Applicability of Metal Nanoparticles in the Detection and Monitoring of Hepatitis B Virus Infection
Abstract
:1. Introduction
2. Gold Nanoparticles
2.1. Detection of Hepatitis B Virus Antigens by Gold Nanoparticles Surface Plasmon Resonance
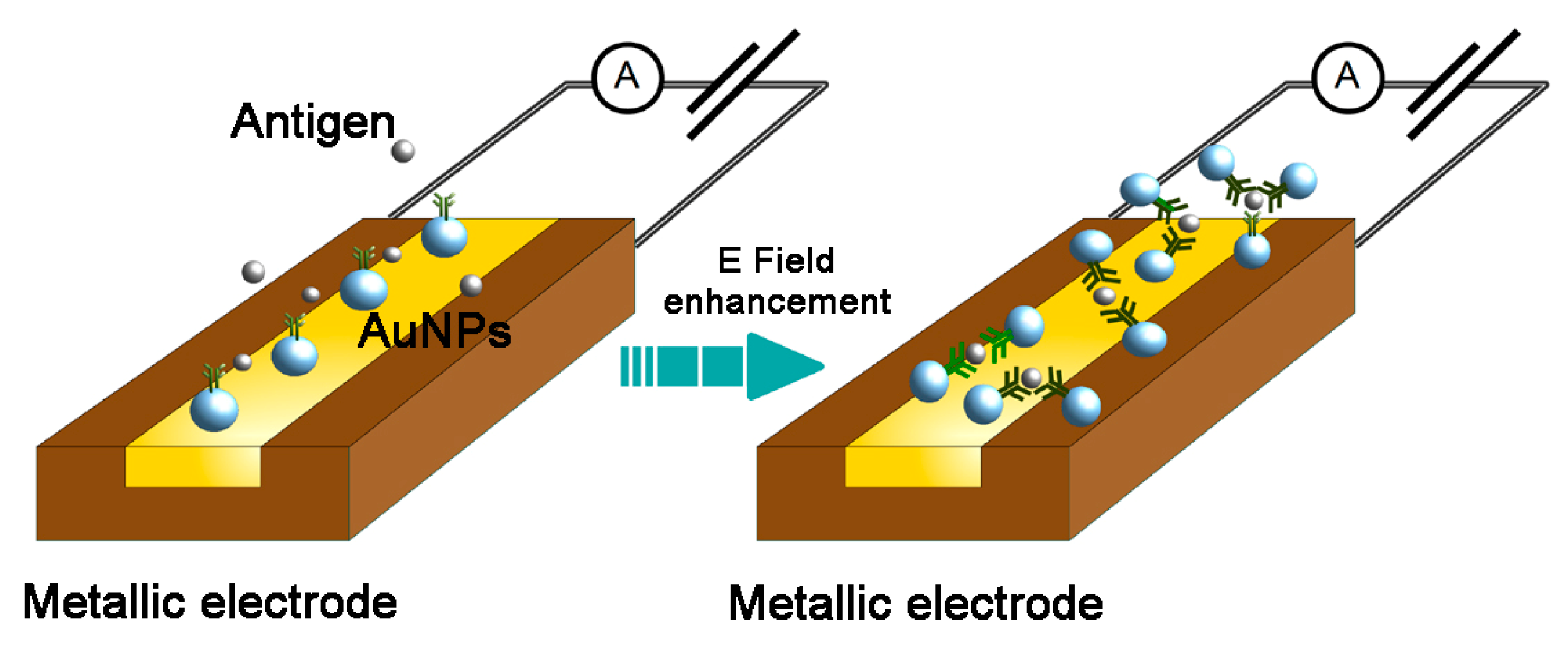
2.2. Use of Gold Nanoparticles in Electrochemical Detectors
2.3. Gold Nanoparticles-Based Lateral Flow Assay
2.4. Gold Nanoparticles-Enhanced Raman Spectroscopy
3. Magnetic Nanoparticles
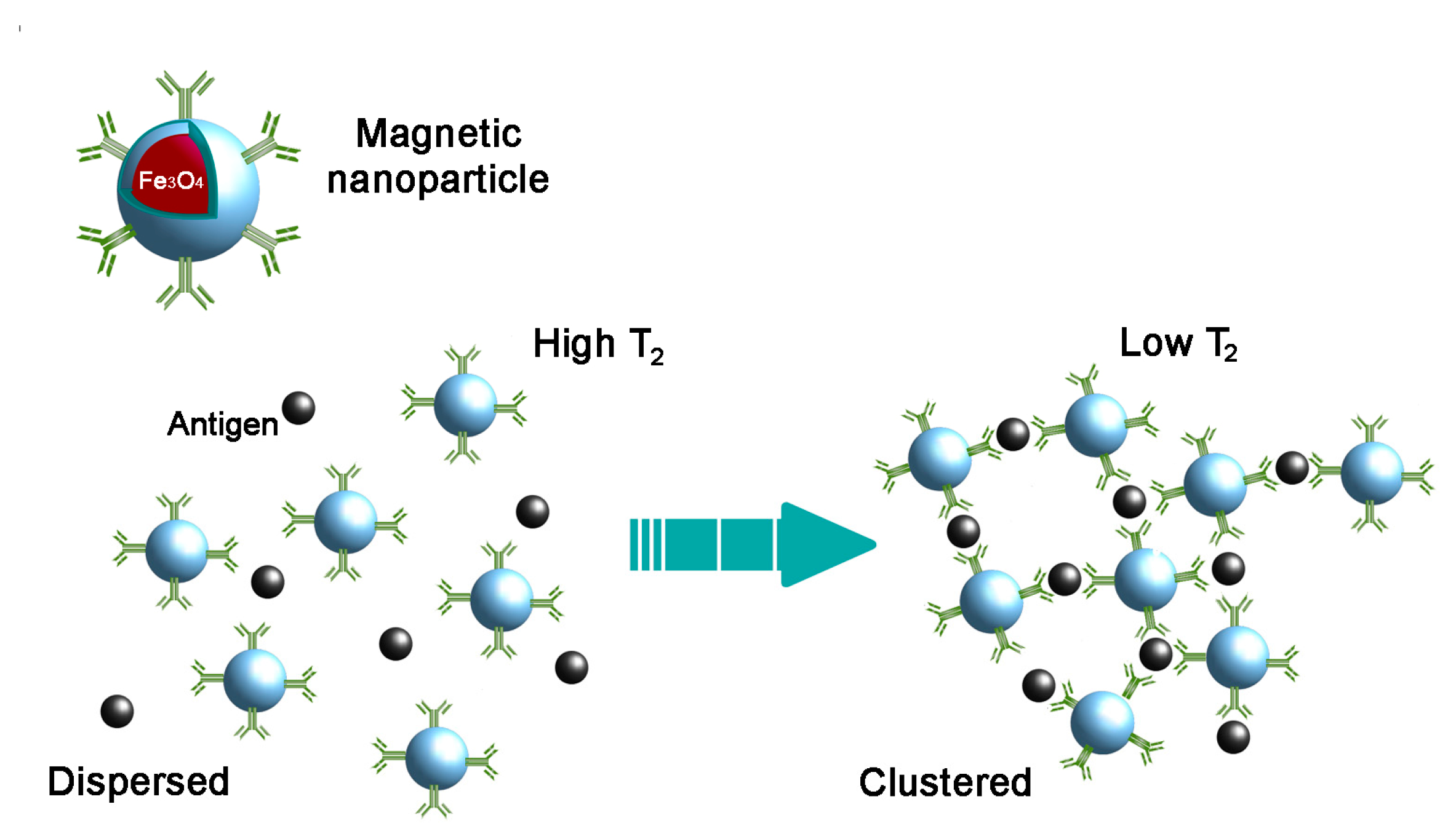
3.1. Spin–Spin Relaxation Time-Based Detection Methods
3.2. Electrochemical Detection
3.3. Lateral Flow Assay
4. Quantum Dots
5. Combinations of Different Nanoparticles
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lobaina, Y.; Michel, M.L. Chronic hepatitis B: Immunological profile and current therapeutic vaccines in clinical trials. Vaccine 2017, 35, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Wang, C.; Lau, G. Treatment of chronic hepatitis B infection-2017. Liver Int. 2017, 37 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Debarry, J.; Cornberg, M.; Manns, M.P. Challenges in warranting access to prophylaxis and therapy for hepatitis B virus infection. Liver Int. 2017, 37 (Suppl. 1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Krajden, M.; McNabb, G.; Petric, M. The laboratory diagnosis of hepatitis B virus. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Goddard, C.; Clearfield, E.; Mills, C.; Xiao, T.; Guo, H.; Morrey, J.D.; Motter, N.E.; Zhao, K.; Block, T.M.; et al. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J. Med. Chem. 2011, 54, 5660–5670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, L.; Yin, P.; Lu, X.; Wang, X.; Niu, J.; Gao, P.; Xu, G. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J. Proteome Res. 2012, 11, 5433–5442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, L.; Tan, Z.; Zlotnick, A.; Jacobson, S.C. Characterization of hepatitis B virus capsids by resistive-pulse sensing. J. Am. Chem. Soc. 2011, 133, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Inoue, K.; Tanaka, T.; Kato, J.; Kajiyama, N.; Kawaguchi, R.; Tanaka, S.; Yoshiba, M.; Kohara, M. Quantitation of hepatitis B virus genomic DNA by real-time detection PCR. J. Clin. Microbiol. 1999, 37, 2899–2903. [Google Scholar] [PubMed]
- Reddy, L.H.; Couvreur, P. Nanotechnology for therapy and imaging of liver diseases. J. Hepatol. 2011, 55, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Shingyoji, M.; Gerion, D.; Pinkel, D.; Gray, J.W.; Chen, F. Quantum dots-based reverse phase protein microarray. Talanta 2005, 67, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sood, A.K.; Kumar, N. Carbon nanotube flow sensors. Science 2003, 299, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Li, G.; Sun, S.; Wilson, R.J.; White, R.L.; Pourmand, N.; Wang, S.X. Spin valve sensors for ultrasensitive detection of superparamagnetic nanoparticles for biological applications. Sens. Actuators A Phys. 2006, 126, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Millen, R.L.; Kawaguchi, T.; Granger, M.C.; Porter, M.D.; Tondra, M. Giant magnetoresistive sensors and superparamagnetic nanoparticles: A chip-scale detection strategy for immunosorbent assays. Anal. Chem. 2005, 77, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Horisberger, M.; Rosset, J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J. Histochem. Cytochem. 1977, 25, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, L.M.; Ribeiro, C.M.S.; Zijlstra-Willems, E.M.; de Witte, L.; Fluitsma, D.; Tigchelaar, W.; Everts, V.; Geijtenbeek, T.B.H. Caveolin-1 mediated uptake via langerin restricts HIV-1 infection in human Langerhans cells. Retrovirology 2014, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Ferreira Carlos, F.; Pedrosa, P.; Lopez, A.; Baptista, P. Gold nanoparticles for diagnostics: Advances towards points of care. Diagnostics 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, W.; Peng, W.; Zhao, Q.; Piao, J.; Zhang, B.; Wu, X.; Wang, H.; Gong, X.; Chang, J. Enhanced fluorescence ELISA based on HAT triggering fluorescence “turn-on” with enzyme-antibody dual labeled AuNP probes for ultrasensitive detection of AFP and HBsAg. ACS Appl. Mater. Interfaces 2017, 9, 9369–9377. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Mirkin, C.A.; Letsinger, R.L. Homogeneous, nanoparticle-based quantitative colorimetric detection of oligonucleotides. J. Am. Chem. Soc. 2000, 122, 3795–3796. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Colorimetric biosensors based on dnazyme-assembled gold nanoparticles. J. Fluoresc. 2004, 14, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xue, X.; Li, T.; Zeng, H.; Liu, X. Ultrasensitive and selective colorimetric dna detection by nicking endonuclease assisted nanoparticle amplification. Angew. Chem. 2009, 121, 6981–6984. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, H.; Fu, Q.; Peng, J.; Wang, Y.; Du, J.; Zhou, Y.; Zhan, L. Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of hepatitis B virus in buffer, blood serum and plasma. Biosens. Bioelectron. 2010, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Aguas, H.; Fortunato, E.; Martins, R. Inkjet printed and "doctor blade" TiO2 photodetectors for dna biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Pedrosa, P.; Carlos, F.F.; Mancio-Silva, L.; Grosso, A.R.; Fortunato, E.; Mota, M.M.; Baptista, P.V. One nanoprobe, two pathogens: Gold nanoprobes multiplexing for point-of-care. J. Nanobiotechnol. 2015, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wang, Y.; Li, S.S.-C.; Wan, Z.; Zhai, J. Rapid and simultaneous detection of human hepatitis B virus and hepatitis C virus antibodies based on a protein chip assay using nano-gold immunological amplification and silver staining method. BMC Infect. Dis. 2005, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.W.; Xin, Z.; Yao, L.; Li, X.-F.; Tang, J.-X.; Zhou, X.-J.; Wu, B. Development of clinical highly sensitive biosensor-based microarray system. World Chin. J. Digestol. 2008, 15, 1628–1633. [Google Scholar]
- Jang, K.-J.; Lee, H.; Jin, H.-L.; Park, Y.; Nam, J.-M. Restriction-enzyme-coded gold-nanoparticle probes for multiplexed DNA detection. Small 2009, 5, 2665–2668. [Google Scholar] [CrossRef] [PubMed]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Pänke, O.; Kirbs, A.; Lisdat, F. Voltammetric detection of single base-pair mismatches and quantification of label-free target ssDNA using a competitive binding assay. Biosens. Bioelectron. 2007, 22, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, Y.; Dong, P.; Tang, B. Gold nanoparticles modified electrode via a mercapto-diazoaminobenzene monolayer and its development in dna electrochemical biosensor. Biosens. Bioelectron. 2010, 25, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lai, Z.-L.; Wang, G.-J.; Wu, C.-Y. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosens. Bioelectron. 2016, 77, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Shourian, M.; Ghourchian, H.; Boutorabi, M. Ultra-sensitive immunosensor for detection of hepatitis B surface antigen using multi-functionalized gold nanoparticles. Anal. Chim. Acta 2015, 895, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Baca, A.J.; Hu, J.; Zhou, F.; Yan, W.; Pang, D.-W. Amplified voltammetric detection of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Anal. Chem. 2003, 75, 3941–3945. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y.; Zhong, X.; Liu, Y.; Dai, J. Electrochemical detection of hepatitis B surface antigen using colloidal gold nanoparticles modified by a sol–gel network interface. Clin. Biochem. 2006, 39, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Li, H.; Liao, J. Ionic liquid and nanogold-modified immunosensing interface for electrochemical immunoassay of hepatitis B surface antigen in human serum. Microfluid. Nanofluid. 2008, 6, 403. [Google Scholar] [CrossRef]
- Fu, Y.-Z.; Yuan, R.; Chai, Y.-Q. Reagentless immunosensing assay via electrochemical impedance for hepatitis B surface antigen monitoring based on polypyrrole and gold nanoparticles as matrices. Chin. J. Chem. 2006, 24, 59–64. [Google Scholar] [CrossRef]
- Tang, D.P.; Yuan, R.; Chai, Y.Q.; Zhong, X.; Liu, Y.; Dai, J.Y.; Zhang, L.Y. Novel potentiometric immunosensor for hepatitis B surface antigen using a gold nanoparticle-based biomolecular immobilization method. Anal. Biochem. 2004, 333, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yuan, R.; Chai, Y.; Zhang, Y.; Li, X.L.; Zhu, Q.; Wang, N. An amperometric immunosensor based on immobilization of hepatitis B surface antibody on gold electrode modified gold nanoparticles and horseradish peroxidase. Anal. Chim. Acta 2005, 548, 205–210. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Li, F.; Han, Y.L.; Lin, M.; Lu, T.J.; Xu, F. Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab Chip 2013, 13, 4352–4357. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Hong, S.; Kim, J.; Heo, N.; Lee, M.-K.; Lee, S.; Kim, B.; Kim, I.; Huh, Y.; et al. Development of lateral flow assay based on size-controlled gold nanoparticles for detection of hepatitis B surface antigen. Sensors 2016, 16, 2154. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Witkowska, E.; Winkler, K.; Dzięcielewski, I.; Weyher, J.L.; Waluk, J. Detection of hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system. Biosens. Bioelectron. 2015, 66, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tamanaha, C.R.; Mulvaney, S.P.; Rife, J.C.; Whitman, L.J. Magnetic labeling, detection, and system integration. Biosens. Bioelectron. 2008, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Multhoff, G. Recent developments of magnetic nanoparticles for theranostics of brain tumor. Curr. Drug Metab. 2016, 17, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Hofmann, H. Superparamagnetic iron oxide nanoparticles (SPIONs): From synthesis to in vivo studies—A summary of the synthesis, characterization, in vitro and in vivo investigations of spions with particular focus on surface and colloidal properties. IEEE Trans. Nanobiosci. 2007, 6, 289–953. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, E. Electrochemical biosensors based on magnetic micro/nano particles. Electrochim. Acta 2012, 84, 62–73. [Google Scholar] [CrossRef]
- Jaffrezic-Renault, N.; Martelet, C.; Chevolot, Y.; Cloarec, J.-P. Biosensors and bio-bar code assays based on biofunctionalized magnetic microbeads. Sensors 2007, 7, 589. [Google Scholar] [CrossRef]
- Hsing, I.M.; Xu, Y.; Zhao, W. Micro- and nano-magnetic particles for applications in biosensing. Electroanalysis 2007, 19, 755–768. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. WIRE Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yamini, Y.; Rezaee, M. Cheminform abstract: Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. ChemInform 2010, 41. [Google Scholar] [CrossRef]
- Xi, Z.; Huang, R.; Li, Z.; He, N.; Wang, T.; Su, E.; Deng, Y. Selection of HBsAG-specific DNA aptamers based on carboxylated magnetic nanoparticles and their application in the rapid and simple detection of hepatitis B virus infection. ACS Appl. Mater. Interfaces 2015, 7, 11215–11223. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, P.; Dong, H.; Krause, H.-J.; Zhang, Y.; Willbold, D.; Offenhaeusser, A.; Gu, Z. A magnetic nanoparticles relaxation sensor for protein–protein interaction detection at ultra-low magnetic field. Biosens. Bioelectron. 2016, 80, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, K.; Ghourchian, H.; Ziaee, A.-A.; Samiei, S.; Hanaee, H. Paramagnetic nanoparticle-based detection of hepatitis B virus using cathodic stripping voltammetry. Biotechnol. Appl. Biochem. 2009, 52, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Nourani, S.; Ghourchian, H.; Boutorabi, S.M. Magnetic nanoparticle-based immunosensor for electrochemical detection of hepatitis B surface antigen. Anal. Biochem. 2013, 441, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vaculovicova, M.; Smerkova, K.; Sedlacek, J.; Vyslouzil, J.; Hubalek, J.; Kizek, R.; Adam, V. Integrated chip electrophoresis and magnetic particle isolation used for detection of hepatitis B virus oligonucleotides. Electrophoresis 2013, 34, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Nägele, E.; Moritz, R. Structure elucidation of degradation products of the antibiotic amoxicillin with ion trap MSn and accurate mass determination by ESI TOF. J. Am. Soc. Mass Spectrom. 2005, 16, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.M.; Chaix, C.; Abdelghani, A.; Bessueille, F.; Leonard, D.; Jaffrezic-Renault, N. An impedimetric dna sensor based on functionalized magnetic nanoparticles for HIV and HBV detection. Sens. Actuators B Chem. 2008, 134, 755–760. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Zhang, C.; Li, D.; Wang, C.; Gao, F.; Cui, D. A silicon dioxide modified magnetic nanoparticles—Labeled lateral flow strips for HBs antigen. J. Biomed. Nanotechnol. 2011, 7, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qiu, H.; Xiao, Q.; Huang, C.; Su, W.; Hu, B. A simple QD-FRET bioprobe for sensitive and specific detection of hepatitis B virus DNA. J. Fluoresc. 2013, 23, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Wang, C.; Zhi, X.; Zhang, C.; Wang, K.; Cui, D. A CCD-based reader combined quantum dots-labeled lateral flow strips for ultrasensitive quantitative detection of anti-HBs antibody. J. Biomed. Nanotechnol. 2012, 8, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Liang, X.; Zhang, G.; Ma, H.; Nie, L.; Wang, Y. Detection of hepatitis B virus M204I mutation by quantum dot-labeled DNA probe. Sensors 2017, 17, 961. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-F.; Turk, J. Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: Assignment of fatty acyl groups on the glycerol backbone and location of double bonds. J. Am. Soc. Mass Spectrom. 2010, 21, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, Y.; Fu, F.; Xu, H.; Lv, J.; Xiong, Y.; Wang, A. Immunochromatographic assay for quantitative and sensitive detection of hepatitis B virus surface antigen using highly luminescent quantum dot-beads. Talanta 2015, 142, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mashhadizadeh, M.H.; Talemi, R.P. Synergistic effect of magnetite and gold nanoparticles onto the response of a label-free impedimetric hepatitis B virus DNA biosensor. Mater. Sci. Eng. C 2016, 59, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Oishi, M. Ultrasensitive detection of DNA and RNA based on enzyme-free click chemical ligation chain reaction on dispersed gold nanoparticles. ACS Nano 2014, 8, 9988–9997. [Google Scholar] [CrossRef] [PubMed]
- Price, H.; Dunn, D.; Zachary, T.; Vudriko, T.; Chirara, M.; Kityo, C.; Munderi, P.; Spyer, M.; Hakim, J.; Gilks, C.; et al. Hepatitis B serological markers and plasma DNA concentrations. AIDS (Lond. Engl.) 2017, 31, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, Y. Highly sensitive electrochemical stripping detection of hepatitis B surface antigen based on copper-enhanced gold nanoparticle tags and magnetic nanoparticles. Anal. Chim. Acta 2010, 674, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X.; Ma, Q.; Lin, Z.; Chen, S.; Li, Y.; Lu, L.; Qu, H.; Su, X. Multiplex electrochemiluminescence DNA sensor for determination of hepatitis B virus and hepatitis C virus based on multicolor quantum dots and Au nanoparticles. Anal. Chim. Acta 2016, 916, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Hallaj, R.; Salimi, A. A highly sensitive electrochemical immunosensor for hepatitis B virus surface antigen detection based on Hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme-signal amplification. Biosens. Bioelectron. 2017, 94, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Scida, K.; Crooks, R.M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015, 87, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
| Method | Nanoparticle Use | Detected Antigen | Detection Method | Lower Limit of Detection | Substrates Tested | Ref. |
|---|---|---|---|---|---|---|
| Conventional methods | - | Anti-HBsAg | ELISA | Plasma, serum | [73] | |
| - | HBsAg | ELISA | 0.5 IU/mL | Plasma, serum | [19] | |
| - | HBV DNA | PCR | 2000 IU/mL | Plasma, serum | [74] | |
| Gold nanoparticles | DNA-coated AuNP | HBsAg | Direct detection of SPR peak | 0.1 IU/mL | Blood, serum, plasma | [26] |
| DNA-coated AuNP | HBV DNA | Voltammetry | 2 × 10−9 M | PCR product | [37] | |
| Anti-HBs and HAT-coated AuNP | HBsAg | FELISA | 5 × 10−4 IU/mL | HBsAg in PBS | ||
| Oligo-coated AuNP | DNA | Colorimetric, disposable paper strips | 1 × 10−9 M | N.A. | [27] | |
| Oligo-coated AuNP | HBV DNA | Colorimetric, dark-field microscope | 1 × 10−13 M | PCR product | ||
| HBsAg | Electrochemical | 0.343 pg/mL | [36] | |||
| Gold Nanostructure | HBsAg | SERS | 0.01 IU/mL | Serum | [46] | |
| Oligo-coated AuNP | HBV DNA | Electrochemical (impedance) | 111 copies/mL | Serum | [35] | |
| Magnetic nanoparticles | Immobilised, probe-conjungated NP | HBV DNA | Non-faradic impedance spectroscopy | 50 pMol in 20 µL; 2.5 × 10−6 M | Plasma and serum | [63] |
| Anti-HBsAg coated MNP | HBsAg | (cyclic) voltammetry | 0.9 pg/mL | HBsAg in PBS | [60] | |
| QDs | HBsAg-coated QDs | Anti-HBsAg | Lateral flow | 2 pg/mL | Anti-HBsAg | [67] |
| Magnetite and gold nanoparticles | Immobilised gold NP, competition between target DNA and MNP | HBV DNA | RCT | 3.1 (±0.1) × 10−13 M | Urine, plasma | [72] |
| Anti-HBsAg coated MNP and AuNP aggregation | HBsAg | Anodic stripping voltammetry | 87 pg/mL | HBsAg in PBS | [75] | |
| AuNPs and QDs | Immobilised QD, competition between target DNA and AuNP | Simultaneous HBV DNA and HCV RNA | Colorimetric, ECL quenching | 8.2 × 10−14 M (HBV) and 3.4 × 10−13 M (HCV) | Plasma | [76] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevtsov, M.; Zhao, L.; Protzer, U.; Klundert, M.A.A.v.d. Applicability of Metal Nanoparticles in the Detection and Monitoring of Hepatitis B Virus Infection. Viruses 2017, 9, 193. https://doi.org/10.3390/v9070193
Shevtsov M, Zhao L, Protzer U, Klundert MAAvd. Applicability of Metal Nanoparticles in the Detection and Monitoring of Hepatitis B Virus Infection. Viruses. 2017; 9(7):193. https://doi.org/10.3390/v9070193
Chicago/Turabian StyleShevtsov, Maxim, Lili Zhao, Ulrike Protzer, and Maarten A. A. van de Klundert. 2017. "Applicability of Metal Nanoparticles in the Detection and Monitoring of Hepatitis B Virus Infection" Viruses 9, no. 7: 193. https://doi.org/10.3390/v9070193







