, Porcine UK (N. Ireland): Not FMD, SVD. ProMED-Mail Archive Number: 20070110.0099. Available online: http://www.promedmail.org/direct.php?id=6471 (accessed on 8 February 2015).
- Sensi, M.; Catalano, A.; Tinaro, M.; Mariotti, C.; Panzieri, C.; Marchi, S.; Costarelli, S. Idiopathic vesicular disease (IVD): A case report in the centre of Italy. In Proceedings of the 21st International Pig Veterinary Society (IPVS) Congress, Vancouver, BC, Canada, 18–21 July 2010; p. 46. [Google Scholar]
- Leme, R.A.; Zotti, E.; Alcantara, B.K.; Oliveira, M.V.; Freitas, L.A.; Alfieri, A.F.; Alfieri, A.A. Senecavirus A: An emerging vesicular infection in Brazilian pig herds. Transbound. Emerg. Dis. 2015, 62, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Amass, S.F.; Schneider, J.L.; Miller, C.A.; Shawky, S.A.; Stevenson, G.W.; Woodruff, M.E. Idiopathic vesicular disease in a swine herd in Indiana. J. Swine Health Prod. 2004, 12, 192–196. [Google Scholar]
- Vannucci, F.A.; Linhares, D.C.; Barcellos, D.E.; Lam, H.C.; Collins, J.; Marthaler, D. Identification and complete genome of Seneca Valley virus in vesicular fluid and sera of pigs affected with idiopathic vesicular disease, Brazil. Transbound. Emerg. Dis. 2015, 62, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Oliveira, T.E.S.; Alcântara, B.K.; Headley, S.A.; Alfieri, A.F.; Yang, M.; Alfieri, A.A. Clinical manifestations of Senecavirus A infection in neonatal pigs, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Oliveira, T.E.; Alfieri, A.F.; Headley, S.A.; Alfieri, A.A. Pathological, immunohistochemical and molecular findings associated with Senecavirus A-induced lesions in neonatal piglets. J. Comp. Pathol. 2016, 155, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Headley, S.A.; Oliveira, T.E.; Yang, M.; Gomes, R.; Feronato, C.; Alfieri, A.F.; Alfieri, A.A. Molecular, pathological, and immunohistochemical evidence of Senecavirus A-induced infections in pigs of different age groups with vesicular disease from Brazil. In Proceedings of the Allen D. Leman Swine Conference, Saint Paul, MN, USA, 19–22 September 2015; p. 26. [Google Scholar]
- Ministry of Agriculture, Livestock, and Food Supply. Circular n°12/2015, in 13 February 2015. Ocurrence of Pig Mortality. Available online: http://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/arquivos-publicacoes-dipoa/relatorio-de-gestao-dipoa-atualizado.pdf (accessed on 14 July 2015).
- Laguardia-Nascimento, M.; Gasparini, M.R.; Sales, E.B.; Rivetti, A.V., Jr.; Sousa, N.M.; Oliveira, A.M.; Camargos, M.F.; Pinheiro de Oliveira, T.F.; Goncalves, J.P.; Madureira, M.C.; et al. Molecular epidemiology of Senecavirus A associated with vesicular disease in pigs in Brazil. Vet. J. 2016, 216, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Saporiti, V.; Fritzen, J.T.T.; Feronato, C.; Leme, R.A.; Alfieri, A.F.; Alfieri, A.A. A ten years (2007–2016) retrospective serological survey for Senecavirus A infection in Brazilian pig herds. Vet. Res. Commun. Under review.
- Wu, Q.; Zhao, X.; Chen, Y.; He, X.; Zhang, G.; Ma, J. Complete genome sequence of Seneca Valley virus CH-01-2015 identified in China. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, X.; Bai, Y.; Sun, B.; Xie, Q.; Ma, J. The first identification and complete genome of Senecavirus A affecting pig with Idiopathic Vesicular Disease in China. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, W.; Qian, P.; Chen, H.; Li, X. Isolation and full-genome sequencing of Seneca Valley virus in piglets from China, 2016. Virol. J. 2016, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Fernandes, M.H.; Clement, T.; Lawson, S.; Pillatzki, A.; Resende, T.P.; Vannucci, F.A.; Kutish, G.F.; Nelson, E.A.; Diel, D.G. Pathogenesis of Senecavirus A infection in finishing pigs. J. Gen. Virol. 2016, 97, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Canon, A.; Bates, J.L.; Gerardy, K.; Linhares, D.C.; Pineyro, P.E.; Schwartz, K.J.; Yoon, K.J.; Rademacher, C.J.; Holtkamp, D.; et al. Neonatal mortality, vesicular lesions and lameness associated with Senecavirus A in a U.S. sow farm. Transbound. Emerg. Dis. 2016, 63, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Lirola, L.G.; Rademacher, C.; Linhares, D.; Harmon, K.; Rotolo, M.; Sun, Y.; Baum, D.H.; Zimmerman, J.; Pineyro, P. Serological and molecular detection of Senecavirus A associated with an outbreak of swine idiopathic vesicular disease and neonatal mortality. J. Clin. Microbiol. 2016, 54, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- International Society for Infectious Diseases. Senecavirus A Canada: (Ontario) Swine, 2016. ProMED-Mail Archive Number: 20161009.4546471. Available online: http://www.promedmail.org/direct.php?id=20161009.4546471 (accessed on 28 December 2016).
- Saeng-Chuto, K.; Rodtian, P.; Temeeyasen, G.; Wegner, M.; Nilubol, D. The first detection of Senecavirus A in pigs in Thailand, 2016. Transbound. Emerg. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.L.; Mowrer, C.; Canon, A.; Linhares, D.C.; Rademacher, C.; Karriker, L.A.; Holtkamp, D.J. Systematic epidemiological investigations of cases of Senecavirus A in US swine breeding herds. Transbound. Emerg. Dis. 2017, 64, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Myers, O.; Duff, J.; Hesse, R.A. Senecavirus A in Pigs, United States, 2015. Emerg. Infect. Dis. 2016, 22, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Pineyro, P.E.; Rademacher, C.J.; Zheng, Y.; Li, G.; Yuan, J.; Hoang, H.; Gauger, P.C.; Madson, D.M.; Schwartz, K.J.; et al. Novel Senecavirus A in swine with vesicular disease, United States, July 2015. Emerg. Infect. Dis. 2016, 22, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Mohr, K.A.; Clement, T.; Hain, K.S.; Myers, B.; Yaros, J.; Nelson, E.A.; Christopher-Hennings, J.; Gava, D.; Schaefer, R.; et al. Detection of the emerging picornavirus Senecavirus A in pigs, mice, and houseflies. J. Clin. Microbiol. 2016, 54, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pineyro, P.; Chen, Q.; Zheng, Y.; Li, G.; Rademacher, C.; Derscheid, R.; Guo, B.; Yoon, K.J.; Madson, D.; et al. Full-length genome sequences of Senecavirus A from recent idiopathic vesicular disease outbreaks in U.S. swine. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Prarat, M.; Hayes, J.; Zhang, Y. Detection and genomic characterization of Senecavirus A, Ohio, USA, 2015. Emerg. Infect. Dis. 2016, 22, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Q.; Bai, Y.; Chen, G.; Zhou, L.; Wu, Z.; Li, Y.; Zhou, W.; Yang, H.; Ma, J. Phylogenetic and genome analysis of seven Senecavirus A isolates in China. Transbound. Emerg. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Montiel, N.; Buckley, A.; Guo, B.; Kulshreshtha, V.; VanGeelen, A.; Hoang, H.; Rademacher, C.; Yoon, K.J.; Lager, K. Vesicular disease in 9-week-old pigs experimentally infected with Senecavirus A. Emerg. Infect. Dis. 2016, 22, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; van Bruggen, R.; Xu, W. Generation and diagnostic application of monoclonal antibodies against Seneca Valley virus. J. Vet. Diagn. Investig. 2012, 24, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bracht, A.J.; O’Hearn, E.S.; Fabian, A.W.; Barrette, R.W.; Sayed, A. Real-Time reverse transcription PCR assay for detection of Senecavirus A in swine vesicular diagnostic specimens. PLoS ONE 2016, 11, e0146211. [Google Scholar] [CrossRef] [PubMed]
- Dall Agnol, A.M.; Otonel, R.A.; Leme, R.A.; Alfieri, A.A.; Alfieri, A.F. A TaqMan-based qRT-PCR assay for Senecavirus A detection in tissue samples of neonatal piglets. Mol. Cell. Probes 2017. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.L.; Ransburgh, R.H.; Poulsen, E.G.; Wadsworth, J.; King, D.P.; Mioulet, V.; Knowles, N.J.; Williamson, S.; Liu, X.; Anderson, G.A.; et al. Development of a novel real-time RT-PCR assay to detect Seneca Valley virus-1 associated with emerging cases of vesicular disease in pigs. J. Virol. Methods 2017, 239, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, P.; Rademacher, C.; Derscheid, R.J.; Guo, B.; Yoon, K.J.; Zhang, J.; Harmon, K.M.; Magstadt, D.R.; Gauger, P.C.; Chen, Q.; et al. Senecavirus A vesicular disease outbreak in Midwest show and commercial pigs. In Proceedings of the 58th Annual Conference of the American Association of Veterinary Laboratory Diagnosticians, Providence, RI, USA, 22–28 October 2015; 2015. [Google Scholar]
- Resende, T.P.; Marthaler, D.G.; Vannucci, F.A. A novel RNA-based in situ hybridization to detect Seneca Valley virus in neonatal piglets and sows affected with vesicular disease. PLoS ONE 2017, 12, e0173190. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. Development and Otimisation of Antibody Detection Assays. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.6.01_ANTIBODY_DETECT.pdf (accessed on 10 April 2017).
- Dvorak, C.M.; Akkutay-Yoldar, Z.; Stone, S.R.; Tousignant, S.J.; Vannucci, F.A.; Murtaugh, M.P. An indirect enzyme-linked immunosorbent assay for the identification of antibodies to Senecavirus A in swine. BMC Vet. Res. 2017, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Goolia, M.; Vannucci, F.; Yang, M.; Patnayak, D.; Babiuk, S.; Nfon, C.K. Validation of a competitive ELISA and a virus neutralization test for the detection and confirmation of antibodies to Senecavirus A in swine sera. J. Vet. Diagn. Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zanella, J.R.C.; Morés, N. Epidemic Transient Neonatal Losses and Vesicular Disease Associated with Seneca Valley Virus (Senecavirus A) Infection. Available online: http://www.infoteca.cnptia.embrapa.br/handle/doc/1040626 (accessed on 9 July 2016).
- World Organisation for Animal Health. Technical Disease Card: Foot-and-Mouth Disease. Available online: http://www.oie.int/animal-health-in-the-world/technical-disease-cards/ (accessed on 10 January 2017).
- Singh, A.; Mor, S.K.; Aboubakr, H.; Vannucci, F.; Patnayak, D.P.; Goyal, S.M. Efficacy of three disinfectants against Senecavirus A on five surfaces and at two temperatures. J. Swine Health Prod. 2017, 25, 64–68. [Google Scholar]
- Hole, K.; Ahmadpour, F.; Krishnan, J.; Stansfield, C.; Copps, J.; Nfon, C. Efficacy of accelerated hydrogen peroxide disinfectant on foot-and-mouth disease virus, swine vesicular disease virus and Senecavirus A. J. Appl. Microbiol. 2017, 122, 634–639. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Veterinary Service Guidance (7406.2)—Recommendations for Swine with Potential Vesicular Disease. Available online: https://www.aasv.org/documents/VSG7406.2_SVA_Guidelines_4516.pdf (accessed on 25 June 2016).
- Ministry of Agriculture, Livestock, and Food Supply. Circular n°18/2015/CGI/DIPOA/SDA, in 07 July 2015. Establish Guideline Procedures to Be Adopted in Cases of Vesicular Lesions in Swine. Available online: http://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/arquivos-publicacoes-dipoa/relatorio-de-gestao-dipoa-atualizado.pdf (accessed on 7 December 2015).
- Meng, X.J. Emerging and re-emerging swine viruses. Transbound. Emerg. Dis. 2012, 59 (Suppl. 1), 85–102. [Google Scholar] [CrossRef] [PubMed]
- Segales, J. Emerging swine diseases and infections: An increasing zoonotic threat. In Proceedings of the Safepork 2015 Conference, Porto, Portugal, 7–10 September 2015; pp. 97–100. [Google Scholar]
- Morales, R.G.; Umandal, A.C.; Lantican, C.A. Emerging and Re-emerging diseases in Asia and the Pacific with special emphasis on porcine epidemic diarrhoea. In Proceedings of the 25th Conference of the OIE Regional Commission for Asia, the Far East and Oceania, Queenstown, New Zealand, 26–30 November 2007; pp. 185–189. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leme, R.A.; Alfieri, A.F.; Alfieri, A.A. Update on Senecavirus Infection in Pigs. Viruses 2017, 9, 170. https://doi.org/10.3390/v9070170
Leme RA, Alfieri AF, Alfieri AA. Update on Senecavirus Infection in Pigs. Viruses. 2017; 9(7):170. https://doi.org/10.3390/v9070170
Chicago/Turabian StyleLeme, Raquel A., Alice F. Alfieri, and Amauri A. Alfieri. 2017. "Update on Senecavirus Infection in Pigs" Viruses 9, no. 7: 170. https://doi.org/10.3390/v9070170





