The Mouse Papillomavirus Infection Model
Abstract
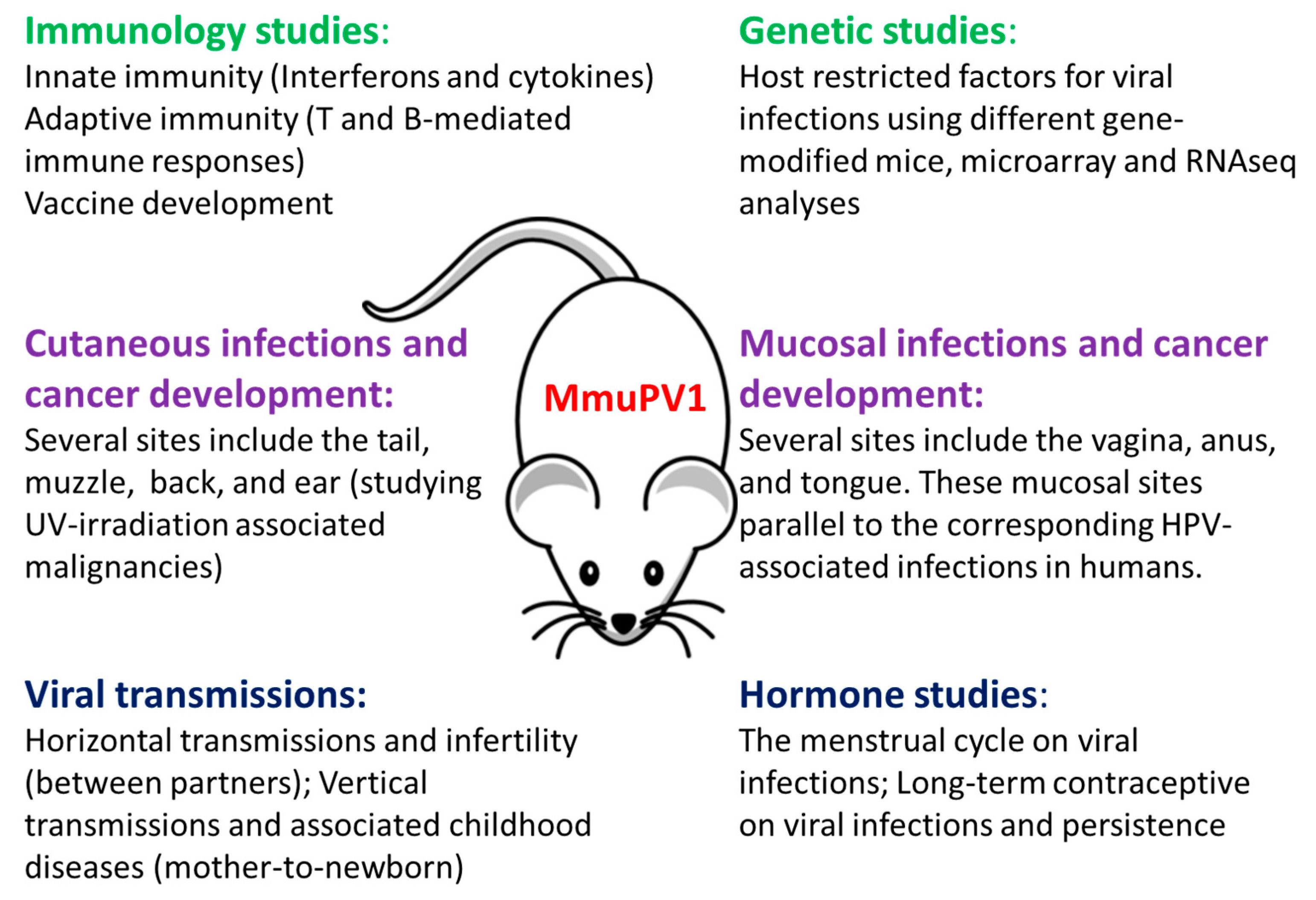
:1. Introduction
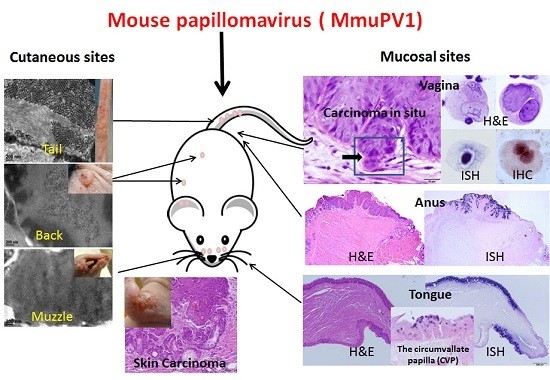
2. Mouse Papillomavirus Exhibits both Cutaneous and Mucosal Tropism
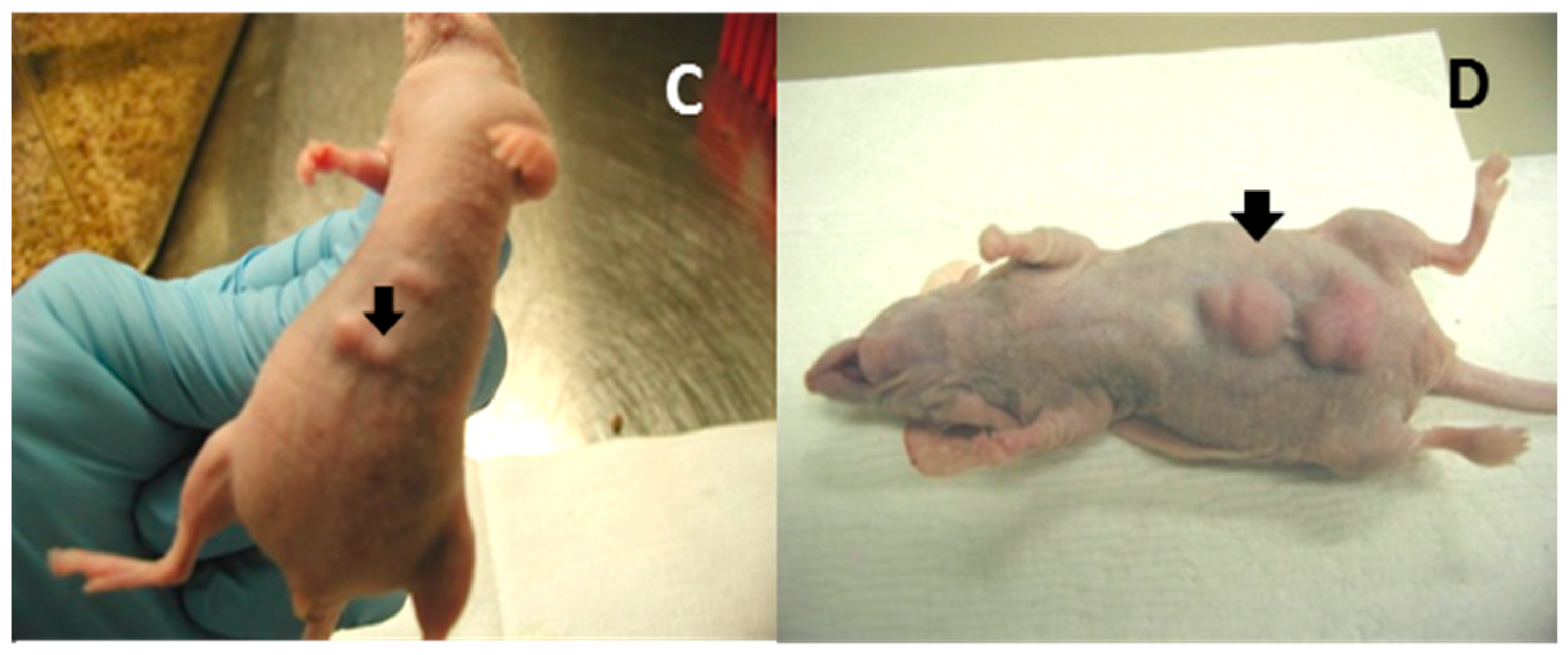
3. Certain Strains of Immunocompetent Mice Are Susceptible to MmuPV1 Infection
4. MmuPV1 Has Malignant Potential
4.1. Mouse Papillomavirus Oncogenes E6 and E7 Are Tumorigenic
4.2. Cutaneous Lesions Can Develop into Cancers
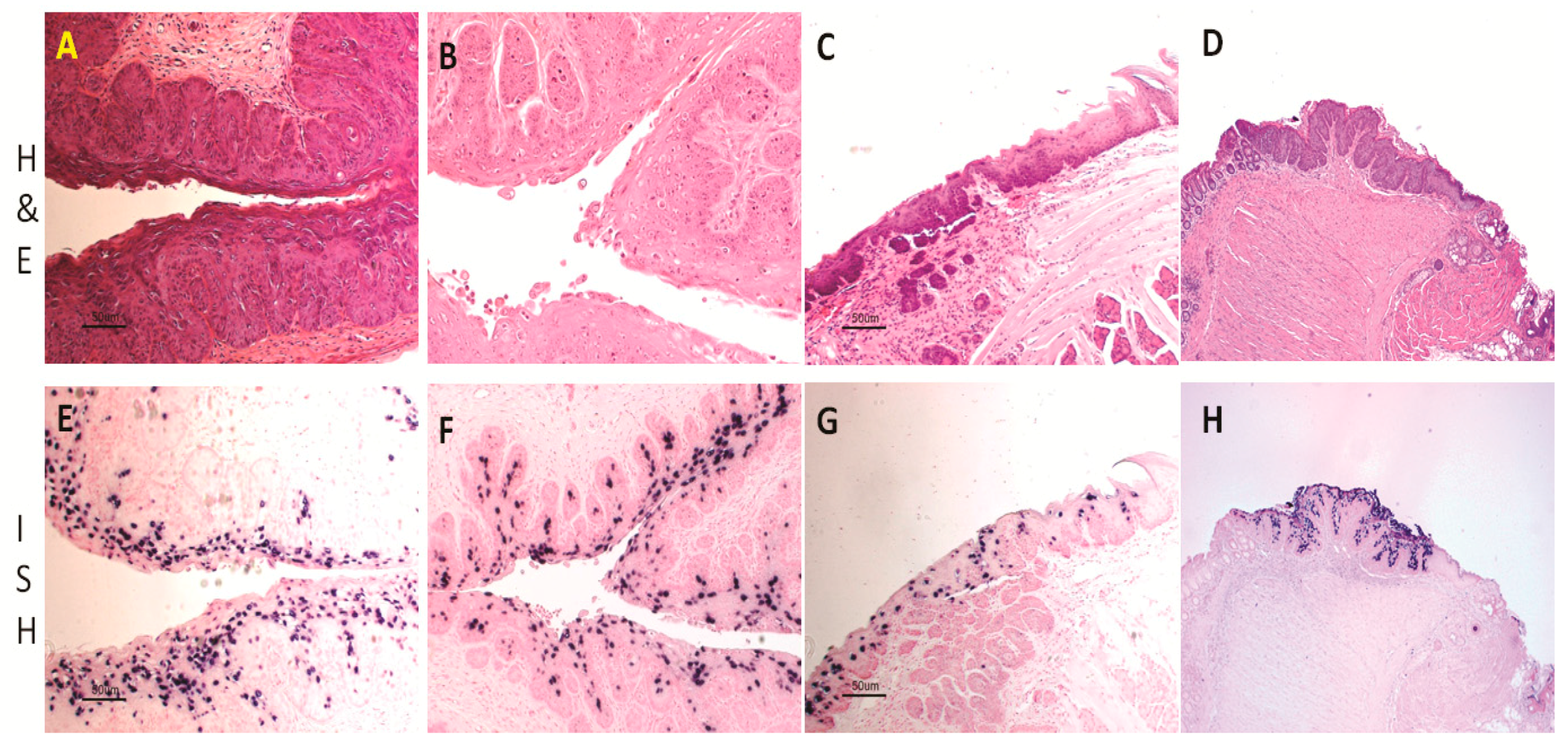
4.3. Mucosal Lesions Can Develop into Cancers
5. Immune Responses to MmuPV1 Infections
5.1. T- and B-Cell Mediated Immunity in the Control of MmuPV1 Infections
5.2. Innate Immunity in the Control of MmuPV1 Infection at Mucosal Sites
5.2.1. Neutrophils and NK Cells Are Associated with Decreased Local MmuPV1 Mucosal Infection in Immunocompetent Heterozygous (Foxn1nu/+) NU/J Mice
5.2.2. RNA Sequencing Data Support the Involvement of NK Cells and Neutrophils as Well as Type I IFNs in MmuPV1-Infected Tissues
6. Other Host-Restricted Factors in Local MmuPV1 Infections
7. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Shope, R.E.; Hurst, E.W. Infectious Papillomatosis of Rabbits: With a Note on the Histopathology. J. Exp. Med. 1933, 58, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; van Ranst, M. Animal papillomaviruses. Virology 2013, 445, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, P.A.; Greenoak, G.E.; Reeve, V.E.; Canfield, P.J.; Gissmann, L.; Gallagher, C.H.; Kulski, J.K. Identification of papillomaviral DNA sequences in hairless mouse tumours induced by ultraviolet irradiation. J. Gen. Virol. 1989, 70, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Reeve, V.E.; Greenoak, G.E.; Canfield, P.J.; Boehm-Wilcox, C.; Tilbrook, P.A.; Kulski, J.K.; Gallagher, C.H. Enhancement of UV-induced skin carcinogenesis in the hairless mouse by inoculation with cell-free extracts of skin tumours. Immunol. Cell Biol. 1989, 67, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Ghim, S.; Joh, J.; Chepkoech, I.; Bennett Jenson, A.; Sundberg, J.P. Novel laboratory mouse papillomavirus (MusPV) infection. Vet. Pathol. 2011, 48, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Jenson, A.B.; King, W.; Proctor, M.; Ingle, A.; Sundberg, J.P.; Ghim, S.J. Genomic analysis of the first laboratory-mouse papillomavirus. J. Gen. Virol. 2011, 92, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gottschling, M.; Ulrich, R.G.; Richter, D.; Stockfleth, E.; Nindl, I. Isolation of three novel rat and mouse papillomaviruses and their genomic characterization. PLoS ONE 2012, 7, e47164. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Ghim, S.J.; Chilton, P.M.; Sundberg, J.P.; Park, J.; Wilcher, S.A.; Proctor, M.L.; Bennett Jenson, A. MmuPV1 infection and tumor development of T cell-deficient mice is prevented by passively transferred hyperimmune sera from normal congenic mice immunized with MmuPV1 virus-like particles (VLPs). Exp. Mol. Pathol. 2016, 100, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.B.; Suo, L.; Ghim, S.; Bennett Jenson, A.; Sundberg, J.P. Retinoic acid metabolism proteins are altered in trichoblastomas induced by mouse papillomavirus 1. Exp. Mol. Pathol. 2015, 99, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Jenson, A.B.; Ingle, A.; Sundberg, J.P.; Ghim, S.J. Searching for the initiating site of the major capsid protein to generate virus-like particles for a novel laboratory mouse papillomavirus. Exp. Mol. Pathol. 2014, 96, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Stearns, T.M.; Joh, J.; Proctor, M.; Ingle, A.; Silva, K.A.; Dadras, S.S.; Jenson, A.B.; Ghim, S.J. Immune status, strain background, and anatomic site of inoculation affect mouse papillomavirus (MmuPV1) induction of exophytic papillomas or endophytic trichoblastomas. PLoS ONE 2014, 9, e113582. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Jenson, A.B.; Proctor, M.; Ingle, A.; Silva, K.A.; Potter, C.S.; Sundberg, J.P.; Ghim, S.J. Molecular diagnosis of a laboratory mouse papillomavirus (MusPV). Exp. Mol. Pathol. 2012, 93, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Yoshida, S.; Frazer, I.H.; Pitot, H.C.; Lambert, P.F. Role of Ultraviolet Radiation in Papillomavirus-Induced Disease. PLoS Pathog. 2016, 12, e1005664. [Google Scholar] [CrossRef] [PubMed]
- Handisurya, A.; Day, P.M.; Thompson, C.D.; Buck, C.B.; Pang, Y.Y.; Lowy, D.R.; Schiller, J.T. Characterization of Mus musculus papillomavirus 1 infection in situ reveals an unusual pattern of late gene expression and capsid protein localization. J. Virol. 2013, 87, 13214–13225. [Google Scholar] [CrossRef] [PubMed]
- Handisurya, A.; Day, P.M.; Thompson, C.D.; Bonelli, M.; Lowy, D.R.; Schiller, J.T. Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Mus musculus Papillomavirus 1. PLoS Pathog. 2014, 10, e1004314. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.M.; Uberoi, A.; Grace, M.; Lambert, P.F.; Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-β Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLoS Pathog. 2017, 13, e1006171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Jiang, R.; Peng, S.; Chang, Y.N.; Hung, C.F.; Roden, R.B. Immunologic Control of Mus musculus Papillomavirus Type 1. PLoS Pathog. 2015, 11, e1005243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.T.; Wang, J.W.; Peng, S.; Huang, T.C.; Wang, C.; Cannella, F.; Chang, Y.N.; Viscidi, R.P.; Best, S.R.A.; Hung, C.F.; et al. Spontaneous and vaccine-induced clearance of Mus musculus Papillomavirus type 1 (MmuPV1/MusPV1) infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.D.; Budgeon, L.R.; Cladel, N.M.; Hu, J. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 2017, 231, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. Mouse papillomavirus MmuPV1 infects oral mucosa and preferentially targets the base of the tongue. Virology 2016, 488, 73–80. [Google Scholar] [CrossRef] [PubMed]
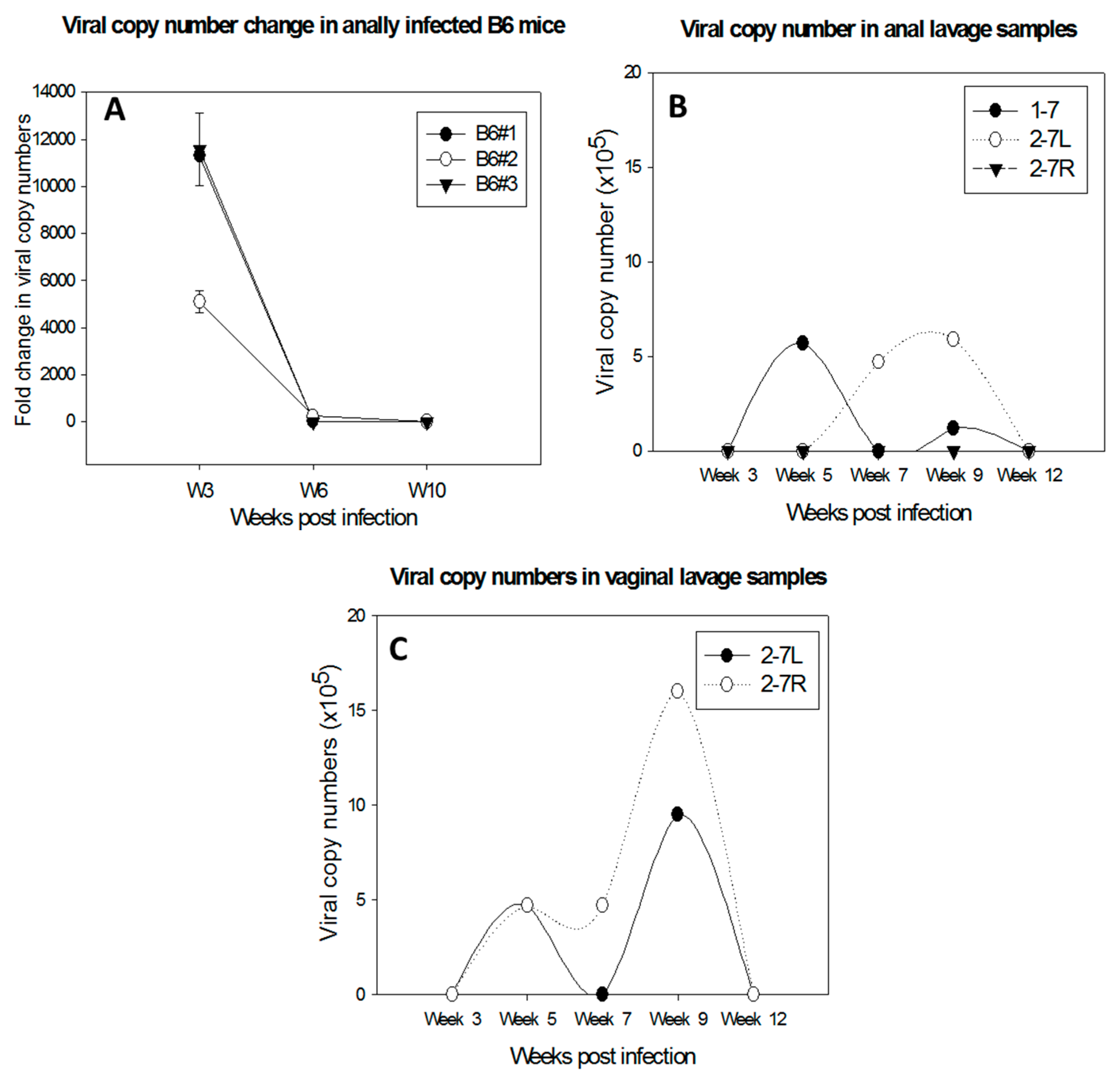
- Hu, J.; Budgeon, L.R.; Cladel, N.M.; Balogh, K.; Myers, R.; Cooper, T.K.; Christensen, N.D. Tracking vaginal, anal and oral infection in a mouse papillomavirus infection model. J. Gen. Virol. 2015, 96, 3554–3565. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. A novel pre-clinical murine model to study the life cycle and progression of cervical and anal papillomavirus infections. PLoS ONE 2015, 10, e0120128. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Cooper, T.K.; Balogh, K.K.; Hu, J.; Christensen, N.D. Secondary infections, expanded tissue tropism, and evidence for malignant potential in immunocompromised mice infected with Mus musculus papillomavirus 1 DNA and virus. J. Virol. 2013, 87, 9391–9395. [Google Scholar] [CrossRef] [PubMed]
- Woods, R., Sr.; O’Regan, E.M.; Kennedy, S.; Martin, C.; O’Leary, J.J.; Timon, C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J. Clin. Cases 2014, 2, 172–193. [Google Scholar] [PubMed]
- Hu, J.; Cladel, N.M.; Budgeon, L.R.; Christensen, N.D. Characterization of three rabbit oral papillomavirus oncogenes. Virology 2004, 325, 48–55. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Munger, K.; Howley, P.M. High-Risk Human Papillomavirus E7 Proteins Target PTPN14 for Degradation. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Peltre, B.; Cribier, B. Composite tumors associating trichoblastoma and benign epidermal/follicular neoplasm: Another proof of the follicular nature of inverted follicular keratosis. J. Cutan. Pathol. 2010, 37, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferons and viral infections. BioFactors 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Durbin, R.K.; Kotenko, S.V.; Durbin, J.E. Interferon induction and function at the mucosal surface. Immunol. Rev. 2013, 255, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef] [PubMed]
- De Craen, A.J.; Posthuma, D.; Remarque, E.J.; van den Biggelaar, A.H.; Westendorp, R.G.; Boomsma, D.I. Heritability estimates of innate immunity: An extended twin study. Genes Immun. 2005, 6, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.; Feng, P. Dissecting innate immune signaling in viral evasion of cytokine production. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sturgis, E.M.; Cao, X.; Song, X.; Salahuddin, T.; Wei, Q.; Li, G. Interleukin-10 promoter variants predict HPV-positive tumors and survival of squamous cell carcinoma of the oropharynx. FASEB J. 2013, 27, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chou, Y.Y.; Chang, T.L. Defensins in viral infections. J. Innate Immun. 2009, 1, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk, K.P.; Krzyzowska, M. Innate immunity to infection in the lower female genital tract. Postepy Hig. Med. Dosw. 2013, 67, 388–401. [Google Scholar] [CrossRef]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Miura, K.; Kinoshita, A.; Mishima, H.; Miura, S.; Yamasaki, K.; Hasegawa, Y.; Higashijima, A.; Jo, O.; Sasaki, K.; et al. Copy number variation of the antimicrobial-gene, defensin β4, is associated with susceptibility to cervical cancer. J. Hum. Genet. 2013, 58, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Erhart, W.; Alkasi, O.; Brunke, G.; Wegener, F.; Maass, N.; Arnold, N.; Arlt, A.; Meinhold-Heerlein, I. Induction of human β-defensins and psoriasin in vulvovaginal human papillomavirus-associated lesions. J. Infect. Dis. 2011, 204, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Skrygan, M.; Gambichler, T.; Brockmeyer, N.H.; Stucker, M.; Herzler, C.; Potthoff, A.; Altmeyer, P.; Pfister, H.; Wieland, U. Human papillomavirus-associated induction of human β-defensins in anal intraepithelial neoplasia. Br. J. Dermatol. 2009, 160, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Daud, I.I.; Scott, M.E.; Ma, Y.; Shiboski, S.; Farhat, S.; Moscicki, A.B. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int. J. Cancer 2011, 128, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tummers, B.; van Esch, E.M.; Goedemans, R.; Melief, C.J.; Meyers, C.; Boer, J.M.; van der Burg, S.H. Human Papillomavirus Downregulates the Expression of IFITM1 and RIPK3 to Escape from IFNγ- and TNFα-Mediated Antiproliferative Effects and Necroptosis. Front Immunol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, H.; Walther-Jallow, L.; Klareskog, E.; Baum, L.; French, A.L.; Patterson, B.K.; Garcia, P.; Spetz, A.L.; Landay, A.; Andersson, J. Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. J. Acquir. Immune. Defic. Syndr. 2007, 45, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Burg, S.H. High-risk human papillomavirus targets crossroads in immune signaling. Viruses 2015, 7, 2485–2506. [Google Scholar] [CrossRef] [PubMed]
- Amador-Molina, A.; Hernandez-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; DE Medeiros Fernandes, T.A.; DE Azevedo, J.C.; Cobucci, R.N.; DE Carvalho, M.G.; Andrade, V.S.; DE Araújo, J.M. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Torres-Poveda, K.; Bahena-Roman, M.; Madrid-Gonzalez, C.; Burguete-Garcia, A.I.; Bermudez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.D.; Lakenberg, N.; van der Breggen, R.; Houwing-Duistermaat, J.J.; Kloppenburg, M.; de Craen, A.J.; Beekman, M.; Meulenbelt, I.; Slagboom, P.E. A genome-wide linkage scan reveals CD53 as an important regulator of innate TNF-alpha levels. Eur. J. Hum. Genet. 2010, 18, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.G.; Troconis, J.N. Natural history of the infection for human papillomavirus: An actualization. Investig. Clin. 2014, 55, 82–91. [Google Scholar]
- Ting, J.; Rositch, A.F.; Taylor, S.M.; Rahangdale, L.; Soeters, H.M.; Sun, X.; Smith, J.S. Worldwide incidence of cervical lesions: A systematic review. Epidemiol. Infect. 2015, 143, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Dochez, C.; Bogers, J.J.; Verhelst, R.; Rees, H. HPV vaccines to prevent cervical cancer and genital warts: An update. Vaccine 2014, 32, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. Update on the treatment of genital warts. Dermatol. Online J. 2013, 19, 18559. [Google Scholar] [PubMed]
- Liu, H.; Li, J.; Zhou, Y.; Hu, Q.; Zeng, Y.; Mohammadreza, M.M. Human papillomavirus as a favorable prognostic factor in a subset of head and neck squamous cell carcinomas: A meta-analysis. J. Med. Virol. 2017, 89, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Sarti, E.; Barni, S. Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: An updated systematic review and meta-analysis of 30 trials. Head Neck 2014, 36, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Dalianis, T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review). Int. J. Oncol. 2014, 44, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Reddy, V.; Einarsdottir, H.; Longo, W.E. Anorectal Human Papillomavirus: Current Concepts. Yale J. Biol. Med. 2014, 87, 537–547. [Google Scholar] [PubMed]
- Varada, S.; Posnick, M.; Alessa, D.; Ramirez-Fort, M.K. Management of cutaneous human papillomavirus infection in immunocompromised patients. Curr. Probl. Dermatol. 2014, 45, 197–215. [Google Scholar] [PubMed]
- Mammas, I.N.; Sourvinos, G.; Spandidos, D.A. The paediatric story of human papillomavirus (Review). Oncol. Lett. 2014, 8, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Lo, C.M.; Lau, H.Y.; Lam, T.H. Vertically transmitted nasopharyngeal infection of the human papillomavirus: Does it play an aetiological role in nasopharyngeal cancer? Oral Oncol. 2014, 50, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Kaushic, C.; Ashkar, A.A.; Reid, L.A.; Rosenthal, K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 2003, 77, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- Gallichan, W.S.; Rosenthal, K.L. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 1996, 224, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Teepe, A.G.; Allen, L.B.; Wordinger, R.J.; Harris, E.F. Effect of the estrous cycle on susceptibility of female mice to intravaginal inoculation of herpes simplex virus type 2 (HSV-2). Antivir. Res. 1990, 14, 227–235. [Google Scholar] [CrossRef]
- Tang, W.W.; Young, M.P.; Mamidi, A.; Regla-Nava, J.A.; Kim, K.; Shresta, S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep. 2016, 17, 3091–3098. [Google Scholar] [CrossRef] [PubMed]

| Transcripts | MmuPV1 Infected Tissues | ||
|---|---|---|---|
| Muzzle | Tongue | Vagina | |
| IL15 | Down | Down | N.S. |
| Il1rn | UP | UP | N.S. |
| Il4ra | N.S. | UP | UP |
| IFNar1 | N.S. | UP | N.S. |
| Ifi27l2b | N.S. | Down | Down |
| Ifi27 | Down | N.S. | N.S. |
| Ifit2 | N.S. | N.S. | Down |
| TLR5 | N.S. | UP | N.S. |
| CXCR2 | UP | N.S. | N.S. |
| CD53 | Down | Down | Down |
| Stat3 | UP | UP | N.S. |
| Stat6 | UP | UP | N.S. |
| Trim23 | Down | Down | N.S. |
| Trim29 | UP | N.S. | UP |
| Defb4 | Down | Down | N.S. |
| Defb6 | Down | Down | N.S. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Christensen, N.D. The Mouse Papillomavirus Infection Model. Viruses 2017, 9, 246. https://doi.org/10.3390/v9090246
Hu J, Cladel NM, Budgeon LR, Balogh KK, Christensen ND. The Mouse Papillomavirus Infection Model. Viruses. 2017; 9(9):246. https://doi.org/10.3390/v9090246
Chicago/Turabian StyleHu, Jiafen, Nancy M. Cladel, Lynn R. Budgeon, Karla K. Balogh, and Neil D. Christensen. 2017. "The Mouse Papillomavirus Infection Model" Viruses 9, no. 9: 246. https://doi.org/10.3390/v9090246