A Recombinant HAV Expressing a Neutralization Epitope of HEV Induces Immune Response against HAV and HEV in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
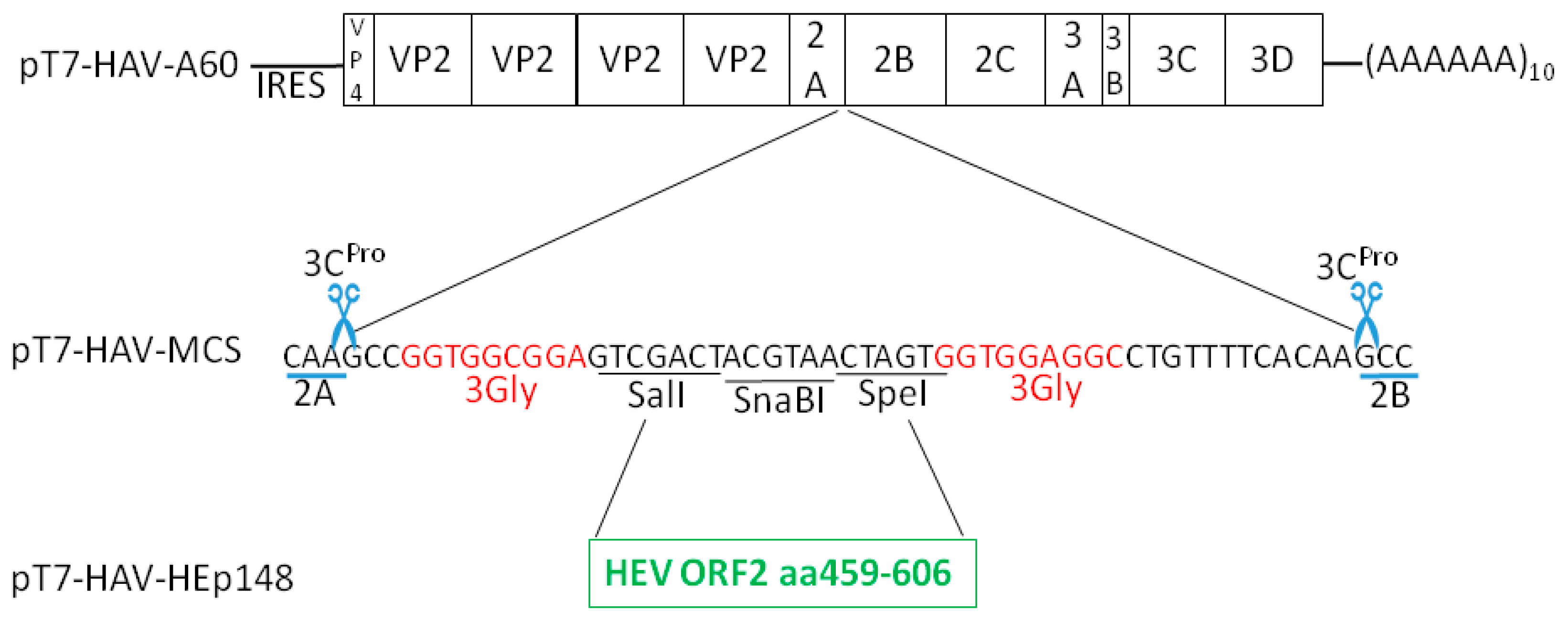
2.2. Plasmids and Constructs
2.3. pT7-HAV-Multiple Cloning Site
2.4. pT7-HAV-HEp148
2.5. RNA Transcription and Transfection
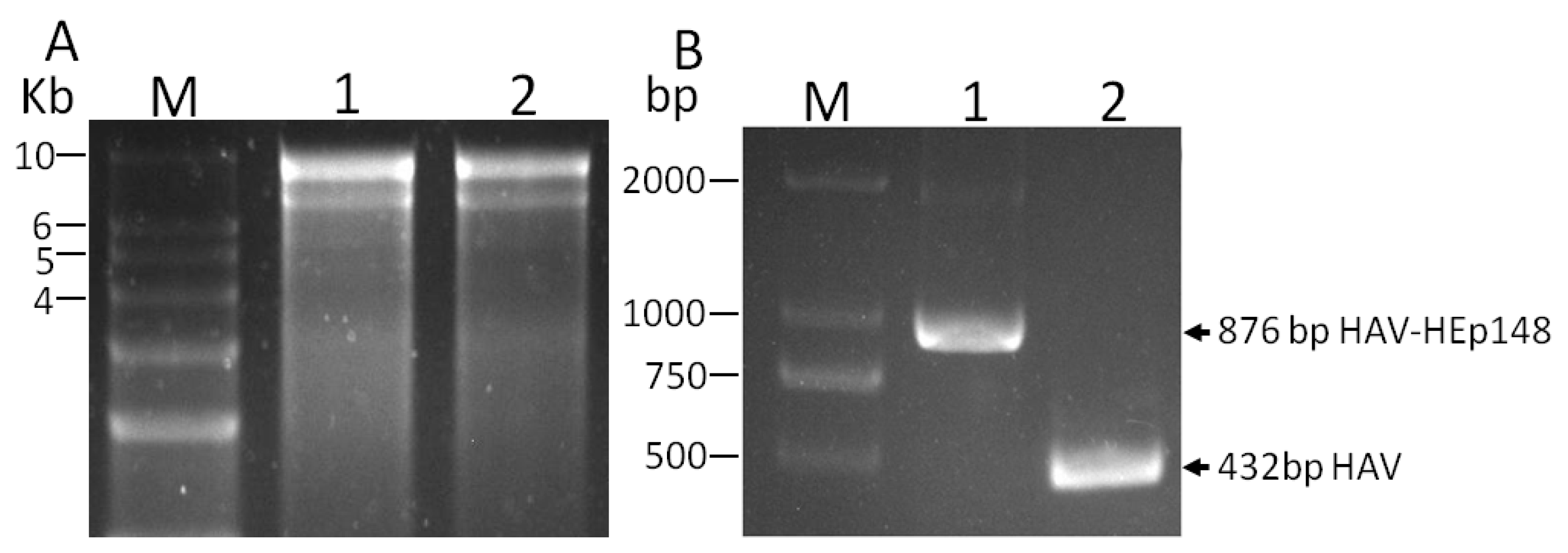
2.6. RT-PCR and Sequence Analysis of Viral RNA
2.7. Immunofluorescence for HAV and HEV Capsid Protein
2.8. Non-Denaturing Gel Electrophoresis and Western Blotting
2.9. Recombinant HAV Amplification and Titer Determination
2.10. Immunogenicity Analysis of the Recombinant Virus and Serum Collection
2.11. Anti-HAV and HEV Antibody Detection
3. Results
3.1. Insertions at the 2A/2B Junction
3.2. Rescue of Recombinant Hepatitis A Virus
3.3. Western Blot Identification of the HEV ORF2 Antigen
3.4. Recombinant Virus Elicits HAV- and HEV-Specific IgG
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, M.J. Summary of current nomenclature, taxonomy, and classification of various microbial agents. Viral taxonomy. Clin. Infect. Dis. 1993, 16, 612–613. [Google Scholar] [CrossRef] [PubMed]
- Wasley, A.; Fiore, A.; Bell, B.P. Hepatitis A in the era of vaccination. Epidemiol. Rev. 2006, 28, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Teng, J.L.L.; Chiu, T.H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E virus genotypes and evolution: Emergence of camel Hepatitis E variants. Int. J. Mol. Sci. 2017, 18, 869. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Hepatitis E vaccine: WHO position paper, May 2015. Wkly. Epidemiol. Rec. 2015, 90, 185–200. [Google Scholar]
- Wang, Z.; Chen, Y.; Xie, S.; Lv, H. Changing epidemiological characteristics of Hepatitis A in Zhejiang Province, China: Increased susceptibility in adults. PLoS ONE 2016, 11, e0153804. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Al Mohajer, M.; Shata, M.T. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int. 2008, 28, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Labrique, A.B.; Sikder, S.S.; Krain, L.J.; West, K.P., Jr.; Christian, P.; Rashid, M.; Nelson, K.E. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg. Infect. Dis. 2012, 18, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Coursaget, P.; Buisson, Y.; Enogat, N.; Bercion, R.; Baudet, J.M.; Delmaire, P.; Prigent, D.; Desrame, J. Outbreak of enterically-transmitted hepatitis due to hepatitis A and hepatitis E viruses. J. Hepatol. 1998, 28, 745–750. [Google Scholar] [CrossRef]
- Yayli, G.; Kilic, S.; Ormeci, A.R. Hepatitis agents with enteric transmission—An epidemiological analysis. Infection 2002, 30, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Provost, P.J.; Hilleman, M.R. Propagation of human hepatitis A virus in cell culture in vitro. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1979, 160, 213–221. [Google Scholar] [CrossRef]
- Orr, N.; Klement, E.; Gillis, D.; Sela, T.; Kayouf, R.; Derazne, E.; Grotto, I.; Balicer, R.; Huerta, M.; Aviram, L.; et al. Long-term immunity in young adults after a single dose of inactivated Hepatitis A vaccines. Vaccine 2006, 24, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xu, Z.; Yao, X.; Tian, M.; Zhou, L.; He, L.; Wen, Y. Immune responses of anti-HAV in children vaccinated with live attenuated and inactivated hepatitis A vaccines. Vaccine 2004, 22, 1941–1945. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.J.; Irving, G.; Wiersma, S.T. Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine 2012, 31, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Meng, Z.D.; Xu, Z.Y.; Guo, J.J.; Chai, S.A.; Duo, C.G.; Wang, X.Y.; Yao, J.F.; Liu, H.B.; Qi, S.X.; et al. H2 strain attenuated live hepatitis A vaccines: Protective efficacy in a hepatitis A outbreak. World J. Gastroenterol. 2000, 6, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.C.; Mao, Z.A.; Jiang, L.M.; Wu, J.; Chen, Y.Q.; Jiang, Q.; Chen, N.L.; Chai, S.A.; Mao, J.S. Long-term immunogenicity and effectiveness of live attenuated hepatitis A vaccine (H2-strain)-a study on the result of 15 years’ follow up. Zhonghua Liu Xing Bing Xue Za Zhi 2010, 31, 1332–1335. [Google Scholar] [PubMed]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Meng, J.; Dai, X.; Chang, J.C.; Lopareva, E.; Pillot, J.; Fields, H.A.; Khudyakov, Y.E. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology 2001, 288, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.H.; Guo, Q.S.; Ge, S.X.; Gu, Y.; Chen, Y.X.; Miao, J.; Du, H.L.; Shi, W.G.; Zhang, J.; Xia, N.S. The preliminary analysis of the recognition epitopes of anti-HEV monoclonal antibodies on HEV ORF2. Bing Du Xue Bao 2008, 24, 83–87. [Google Scholar] [PubMed]
- Li, S.; Tang, X.; Seetharaman, J.; Yang, C.; Gu, Y.; Zhang, J.; Du, H.; Shih, J.W.; Hew, C.L.; Sivaraman, J.; et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog. 2009, 5, e1000537. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Xia, N. Lessons from hepatitis E vaccine design. Curr. Opin. Virol. 2015, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Behloul, N.; Wen, J.; Zhang, J.; Meng, J. Role of asparagine at position 562 in dimerization and immunogenicity of the hepatitis E virus capsid protein. Infect. Genet. Evolut. J. Mol. Epidemiol. Evolut. Genet. Infect. Dis. 2016, 37, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Dai, X.; Meng, J.H. The first experimental study on a candidate combined vaccine against hepatitis A and hepatitis E. Vaccine 2007, 25, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Kui, X.; Sun, M.; Xie, T.; Wang, W.; Jiang, L.; Yan, M.; Ma, K.; Li, H. The expression, purification, and immunogenicity of a new chimeric virus-like particle. Viral Immunol. 2009, 22, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Su, Q.; Yi, Y.; Jia, Z.; Wang, H.; Lu, X.; Qiu, F.; Bi, S. Enhanced mucosal immune responses induced by a combined candidate mucosal vaccine based on Hepatitis A virus and Hepatitis E virus structural proteins linked to tuftsin. PLoS ONE 2015, 10, e0123400. [Google Scholar] [CrossRef] [PubMed]
- Beneduce, F.; Kusov, Y.; Klinger, M.; Gauss-Muller, V.; Morace, G. Chimeric hepatitis A virus particles presenting a foreign epitope (HIV gp41) at their surface. Antivir. Res. 2002, 55, 369–377. [Google Scholar] [CrossRef]
- Konduru, K.; Nakamura, S.M.; Kaplan, G.G. Hepatitis A virus (HAV) packaging size limit. Virol. J. 2009, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Kusov, Y.; Gosert, R.; Dzagurov, G.; Shatirishvili, G.; Gerhardt, M.; Gauss-Müller, V. Hepatitis A virus genome modified at its 3′ terminus. In Focus in Genome Research; Williams, C.R., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2004; pp. 1–38. [Google Scholar]
- Beard, M.R.; Cohen, L.; Lemon, S.M.; Martin, A. Characterization of recombinant hepatitis A virus genomes containing exogenous sequences at the 2A/2B junction. J. Virol. 2001, 75, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Throop, A.L.; LaBaer, J. Site-specific recombinational cloning using gateway and in-fusion cloning schemes. Curr. Protoc. Mol. Biol. 2015, 110, 3.20.1–3.20.23. [Google Scholar] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Hu, N.Z.; Hu, Y.Z.; Shi, H.J.; Liu, G.D.; Qu, S. Mutational characteristics in consecutive passage of rapidly replicating variants of hepatitis A virus strain H2 during cell culture adaptation. World J. Gastroenterol. 2002, 8, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.-L.; Yan, J.-E.; Chen, H.-B.; Long, R.-X.; Chen, T.-Q. The comparison of infectious titers of Hepatitis A virus (H2 strain) in three kinds of cells. J. Yunnan Univ. 1999, 21, 195–196. [Google Scholar]
- Cohen, J.I.; Ticehurst, J.R.; Feinstone, S.M.; Rosenblum, B.; Purcell, R.H. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J. Virol. 1987, 61, 3035–3039. [Google Scholar] [PubMed]
- Kaplan, G.; Lubinski, J.; Dasgupta, A.; Racaniello, V.R. In vitro synthesis of infectious poliovirus RNA. Proc. Natl. Acad. Sci. USA 1985, 82, 8424–8428. [Google Scholar] [CrossRef] [PubMed]
- Jameel, S.; Zafrullah, M.; Ozdener, M.H.; Panda, S.K. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 1996, 70, 207–216. [Google Scholar] [PubMed]
- Dotzauer, A.; Heitmann, A.; Laue, T.; Kraemer, L.; Schwabe, K.; Paulmann, D.; Flehmig, B.; Vallbracht, A. The role of immunoglobulin A in prolonged and relapsing hepatitis A virus infections. J. Gen. Virol. 2012, 93 Pt 4, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.S.; Walimbe, A.M.; Arankalle, V.A. Retrospective serological analysis of hepatitis E patients: A long-term follow-up study. J. Viral Hepat. 1999, 6, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Jiang, Q.; Gong, Y. Epidemiological effects of live attenuated hepatitis A vaccine (H(2)-strain): Results of A 10-year observation. Zhonghua Liu Xing Bing Xue Za Zhi 2001, 22, 188–190. [Google Scholar] [PubMed]
- Wang, X.Y.; Xu, Z.Y.; Ma, J.C.; von Seidlein, L.; Zhang, Y.; Hao, Z.Y.; Han, O.P.; Zhang, Y.L.; Tian, M.Y.; Ouyang, P.Y.; et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: Results from 8-year follow-up. Vaccine 2007, 25, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Y.; Wang, F.; Gong, X.; Wu, Z.; Miao, N.; Zhang, X.; Li, H.; Chen, C.; Hou, X.; et al. Comparing live attenuated and inactivated hepatitis A vaccines: An immunogenicity study after one single dose. Vaccine 2011, 29, 9098–9103. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Meng, J.H. Expression, purification and immunogenicity of a novel hepatitis E virus-like particle. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006, 22, 339–342. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, K.; Kusov, Y.; Ying, G.; Yan, W.; Shan, Y.; Jinyuan, W.; Na, Y.; Yan, Z.; Hongjun, L.; Maosheng, S. A Recombinant HAV Expressing a Neutralization Epitope of HEV Induces Immune Response against HAV and HEV in Mice. Viruses 2017, 9, 260. https://doi.org/10.3390/v9090260
Xiang K, Kusov Y, Ying G, Yan W, Shan Y, Jinyuan W, Na Y, Yan Z, Hongjun L, Maosheng S. A Recombinant HAV Expressing a Neutralization Epitope of HEV Induces Immune Response against HAV and HEV in Mice. Viruses. 2017; 9(9):260. https://doi.org/10.3390/v9090260
Chicago/Turabian StyleXiang, Kui, Yuri Kusov, Guan Ying, Wang Yan, Yi Shan, Wu Jinyuan, Yin Na, Zhou Yan, Li Hongjun, and Sun Maosheng. 2017. "A Recombinant HAV Expressing a Neutralization Epitope of HEV Induces Immune Response against HAV and HEV in Mice" Viruses 9, no. 9: 260. https://doi.org/10.3390/v9090260




