Vaccination with Recombinant Baculovirus Expressing Ranavirus Major Capsid Protein Induces Protective Immunity in Chinese Giant Salamander, Andrias davidianus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Plasmid, Viruses, Cells and Animals
2.3. Generation of Recombinant Baculovirus
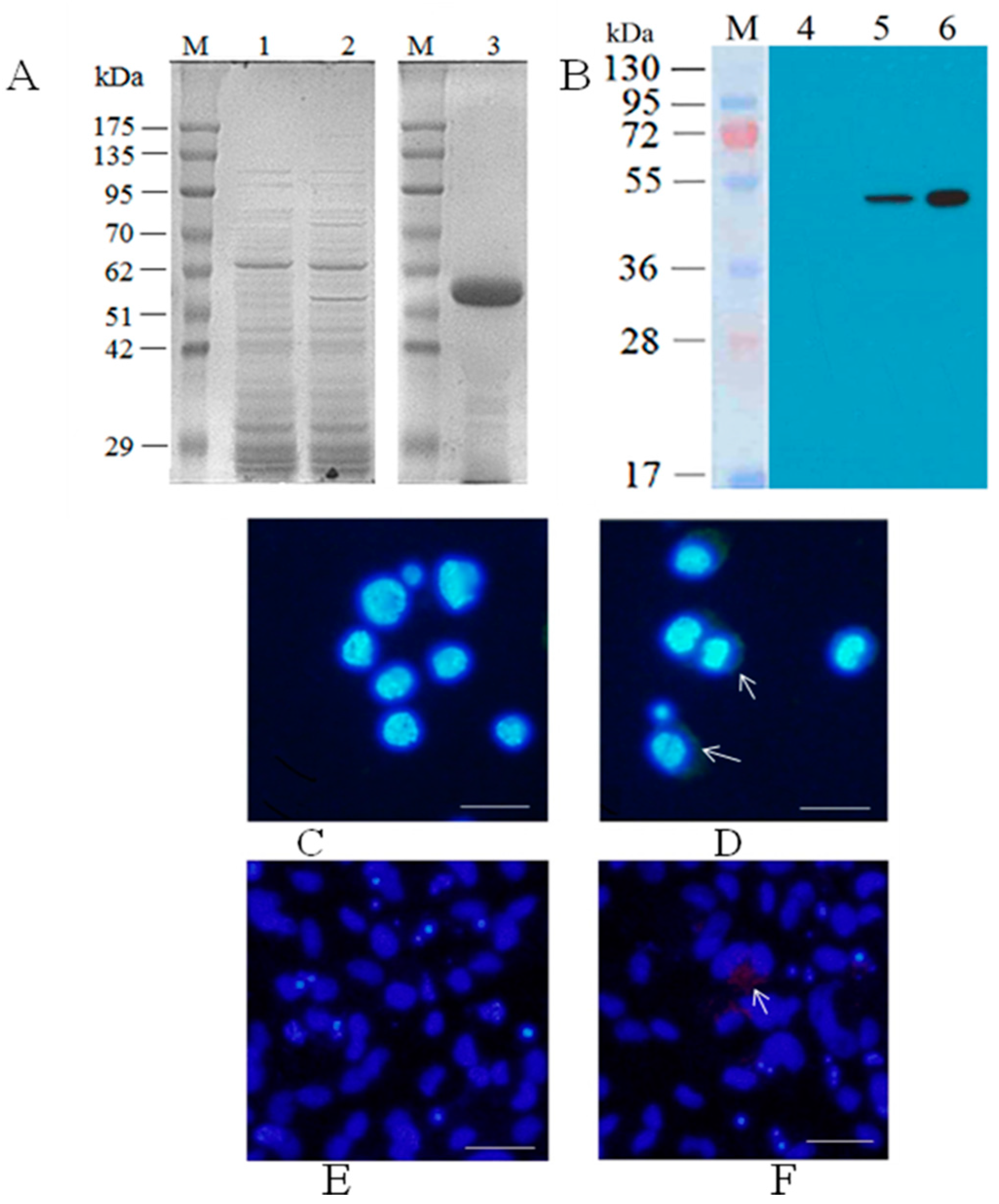
2.4. Expression and Purification of Major Capsid Protein
2.5. Western Blot
2.6. Indirect Immunofluorescence Assay (IIF)
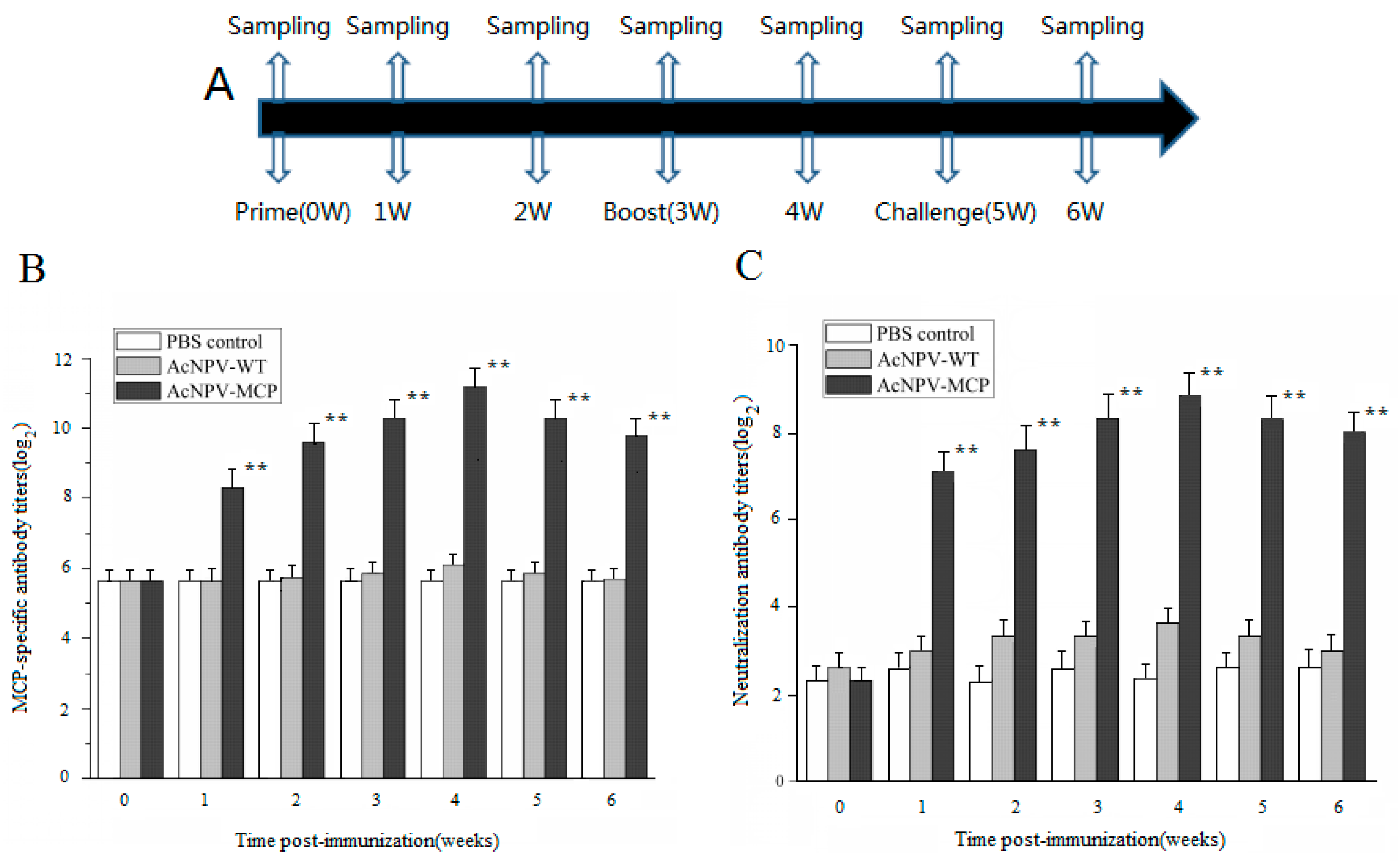
2.7. Animals Immunization and Serum Samples Collection
2.8. Indirect ELISA
2.9. Neutralization Assay
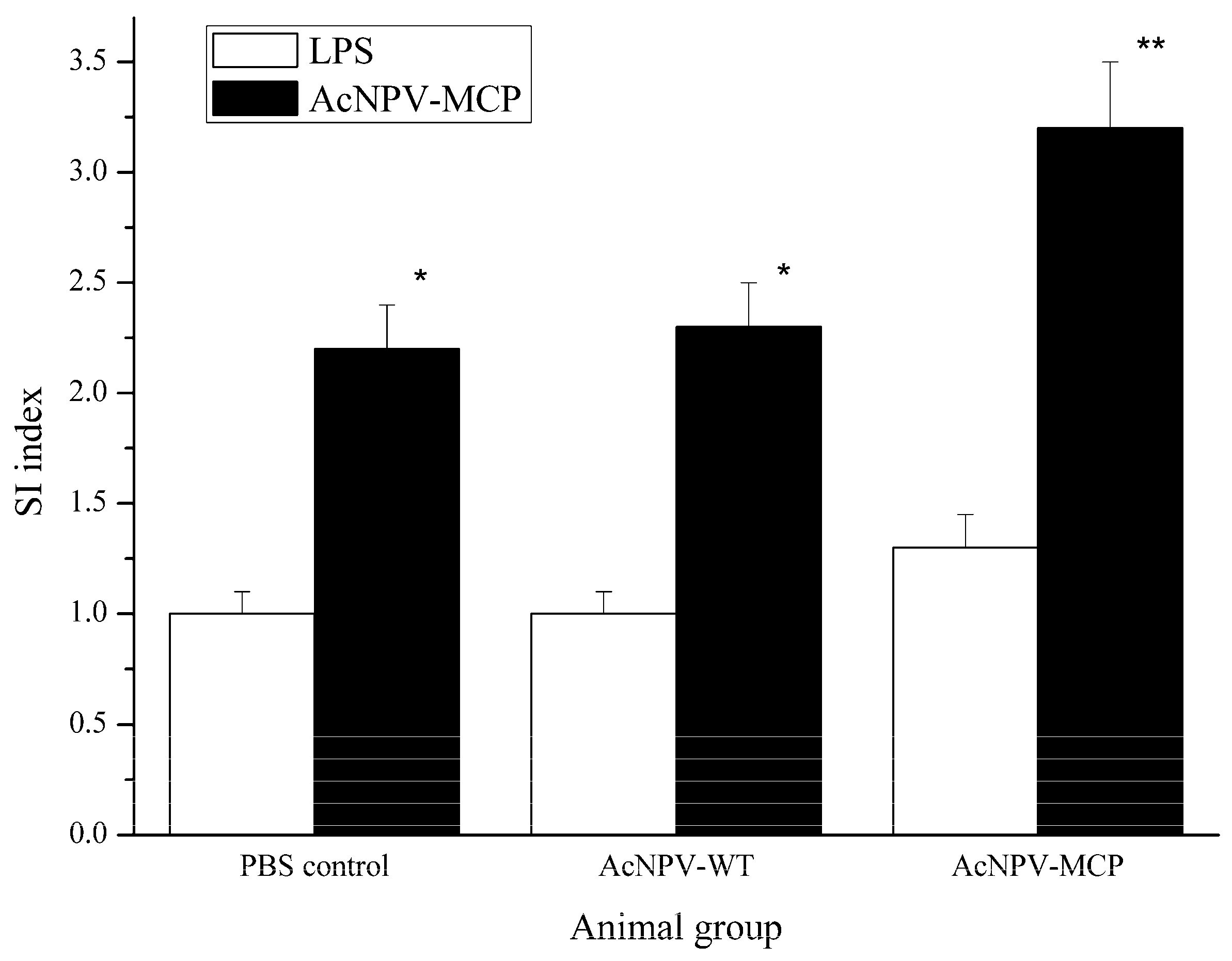
2.10. Splenocyte Proliferation
2.11. Quantitative Real-Time PCR
2.12. Challenge Test
2.13. Statistical Analysis
3. Results
3.1. Generation of Recombinant Baculovirus Expressing CGSIV MCP
3.2. Antibody Responses in Chinese Giant Salamanders Immunized with the Recombinant Baculoviruses
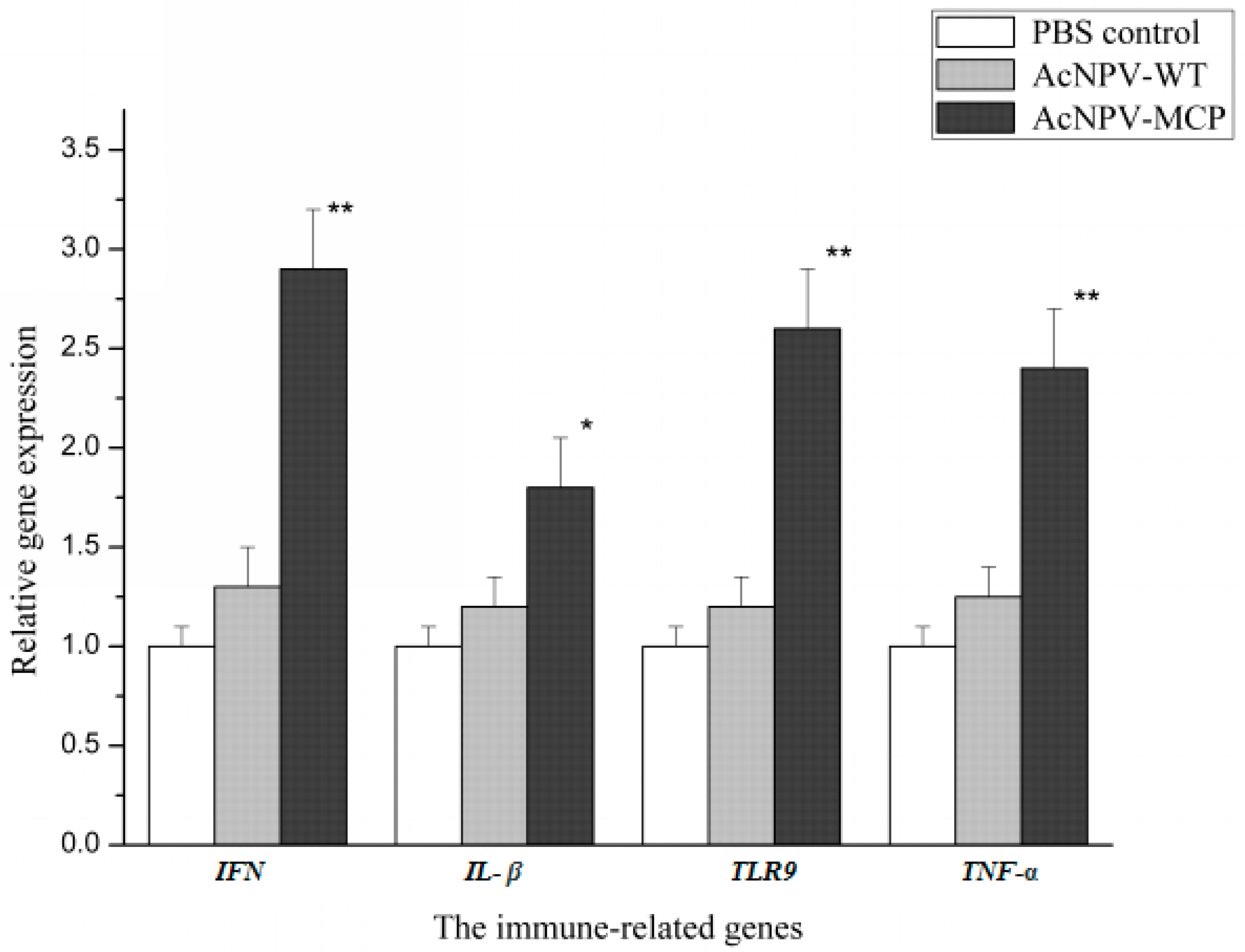
3.3. Cellular Immune Responses in Animals Immunized with the Recombinant Baculovirus of AcNPV-MCP
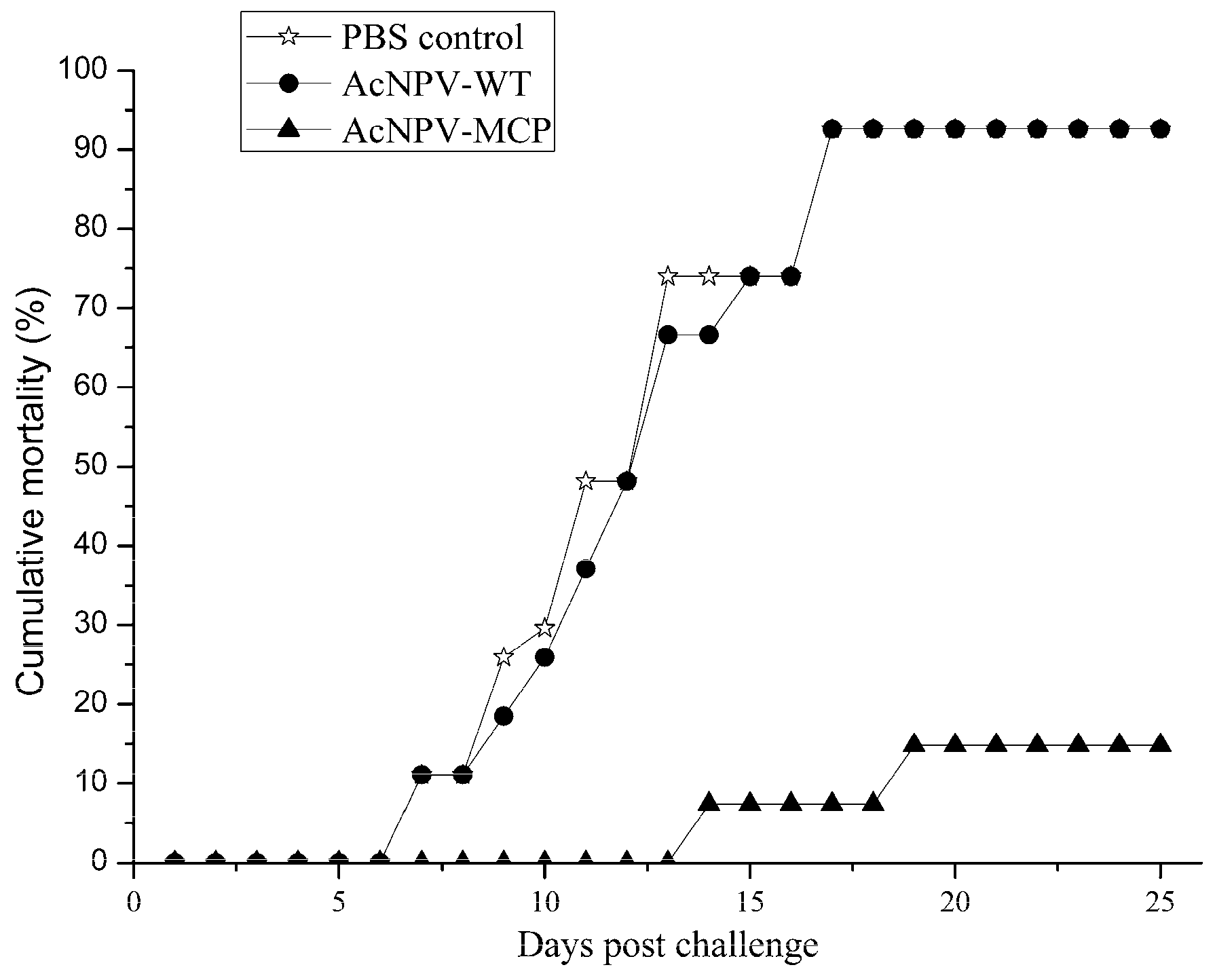
3.4. Protective Efficacy of the Recombinant Baculovirus
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duffus, A.L.J.; Waltzek, T.B.; Stöhr, A.C.; Allender, M.C.; Gotesman, M.; Whittington, R.J.; Hick, P.; Hines, M.K.; Marschang, R.E. Distribution and host range of ranaviruses. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Gray, M.J., Chinchar, V.G., Eds.; Springer: New York, NY, USA, 2015; pp. 9–30. [Google Scholar]
- Geng, Y.; Wang, K.Y.; Zhou, Z.Y.; Li, C.W.; Wang, J.; He, M.; Yin, Z.Q.; Lai, W.M. First report of a ranavirus associated with morbidity and mortality in farmed Chinese giant salamanders (Andrias davidianus). J. Comp. Pathol. 2010, 145, 95–102. [Google Scholar] [CrossRef]
- Dong, W.Z.; Zhang, X.M.; Yang, C.M.; An, J.H.; Qin, J.Z.; Song, F.F.; Zeng, W.X. Iridovirus infection in Chinese giant salamanders, China, 2010. Emerg. Infect. Dis. 2011, 17, 2388–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Weng, S.; Zhao, G.; He, J.; Dong, C. Virion-associated viral proteins of a Chinese giant salamander (Andrias davidianus) iridovirus (genus Ranavirus) and functional study of the major capsid protein (MCP). Vet. Microbiol. 2014, 172, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Ma, J.; Jiang, N.; Zeng, L.B.; Xiao, H.B. Pathological and microbiological findings from mortality of the Chinese giant salamander (Andrias davidianus). Arch. Virol. 2014, 159, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Gui, J.F. Virus genomes and virus-host interactions in aquaculture animals. Sci. China Life Sci. 2015, 58, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Zeng, L.B.; Fan, Y.D.; Meng, Y.; Zhou, Y.; Yang, X. The immunological responses and protection in Chinese giant salamander, Andrias davidianus, induced by the inactivated iridovirus. Vet. Microbiol. 2014, 174, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, S.B.; Fagutao, F.F.; Nho, S.W.; Jang, H.B.; Cha, I.S.; Thompson, K.D.; Adams, A.; Bayley, A.; Jung, T.S. Development of an immunochromatography assay kit for rapid detection of ranavirus. J. Virol. Methods 2014, 223, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-I.; Chiou, P.-P.; Gong, H.-Y.; Chou, H.-Y. Cloning of the major capsid protein (MCP) of grouper iridovirus of Taiwan (TGIV) and preliminary evaluation of a recombinant MCP vaccine against TGIV. Int. J. Mol. Sci. 2015, 16, 28647–28656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, Y.D.; LaPatra, S.E.; Ma, J.; Xu, J.; Meng, Y.; Jiang, N.; Zeng, L.B. Protective immunity of a Pichia pastoris expressed recombinant iridovirus major capsid protein in the Chinese giant salamander, Andrias davidianus. Vaccine 2015, 33, 5662–5669. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.H.; Zeng, L.B.; Zhou, Y.; Fan, Y.D.; Cheng, Q.; Liu, W.Z.; Zhang, X.P.; Zhang, L.L. Construction and immune efficacy of an MCP-containing DNA vaccine for Chinese giant salamander iridovirus. J. Fish. Sci. China 2015, 22, 1055–1067. [Google Scholar]
- Brumfild, S.; Willits, D.; Tang, L.; Johnson, J.E.; Douglas, T.; Young, M. Heterologous expression of the modified coat protein of Cowpea chlorotic mottle bromovirus results in the assembly of protein cages with altered architectures and function. J. Gen. Virol. 2004, 85, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Manna, S.K.; Thomas Allnutt, F.C. Viral vaccines for farmed finfish. Virus Disease 2014, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Blissard, G.W. Baculovirus-insect cell interactions. Cytotechnology 1996, 20, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Mahonen, A.J.; Airenne, K.J.; Purola, S.; Peltomaa, E.; Kaikkonen, M.U.; Riekkinen, M.S.; Heikura, T.; Kinnunen, K.; Roschier, M.M.; Wirth, T.; et al. Post-transcriptional regulatory element boosts baculovirus-mediated gene expression in vertebrate cells. J. Biotechnol. 2007, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brett, I.C.; Johansson, B.E. Immunization against influenza A virus: Comparison of conventional in activated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology 2005, 339, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.; Hüser, A.; Ni, S.; Tuve, S.; Kiviat, N.; Sow, P.S.; Hofmann, C.; Lieber, A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol. Ther. 2007, 15, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Syed Musthaq, S.; Madhan, S.; Sahul Hameed, A.S.; Kwang, J. Localization of VP28 on the baculovirus envelope and its immunogenicity against white spot syndrome virus in Penaeus monodon. Virology 2009, 391, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent end points. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- O’Reilly, D.; Miller, L.K.; Luckow, V.A. Baculovirus Expression Vectors: A Laboratory Manual; Freeman and Company: New York, NY, USA, 1992. [Google Scholar]
- Zhang, H.W.; Qian, P.; Liu, L.F.; Qian, S.H.; Chen, H.C.; Li, X.M. Virus-like particles of chimeric recombinant porcine circovirus type 2 as antigen vehicle carrying foreign epitopes. Viruses 2014, 6, 4839–4855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Zhang, X.L.; Jia, Q.H.; Han, Y.H.; Gao, H.W. Preparation and preliminary application of a monoclonal antibody against MCP COE protein of Chinese giant salamander (Andrias davidianus) iridovirus. Shui Chan Xue Bao 2016, 40, 1923–1930. [Google Scholar]
- Zhou, X.Y.; Zhang, X.L.; Jia, Q.H.; Han, Y.H.; Zhang, H. Establishment and Application of Serological Detection Method for Antibody against Chinese Giant Salamander (Andrias davidianus) Iridoviride; Yellow River Fisheries Research Institute, Chinese Academy of Fishery Sciences: Beijing, China, 2017; Unpublished work. [Google Scholar]
- Feng, Q.; He, Y.; Lu, J.H. Virus-like particle produced in Pichia pastoris induce protective immune responses against Coxsackievirus A16 in mice. Med. Sci. Monit. 2016, 22, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt. Method 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. International Symposium on Fish Biologics: Serodiagnostics and Vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Sample, R.; Bryan, L.; Long, S.; Majji, S.; Hoskins, G.; Sinning, A.; Oliver, J.; Chinchar, V.G. Inhibition of iridovirus protein synthesis and virus replication by antisense morpholino oligonucleotides targeted to the major capsid protein, the 18 kDa immediate-early protein, and a viral homolog of RNA polymerase II. Virology 2007, 358, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Whitley, D.S.; Sample, R.C.; Sinning, A.R.; Henegar, J.; Chinchar, V.G. Antisense approaches for elucidating ranavirus gene function in an infected fish cell line. Dev. Comp. Immunol. 2011, 35, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.; Takano, T.; Hirono, I.; Aoki, T. Genetic vaccines protect red seabream, Pagrus major, upon challenge with red seabream iridovirus (RSIV). Fish Shellfish Immunol. 2006, 21, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Limn, C.K.; Yap, C.C.; Onishi, M.; Nozaki, M.; Nishimune, Y.; Okahashi, N.; Kitagawa, Y.; Watanabe, R.; Mochizuki, R.; et al. In vitro and in vivo gene delivery by recombinant baculovimses. J. Virol. 2003, 77, 9799–9808. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, Z.; Sun, P.; Fu, Y.F.; Tian, F.P.; Hao, X.F.; Bao, H.F.; Liu, X.T.; Liu, Z.X. A pseudotype baculovirus expressing the capsid protein of foot-and-mouth disease virus and a T-Cell immunogen shows enhanced immunogenicity in mice. Virol. J. 2011, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Park, N.; Cho, H.-J.; Yoon, J.K.; Van, N.D.; Oh, Y.K.; Kim, Y.B. Development of a novel viral DNA vaccine against human papillomavirus: AcHERV-HP16L1. Vaccine 2010, 28, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Andino, F.; Chen, G.; Li, Z.; Grayfer, L.; Robert, J. Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 2012, 432, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Maniero, G.D.; Morales, H.; Gantress, J.; Robert, J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev. Comp. Immunol. 2006, 30, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, Z.; Wang, P.; Huang, X.; Cai, J.; Huang, Y.; Wei, S.; Ji, H.; Wei, J.; Zhou, Y.; Qin, Q. Immunogenicity and protective effects of inactivated Singapore grouper iridovirus (SGIV) vaccines in orange-spotted grouper, Epinephelus coioides. Dev. Comp. Immunol. 2012, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.A.; Tems, C.A.; Russell, P.H. Immunohistochemical demonstration of ranavirus antigen in the tissues of infected frogs (Rana temporaria) with systemic hemorrhagic or cutaneous ulcerative disease. J. Comp. Pathol. 2008, 138, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Fan, Y.D.; Zhou, Y.; Liu, W.Z.; Ma, J.; Meng, Y.; Xie, C.X.; Zeng, L.B. Characterization of Chinese giant salamander iridovirus tissue tropism and inflammatory response after infection. Dis. Aquat. Org. 2015, 114, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Caccamo, M.; Laird, G.; Leptin, M. Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 2007, 8, R251. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; de Jesús Andino, F.; Robert, J. The amphibian (Xenopus laevis) type I interferon response to frog virus 3: New insight into ranavirus pathogenicity. J. Virol. 2014, 88, 5766–5777. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, J.; Fan, Y.; Meng, Y.; Xu, J.; Zhou, Y.; Liu, W.; Zeng, X.; Zeng, L. Identification of type I IFN in Chinese giant salamander (Andrias davidianus) and the response to an iridovirus infection. Mol. Immunol. 2015, 65, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Induction of interferon by virus glycoprotein(s) in lymphoid cells through interaction with the cellular receptors via lectin-like action: An alternative interferon induction mechanism. Arch. Virol. 1994, 138, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.A.C.; Kim, C.H.; Johnson, M.C.; Drennan, J.D.; Simon, B.E.; Thomann, E. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol. 2000, 74, 7048–7054. [Google Scholar]
- Lorenzen, N.; Lorenzen, E.; Einer-Jensen, K.; LaPatra, S.E. DNA vaccines as a tool for analysing the protective immune response against rhabdoviruses in rainbow trout. Fish Shellfish Immunol. 2002, 12, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, Z.L.; Wang, P.R.; Huang, Y.H.; Huang, X.H.; Wan, Q.J.; Zhou, S.; Wei, J.G.; Zhou, Y.C.; Qin, Q.W. Selection and identification of Singapore grouper iridovirus vaccine candidate antigens using bioinformatics and DNA vaccination. Vet. Immunol. Immunopathol. 2012, 149, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Gui, J.F. Molecular regulation of interferon antiviral response in fish. Dev. Comp. Immunol. 2012, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hemmi, H.; Miyamoto, H.; Moriishi, K.; Tamura, S.; Takaku, H.; Akira, S.; Matsuura, Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 2005, 79, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.D.; Abramowitz, L.; Gertz, J.; Sowa, J.; Vogel, A.; Robert, J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J. Virol. 2010, 84, 4912–4922. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Abe, T.; Matsunaga, T.M.; Matsuura, Y. Baculovirus vector for gene delivery and vaccine development. Future Virol. 2008, 3, 35–43. [Google Scholar] [CrossRef]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Nucleotide Sequence (5′→3′) | Restriction Site | Usage | Accession Number |
|---|---|---|---|---|
| MCP | Fw CCGGAATTCATGTCTTCTGTAACTGG Rev CCCAAGCTTTTACAAGATTGGGAATCC | EcoR I Hind III | PCR | KF023635 |
| IFN | Fw ATTGGCGTGCCTTTTCGTGCTATT Rev GGGAAAGTGTCCACCCATCTGCTC | qRT-PCR | KM267637 | |
| IL-1 β | FwACCTTCCGGAAGGCAGTGGT Rev TTCTGCCATGGAGGTGACGT | qRT-PCR | KM103345 | |
| TNF-α | Fw ATGCCAGGACCAATGCTGGA Rev GGCCAGGTGCCTGGTAAGAA | qRT-PCR | KM079078 | |
| TLR9 | Fw GGTGGTTTTGATGCGTTTATTGTATTTC Rev ATTGTGTTGTTCGTGTTTCCTCCAGGTG | qRT-PCR | JN969980 | |
| β-actin | Fw TGAACCCAAAAGCCAACCGAGAAAAGAT Rev TACGACCAGAGGCATACAGGGACAGGAC | qRT-PCR | HQ822274 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, X.; Han, Y.; Jia, Q.; Gao, H. Vaccination with Recombinant Baculovirus Expressing Ranavirus Major Capsid Protein Induces Protective Immunity in Chinese Giant Salamander, Andrias davidianus. Viruses 2017, 9, 195. https://doi.org/10.3390/v9080195
Zhou X, Zhang X, Han Y, Jia Q, Gao H. Vaccination with Recombinant Baculovirus Expressing Ranavirus Major Capsid Protein Induces Protective Immunity in Chinese Giant Salamander, Andrias davidianus. Viruses. 2017; 9(8):195. https://doi.org/10.3390/v9080195
Chicago/Turabian StyleZhou, Xiaoyuan, Xinglang Zhang, Yahui Han, Qiuhong Jia, and Hongwei Gao. 2017. "Vaccination with Recombinant Baculovirus Expressing Ranavirus Major Capsid Protein Induces Protective Immunity in Chinese Giant Salamander, Andrias davidianus" Viruses 9, no. 8: 195. https://doi.org/10.3390/v9080195





