Chromosomally Integrated Human Herpesvirus 6: Models of Viral Genome Release from the Telomere and Impacts on Human Health
Abstract
:1. Introduction
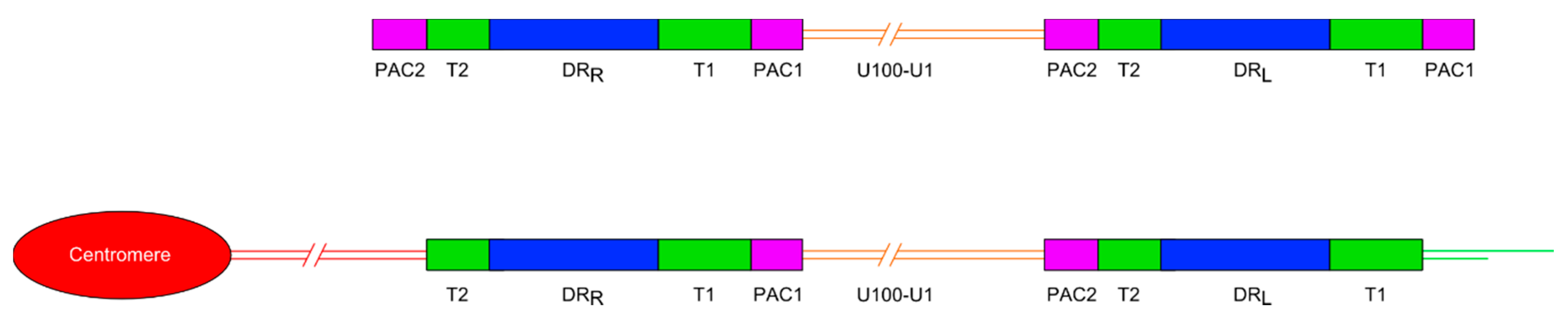
1.1. Organisation of ciHHV-6 Genome
1.2. Germline Versus Somatic Integration and HHV-6 Latency
1.3. Telomeres, Length Regulation and the Effect of ciHHV-6
2. ciHHV-6 and Disease Risk
2.1. Reactivation of Inherited ciHHV-6
2.2. Somatic HHV-6 Reactivation and Encephalitis
2.3. ciHHV-6 Loss, Telomere Instability and Disease
3. Reactivation of Inherited or Somatic ciHHV-6
3.1. Transcription and Reverse Transcription of ciHHV-6
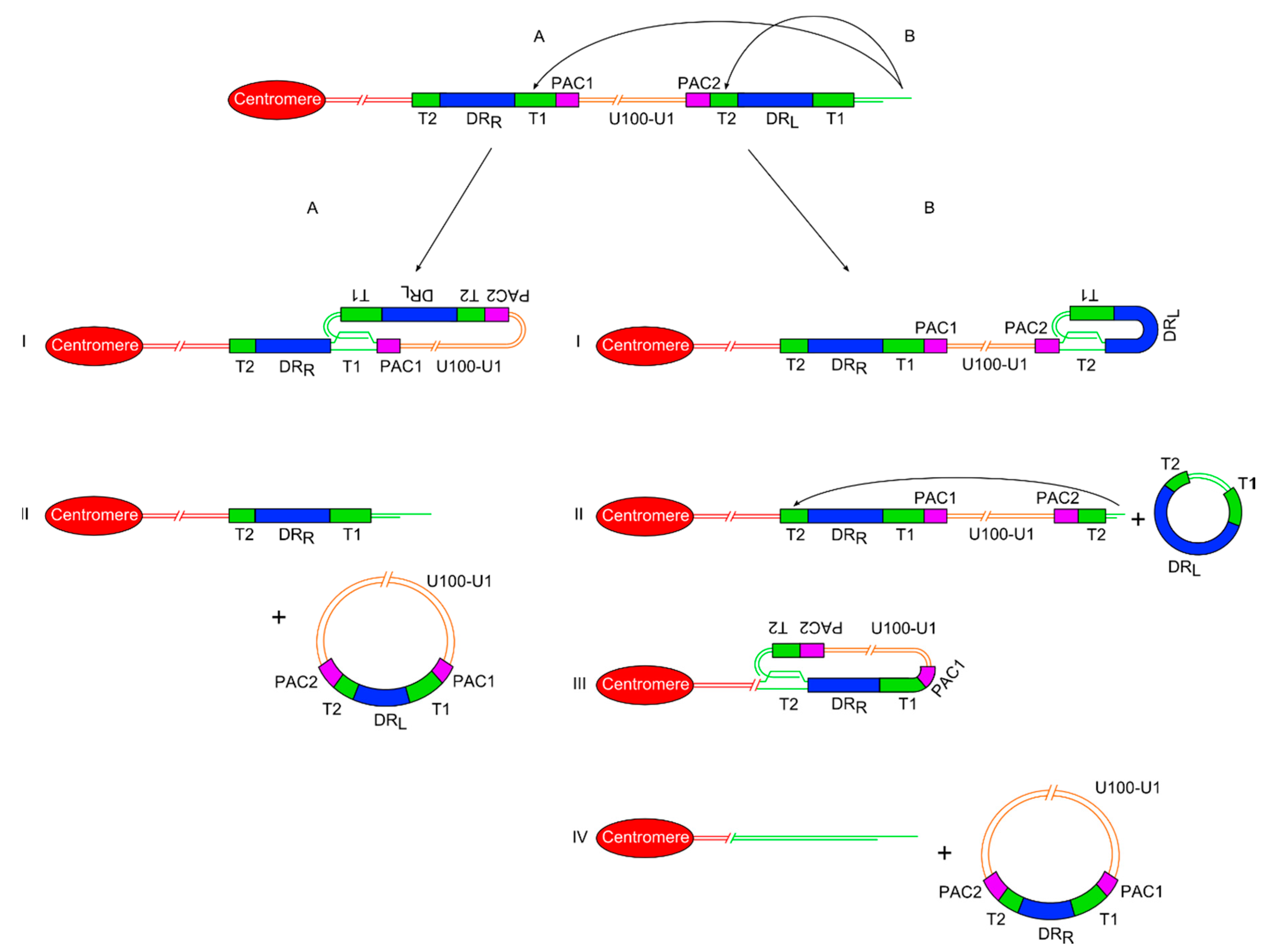
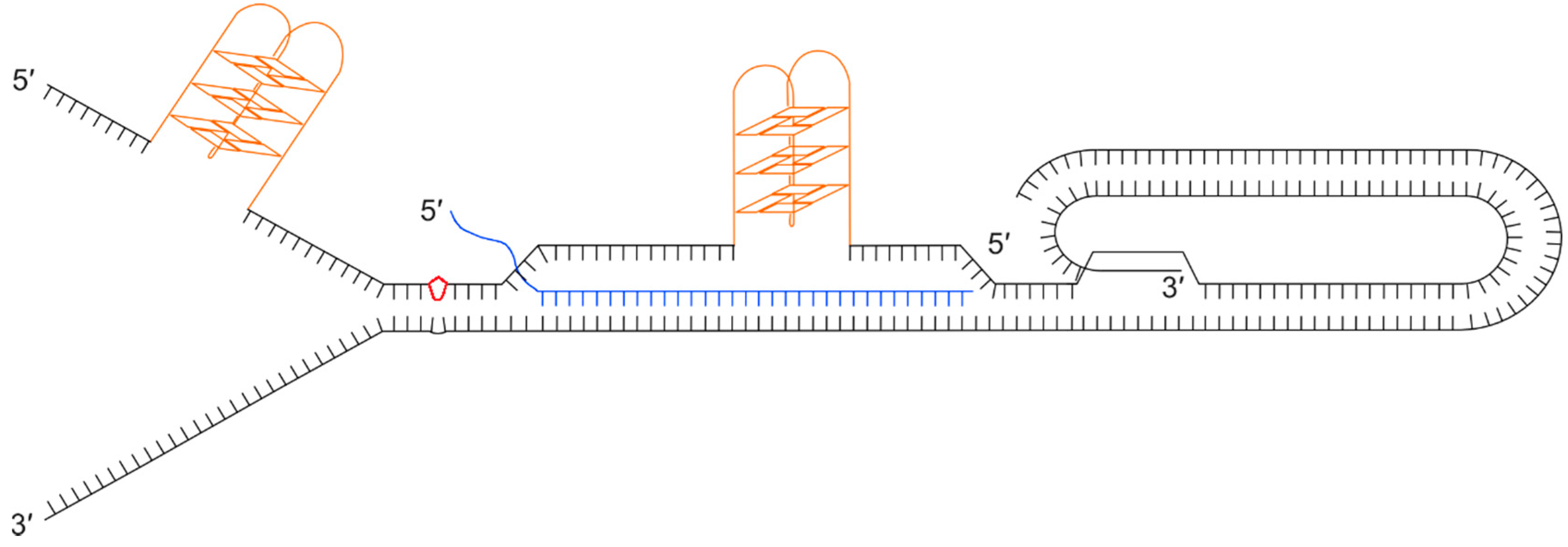
3.2. T-Loop Formation and Excision of the ciHHV-6 Genome
3.2.1. Double T-Loop Formation and Excision
3.2.2. Single T-Loop Formation and Excision
3.3. ciHHV-6, Replisome Stalling, Fork Collapse and Remodelling
4. Conclusions and Future Research Perspectives
Acknowledgements
Conflicts of Interest
References
- Ablashi, D.; Agut, H.; Berneman, Z.; Campadelli-Fiume, G.; Carrigan, D.; Ceccerini-Nelli, L.; Chandran, B.; Chou, S.; Collandre, H.; Cone, R.; et al. Human herpesvirus-6 strain groups: A nomenclature. Arch. Virol. 1993, 129, 363–366. [Google Scholar] [CrossRef]
- Luppi, M.; Marasca, R.; Barozzi, P.; Ferrari, S.; Ceccherini-Nelli, L.; Batoni, G.; Merelli, E.; Torelli, G. Three cases of human herpesvirus-6 latent infection: Integration of viral genome in peripheral blood mononuclear cell DNA. J. Med. Virol. 1993, 40, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Torelli, G.; Barozzi, P.; Marasca, R.; Cocconcelli, P.; Merelli, E.; Ceccherini-Nelli, L.; Ferrari, S.; Luppi, M. Targeted Integration of Human Herpesvirus 6 in the p Arm of Chromosome 17 of Humanperipheral Blood Mononuclear Cells In Vivo. J. Med. Virol. 1995, 46, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Daibata, M.; Taguchi, T.; Taguchi, H.; Miyoshi, I. Integration of human herpesvirus 6 in a Burkitt’s lymphoma cell line. Br. J. Haematol. 1998, 102, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Daibata, M.; Taguchi, T.; Nemoto, Y.; Taguchi, H.; Miyoshi, I. Inheritance of chromosomally integrated human herpesvirus 6 DNA. Blood 1999, 94, 1545–1549. [Google Scholar] [PubMed]
- Morris, C.; Luppi, M.; McDonald, M.; Barozzi, P.; Torelli, G. Fine mapping of an apparently targeted latent human herpesvirus type 6 integration site in chromosome band 17p13.3. J. Med. Virol. 1999, 58, 69–75. [Google Scholar] [CrossRef]
- Tanaka-Taya, K.; Sashihara, J.; Kurahashi, H.; Amo, K.; Miyagawa, H.; Kondo, K.; Okada, S.; Yamanishi, K. Human herpesvirus 6 (HHV-6) is transmitted from parent to child in an integrated form and characterization of cases with chromosomally integrated HHV-6 DNA. J. Med. Virol. 2004, 73, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Nacheva, E.P.; Leong, H.N.; Brazma, D.; Li, Y.T.; Tsao, E.H.F.; Buyck, H.C.E.; Atkinson, C.E.; Lawson, H.M.; Potter, M.N.; et al. Transmission of integrated Human Herpesvirus 6 through stem cell transplantation: Implications for laboratory diagnosis. J. Infect. Dis. 2006, 193, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Nacheva, E.P.; Ward, K.N.; Brazma, D.; Virgili, A.; Howard, J.; Leong, H.N.; Clark, D.A. Human herpesvirus 6 integrates within telomeric regions as evidenced by five different chromosomal Sites. J. Med. Virol. 2008, 80, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Hubacek, P.; Virgili, A.; Ward, K.N.; Pohlreich, D.; Keslova, P.; Goldova, B.; Markova, M.; Zajac, M.; Cinek, O.; Nacheva, E.P.; et al. HHV-6 DNA throughout the tissues of two stem cell transplant patients with chromosomally integrated HHV-6 and fatal CMV pneumonitis. Br. J. Haematol. 2009, 145, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, J.H.; Medveczky, M.M.; Luka, J.; Hadley, S.H.; Luegmayr, A.; Ablashi, D.; Lund, T.C.; Tolar, J.; De Meirleir, K.; Montoya, J.G.; et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 5563–5568. [Google Scholar] [CrossRef] [PubMed]
- Strenger, V.; Aberle, S.W.; Nacheva, E.P.; Urban, C. Chromosomal integration of the HHV-6 genome in a patient with nodular sclerosis Hodgkin lymphoma. Br. J. Haematol. 2013, 161, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Watanabe, K.; Ohye, T.; Suzuki, K.; Matsubara, T.; Shimizu, N.; Kurahashi, H.; Yoshikawa, T.; Katano, H.; Inoue, N.; et al. Molecular and virological evidence of viral activation from chromosomally integrated human herpesvirus 6A in a patient with X-linked severe combined immunodeficiency. Clin. Infect. Dis. 2014, 59, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Ohye, T.; Inagaki, H.; Ihira, M.; Higashimoto, Y.; Kato, K.; Oikawa, J.; Yagasaki, H.; Niizuma, T.; Takahashi, Y.; Kojima, S.; et al. Dual roles for the telomeric repeats in chromosomally integrated human herpesvirus-6. Sci. Rep. 2014, 4, 4559. [Google Scholar] [CrossRef] [PubMed]
- Ohye, T.; Kawamura, Y.; Inagaki, H.; Yoshikawa, A.; Ihira, M.; Yoshikawa, T.; Kurahashi, H. A simple cytogenetic method to detect chromosomally integrated human herpesvirus-6. J. Virol. Methods 2016, 228, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Lindquester, G.J.; Pellett, P.E. Properties of the human herpesvirus 6 strain Z29 genome: G + C content, length, and presence of variable-length directly repeated terminal sequence elements. Virology 1991, 182, 102–110. [Google Scholar] [CrossRef]
- Gomples, U.A.; Macaulay, H.A. Characterization of human telomeric repeat sequences from human herpesvirus 6 and relationship to replication. J. Gen. Virol. 1995, 76, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Malet, I.; Deback, C.; Bonnafous, P.; Boutolleau, D.; Gautheret-Dejean, A.; Agut, H. Length variability of telomeric repeat sequences of human herpesvirus 6 DNA. J. Virol. Methods 2009, 159, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Wallaschek, N.; Sanyal, A.; Pirzer, F.; Gravel, A.; Mori, Y.; Flamand, L.; Kaufer, B.B. The Telomeric Repeats of Human Herpesvirus 6A (HHV-6A) Are Required for Efficient Virus Integration. PLoS Pathog. 2016, 12, e1005666. [Google Scholar] [CrossRef] [PubMed]
- Trempe, F.; Gravel, A.; Dubuc, I.; Wallaschek, N.; Collin, V.; Gilbert-Girard, S.; Morissette, G.; Kaufer, B.B.; Flamand, L. Characterization of human herpesvirus 6A/B U94 as ATPase, helicase, exonuclease and DNA-binding proteins. Nucleic Acids Res. 2015, 43, 6084–6098. [Google Scholar] [CrossRef] [PubMed]
- Wallaschek, N.; Gravel, A.; Flamand, L.; Kaufer, B.B. The putative U94 integrase is dispensable for human herpesvirus 6 (HHV-6) chromosomal integration. J. Gen. Virol. 2016, 97, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, R.H.; Cook, L.; Huang, M.; Magaret, A.; Zerr, D.M.; Boeckh, M.; Jerome, K.R. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin. Chem. 2014, 60, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Gallagher, A.; Mottram, T.; Lake, A.; Kane, E.V.; Lightfoot, T.; Roman, E.; Jarrett, R.F. Germ-line transmitted, chromosomally integrated HHV-6 and classical Hodgkin lymphoma. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hidalgo-Bravo, A.; Zhang, E.; Cotton, V.E.; Mendez-Bermúdez, A.; Wig, G.; Medina-Calzada, Z.; Neumann, R.; Jeffreys, A.J.; Winney, B.; et al. Human telomeres that carry an integrated copy of human herpesvirus 6 are often short and unstable, facilitating release of the viral genome from the chromosome. Nucleic Acids Res. 2014, 42, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Gulve, N.; Frank, C.; Klepsch, M.; Prusty, B.K. Chromosomal integration of HHV-6A during non-productive viral infection. Sci. Rep. 2017, 7, 512. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Caserta, M.T.; Schnabel, K.C.; Boettrich, C.; McDermott, M.P.; Lofthus, G.K.; Carnahan, J.A.; Dewhurst, S. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J. Pediatr. 2004, 145, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.N.; Tuke, P.W.; Tedder, R.S.; Khanom, A.B.; Eglin, R.P.; Atkinson, C.E.; Ward, K.N.; Griffiths, P.D.; Clark, D.A. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of UK blood donors. J. Med. Virol. 2007, 79, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Caserta, M.T.; Schnabel, K.; Shelley, L.M.; Marino, A.S.; Carnahan, J.A.; Yoo, C.; Lofthus, G.K.; McDermott, M.P. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics 2008, 122. [Google Scholar] [CrossRef] [PubMed]
- Hudnall, S.D.; Chen, T.; Allison, P.; Tyring, S.K.; Heath, A. Herpesvirus prevalence and viral load in healthy blood donors by quantitative real-time polymerase chain reaction. Transfusion 2008, 48, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Dubuc, I.; Morissette, G.; Sedlak, R.H.; Jermone, K.R.; Flamand, L. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc. Natl. Acad. Sci. USA 2015, 112, 8058. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, M.D.; Larsen, P.B.; Kofod-Olsen, E.; Fedder, J.; Bonde, J.; Höllsberg, P. Human herpesvirus-6A/B binds to spermatozoa acrosome and is the most prevalent herpesvirus in semen from sperm donors. PLoS ONE 2012, 7, e48810. [Google Scholar] [CrossRef] [PubMed]
- Neofytou, E.; Sourvinos, G.; Asmarianaki, M.; Spandidos, D.A.; Makrigiannakis, A. Prevalence of human herpes virus types 1–7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil. Steril. 2009, 91, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Taya, K.; Kondo, T.; Mukai, T.; Miyoshi, H.; Yamamoto, Y.; Okada, S.; Yamanishi, K. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J. Med. Virol. 1996, 48, 88–94. [Google Scholar] [CrossRef]
- Ward, K.N.; Thiruchelvam, A.D.; Couto-Parada, X. Unexpected occasional persistence of high levels of HHV-6 DNA in sera: detection of variants A and B. J. Med. Virol. 2005, 76, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Caserta, M.T.; Schnabel, K.C.; McDermott, M.P.; Lofthus, G.K.; Carnahan, J.A.; Gilbert, L.M.; Dewhurst, S. Characteristics and acquisition of human herpesvirus (HHV)–7 infections in relation to infection with HHV-6. J. Infect. Dis. 2006, 193, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Monze, M.; Bima, H.; Kapambwe, M.; Clark, D.; Kasolo, F.C.; Gompels, U.A. Predominant human herpesvirus 6 variant A infant infections in an HIV-1 endemic region of Sub-Saharan Africa. J. Med. Virol. 2009, 81, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, E.C.; Brunetto, G.S.; Caruso, B.; Fenton, K.; Ohayon, J.; Reich, D.S.; Jacobson, S. Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS ONE 2014, 9, e92328. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991, 72, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Luppi, M.; Barozzi, P.; Morris, C.; Maiorana, A.; Garber, R.; Bonacorsi, G.; Donelli, A.; Marasca, R.; Tabilio, A.; Torelli, G. Human herpesvirus 6 latently infects early bone marrow progenitors in vivo. J. Virol. 1999, 73, 754–759. [Google Scholar] [PubMed]
- Yasukawa, M.; Ohminami, H.; Sada, E.; Yakushijin, Y.; Kaneko, M.; Yanagisawa, K.; Kohno, H.; Bando, S.; Fujita, S. Latent infection and reactivation of human herpesvirus 6 in two novel myeloid cell lines. Blood 1999, 93, 991–999. [Google Scholar] [PubMed]
- Yoshikawa, T.; Asano, Y.; Akimoto, S.; Ozaki, T.; Iwasaki, T.; Kurata, T.; Goshima, F.; Nishiyama, Y. Latent infection of human herpesvirus 6 in astrocytoma cell line and alteration of cytokine synthesis. J. Med. Virol. 2002, 66, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, J.; Fotheringham, J.; Akhyani, N.; Yao, K.; Fogdell-Hahn, A.; Jacobson, S. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J. Neurovirol. 2005, 11, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, N.; Karlseder, J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat. Struct. Mol. Biol. 2015, 22, 859. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings - what telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell. Biol. 2016, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; de Lange, T. Telomeres in cancer: tumour suppression and genome instability. Nat. Rev. Mol. Cell. Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; de Lange, T. Removal of Shelterin Reveals the Telomere End-Protection Problem. Science 2012, 336, 593. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; de Lange, T. How Shelterin Protects Mammalian Telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Pellett, P.E.; Ablashi, D.V.; Ambros, P.F.; Agut, H.; Caserta, M.T.; Descamps, V.; Flamand, L.; Gautheret-Dejean, A.; Hall, C.B.; Kamble, R.T.; et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev. Med. Virol. 2012, 22, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Hall, C.B.; Flamand, L. Sequence analysis of transplacentally acquired human herpesvirus 6 DNA is consistent with transmission of a chromosomally integrated reactivated virus. J. Infect. Dis. 2013, 207, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Pradeau, K.; Bordessoule, D.; Szelag, J.; Rolle, F.; Ferrat, P.; Meur, Y.L.; Turlure, P.; Denis, F.; Ranger-Rogez, S. A reverse transcription-nested PCR assay for HHV-6 mRNA early transcript detection after transplantation. J. Virol. Methods 2006, 134, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Kikuchi, H.; Satou, T.; Kawano, R.; Ikewaki, J.; Kohno, K.; Kashima, K.; Ohtsuka, E.; Kadota, J. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J. Infect. Dis. 2006, 193, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Miyamoto, T.; Nagafuji, K.; Kamezaki, K.; Yamamoto, A.; Saito, N.; Kato, K.; Takenaka, K.; Iwasaki, H.; Harada, N.; et al. High Incidence of human herpes virus 6-associated encephalitis/myelitis following a second unrelated cord blood transplantation. Biol. Blood Marrow Transplant. 2006, 193, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Satou, T.; Kawano, R.; Takakura, S.; Goto, K.; Ikewaki, J.; Kohno, K.; Ikebe, T.; Ando, T.; Miyazaki, Y.; et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2010, 45, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Kondo, T.; Ishikawa, T.; Yamashita, K.; Takaori-Kondo, A. Human herpesvirus-6 encephalitis during hematopoietic stem cell transplantation leads to poor prognosis. Transplant. Infect. Dis. 2013, 15, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Satou, T.; Kadota, J.; Saito, N.; Yoshida, T.; Okumura, H.; Ueki, T.; Nagafuji, K.; Kako, S.; Uoshima, N.; et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin. Infect. Dis. 2013, 57, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Oruzio, D.; Jager, G.; Schlemmer, M.; Schleuning, M.; Schiel, X.; Hiddemann, W.; Kolb, H. Impact of human herpesvirus-6 after haematopoietic stem cell transplantation. Br. J. Haematol. 2005, 128, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zerr, D.M.; Corey, L.; Kim, H.W.; Huang, M.; Nguy, L.; Boeckh, M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2005, 40, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Betts, B.C.; Young, J.H.; Ustun, C.; Cao, Q.; Weisdorf, D.J. Human herpesvirus 6 infection after hematopoietic cell transplantation: Is routine surveillance necessary? Biol. Blood Marrow Transplant. 2011, 17, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, H.; Agrinier, N.; Guery, M.; Salmon, A.; Clément, L.; Bordigoni, P.; Venard, V. Human herpesvirus 6 infection after allogeneic stem cell transplantation: Incidence, outcome, and factors associated with HHV-6 reactivation. Transplant. J. 2013, 95, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, R.H.; Hill, J.A.; Nguyen, T.; Cho, M.; Levin, G.; Cook, L.; Huang, M.; Flamand, L.; Zerr, D.M.; Boeckh, M. Detection of human herpesvirus 6b (HHV-6b) reactivation in hematopoietic cell transplant recipients with inherited chromosomally integrated HHV-6a by droplet digital PCR. J. Clin. Microbiol. 2016, 54, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Zerr, D.M.; Gooley, T.A.; Yeung, L.; Huang, M.-L.; Carpenter, P.; Wade, J.C.; Corey, L.; Anasetti, C. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 2001, 33, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kamitsuji, Y.; Kuroda, J.; Tsunoda, S.; Uoshima, N.; Kimura, S.; Wada, K.; Matsumoto, Y.; Nomura, K.; Horiike, S.; et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: Report of 2 cases and review of 212 cases in the literature. Acta Haematol. 2007, 117, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.D. Human herpesvirus-8: Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Ludlow, A.T.; Min, J.; Robin, J.D.; Stadler, G.; Mender, I.; Lai, T.; Zhang, N.; Wright, W.E.; Shay, J.W. Regulation of the human telomerase gene TERT by telomere position effect—over long distances (TPE-OLD): Implications for aging and cancer. PLoS Biol. 2016, 14, e2000016. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric Repeat Containing RNA and RNA Surveillance Factors at Mammalian Chromosome Ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Romero, C.; Chartrand, P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Balk, B.; Dees, M.; Bender, K.; Luke, B. The differential processing of telomeres in response to increased telomeric transcription and RNA-DNA hybrid accumulation. RNA Biol. 2014, 11, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Kao, Y.W.; Lin, J.J. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc. Natl. Acad. Sci. USA 2014, 111, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Maicher, A.; Kastner, L.; Dees, M.; Luke, B. Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res. 2012, 40, 6649–6659. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef]
- Sarek, G.; Vannier, J.; Panier, S.; Petrini, J.H.J.; Boulton, S.J. TRF2 recruits RTEL1 to telomeres in S phase to promote t-loop unwinding. Mol. Cell 2015, 57, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Opresko, P.L.; Otterlei, M.; Graakjær, J.; Bruheim, P.; Dawut, L.; Kølvraa, S.; May, A.; Seidman, M.M.; Bohr, V.A. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 2004, 14, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Lillard-Wetherell, K.; Machwe, A.; Langland, G.T.; Combs, K.A.; Behbehani, G.K.; Schonberg, S.A.; German, J.; Turchi, J.J.; Orren, D.K.; Groden, J. Association and regulation of the BLM helicase by the telomere proteins TRF1 and TRF2. Hum. Mol. Genet. 2004, 13, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Vannier, J.; Pavicic-Kaltenbrunner, V.; Petalcorin, M.I.R.; Ding, H.; Boulton, S.J. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 2012, 149, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Krohne, G.; Rudel, T. Reactivation of chromosomally integrated human herpesvirus-6 by telomeric circle formation. PLoS Genetics 2013. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Parikh, D.; Opresko, P. DNA damage processing at telomeres: The ends justify the means. DNA Repair 2016, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, R.J.; Wolf, A.J.; Zakian, V.A. Structural and Temporal Analysis of Telomere Replication in Yeast. Cold Spring Harb. Symp. Quant. Biol. 1993, 58, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ivessa, A.S.; Zhou, J.; Schulz, V.P.; Monson, E.K.; Zakian, V.A. Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 2002, 16, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Makovets, S.; Herskowitz, I.; Blackburn, E.H. anatomy and dynamics of DNA replication fork movement in yeast telomeric regions. Mol. Cell. Biol. 2004, 24, 4019–4031. [Google Scholar] [CrossRef] [PubMed]
- Salas, T.R.; Petruseva, I.; Lavrik, O.; Bourdoncle, A.; Mergny, J.; Favre, A.; Saintomé, C. Human replication protein A unfolds telomeric G-quadruplexes. Nucleic Acids Res. 2006, 34, 4857–4865. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Karow, J.K.; Hickson, I.D.; Maizels, N. The Bloom’s syndrome helicase unwinds G4 DNA. J. Biol. Chem. 1998, 273, 27587–27592. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, P.; Karow, J.K.; Brosh, R.M., Jr.; Bohr, V.A.; Hickson, I.D. The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001, 29, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.A.; Reddel, R.R.; Johnston, R.L.; Cesare, A.J.; Pickett, H.A. Control of telomere length by a trimming mechanism that involves generation of t-circles. EMBO J. 2009, 28, 799–809. [Google Scholar] [CrossRef]
- Campioni, D.; Gentili, V.; Cavazzini, F.; Bortolotti, D.; Nacheva, E.P.; Cuneo, A.; Di Luca, D.; Rizzo, R. Detection of inherited chromosomally integrated HHV-6 (ciHHV-6) in a marker chromosome. Eur. J. Haematol. 2017, 98, 635–637. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, M.L.; Royle, N.J. Chromosomally Integrated Human Herpesvirus 6: Models of Viral Genome Release from the Telomere and Impacts on Human Health. Viruses 2017, 9, 184. https://doi.org/10.3390/v9070184
Wood ML, Royle NJ. Chromosomally Integrated Human Herpesvirus 6: Models of Viral Genome Release from the Telomere and Impacts on Human Health. Viruses. 2017; 9(7):184. https://doi.org/10.3390/v9070184
Chicago/Turabian StyleWood, Michael L., and Nicola J. Royle. 2017. "Chromosomally Integrated Human Herpesvirus 6: Models of Viral Genome Release from the Telomere and Impacts on Human Health" Viruses 9, no. 7: 184. https://doi.org/10.3390/v9070184



