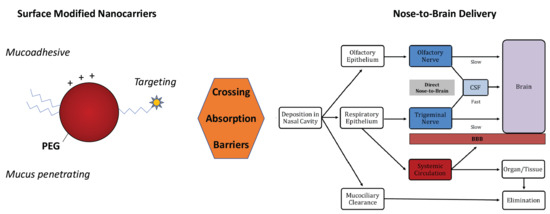
Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting
Abstract
:1. Pharmaceutical Nanotechnologies for Nose-to-Brain Delivery
- Protect the encapsulated drug from biological and/or chemical degradation
- Increase the drug apparent aqueous solubility
- Enhance the residence time at the site of absorption
- Promote mucosal permeation and/or cellular internalization
- Control the release kinetics of the encapsulated drug
- Achieve targeted drug delivery through surface modification with specific ligands
- Reduce the drug distribution to non-target sites, minimizing its systemic side effects.
2. Influence of Physicochemical Properties on Nanoparticles Nose-to-Brain Delivery
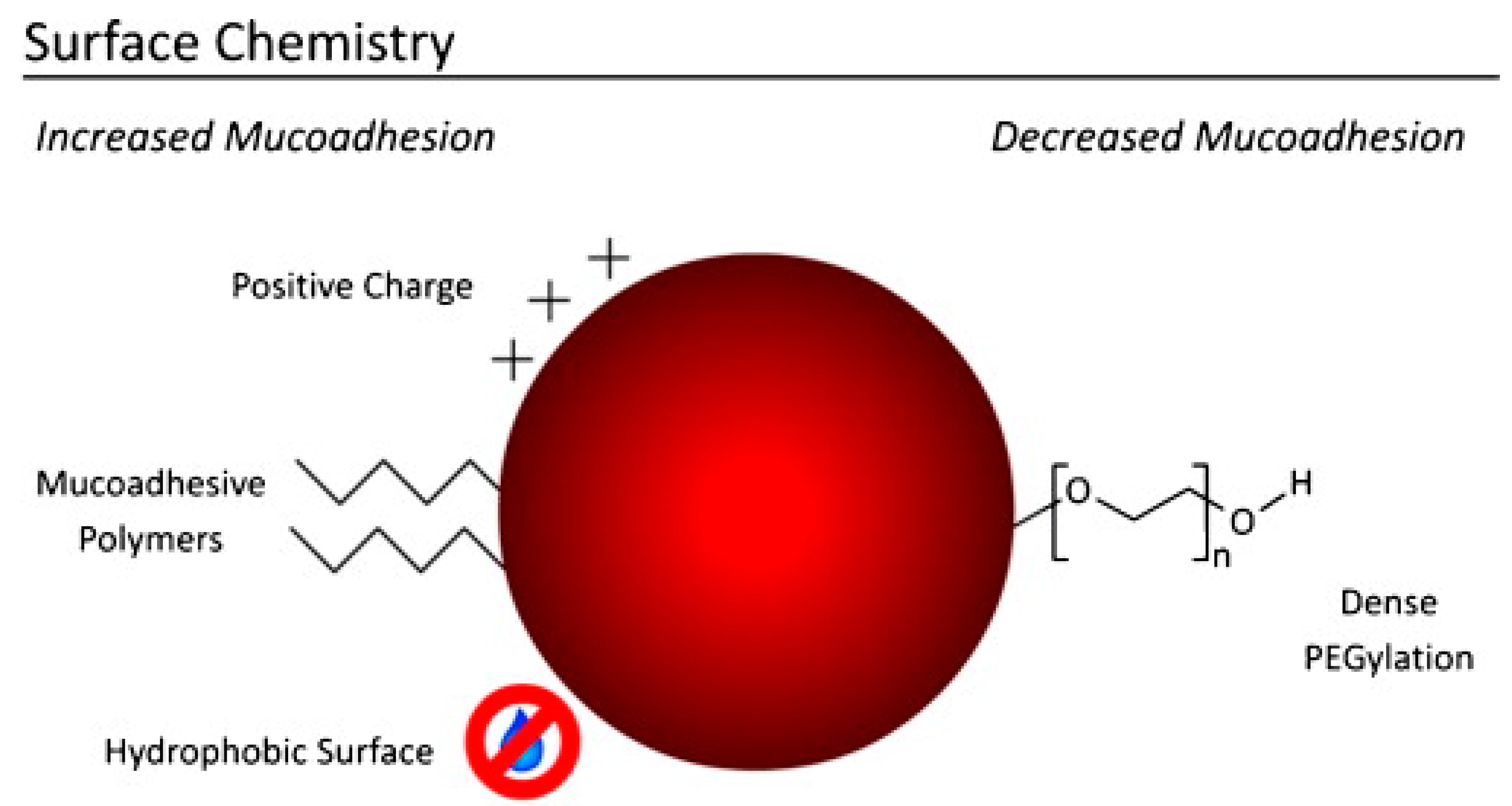
3. Mucoadhesive Nanoparticles
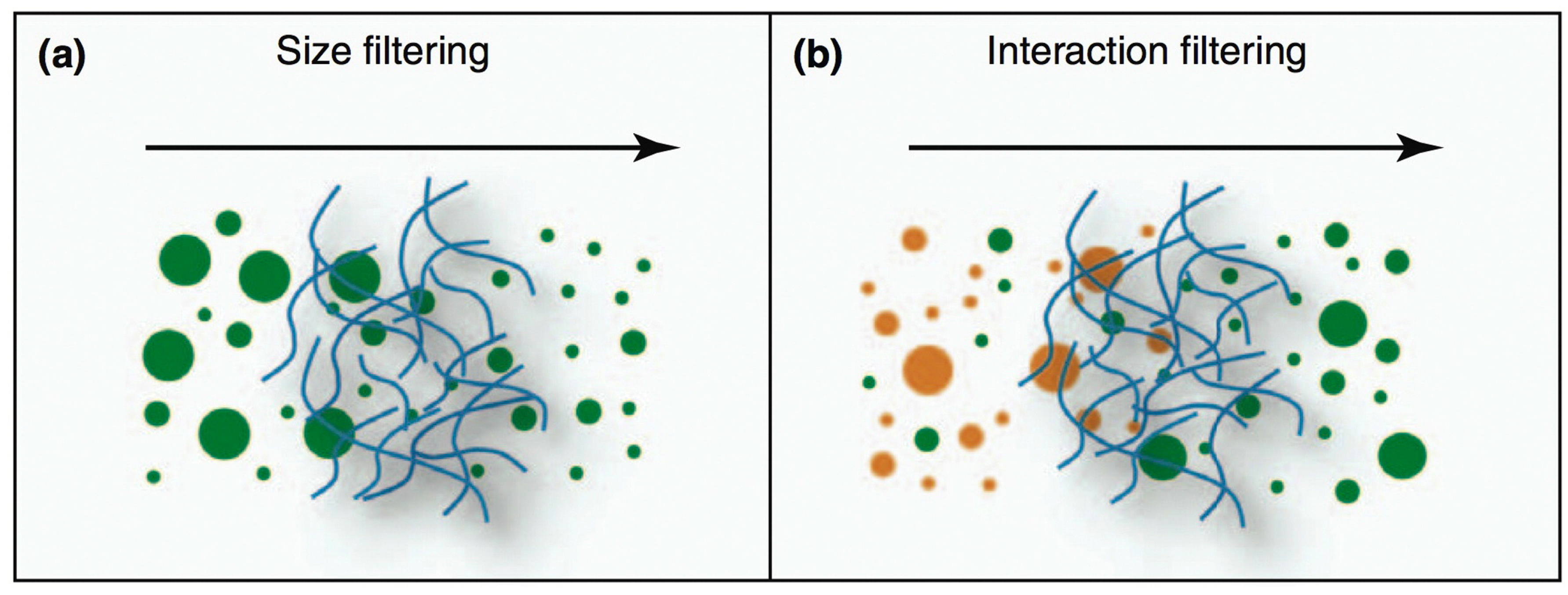
4. Beyond Bioadhesion: Mucus-Penetrating and Penetration-Enhancing Nanocarriers
4.1. Mucus-Penetrating Nanocarriers
4.2. Penetration-Enhancing Nanocarriers
5. Targeting the Nasal Epithelium for Optimizing Nose-to-Brain Delivery
5.1. Lectin-Modified Nanocarriers
5.2. Cell-Penetrating Peptides as Surface Ligands for Targeted Nanocarriers
5.3. Other Targeting Approaches
6. Future Perspectives of Nose-to-Brain Delivery with Nanocarriers
- Select a potent drug with unfavorable physicochemical characteristics for N2B;
- Design particles with biocompatible, biodegradable, Generally Recognized as Safe (GRAS) materials;
- Adopt a robust, validated, and up-scalable fabrication method;
- Determine drug release from nanocarriers in biorelevant conditions;
- Establish early on the safety and biodegradability pattern of the nanocarrier;
- If possible/advisable, adopt particles with sizes of 100–400 nm, as smaller ones are more likely to enter the CNS with consequent concerns related to the nanotoxicology of those materials;
- Develop bioanalytical methods able to detect the drug, instead of fluorescent or radioactive labels, in biodistribution studies, if possible;
- Develop methods allowing to track the particles in the tissues in order to differentiate free drug and nanomaterial biodistribution;
- Carry out the in vivo experiments perfusing the organs before dissection, in order to eliminate blood contamination from the analytical quantitation;
- Establish the pharmacokinetics of the free and nanoencapsulated drug, applying multiple and relevant controls (IV and IN administered solutions or formulations including absorption-promoting excipients);
- Determine relevant parameters such as drug targeting efficiency (DTE) and direct transport percentage (DTP);
- Establish the therapeutic proof of concept through pharmacodynamics studies in a disease model as close as possible to the human condition;
- Combine PK and PD data to critically predict the feasibility of the treatment in terms of drug dose, amount of formulation to administer, posology, etc.;
- Select the candidate formulation for pre-clinical/clinical development.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pozzoli, M.; Rogueda, P.; Zhu, B.; Smith, T.; Young, P.M.; Traini, D.; Sonvico, F. Dry powder nasal drug delivery: Challenges, opportunities and a study of the commercial Teijin Puvlizer Rhinocort device and formulation. Drug Dev. Ind. Pharm. 2016, 42, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, E.; Gil, S.; Andrieux, K.; Desmaële, D.; Nicolas, V.; Taran, F.; Georgin, D.; Andreux, J.P.; Roux, F.; Couvreur, P. A relevant in vitro rat model for the evaluation of blood-brain barrier translocation of nanoparticles. Cell. Mol. Life Sci. 2005, 62, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Boraschi, D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov. Today 2012, 17, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Ying, W. The nose may help the brain: Intranasal drug delivery for treating neurological diseases. Future Neurol. 2008, 3, 1–4. [Google Scholar] [CrossRef]
- Dhuria, S.V. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Comfort, C.; Garrastazu, G.; Pozzoli, M.; Sonvico, F. Opportunities and Challenges for the Nasal Administration of Nanoemulsions. Curr. Top. Med. Chem. 2015, 15, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Cady, R. A novel intranasal breath-powered delivery system for sumatriptan: A review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine. Expert Opin. Drug Deliv. 2015, 12, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hasegawa, M.; Kametani, S.; Ito, S. Nose-to-brain delivery of TS-002, prostaglandin D2 analogue. J. Drug Target. 2007, 15, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Lorenzini, L.; Zironi, E.; Galligioni, V.; Sonvico, F.; Sonvico, F.; Balducci, A.G.; Balducci, A.G.; Pagliuca, G.; Giuliani, A.; et al. Brain distribution of ribavirin after intranasal administration. Antivir. Res. 2011, 92, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; Van Gool, S.W.; De Vleeschouwer, S. Formulations for intranasal delivery of pharmacological agents to combat brain disease: A new opportunity to tackle GBM? Cancers 2013, 5, 1020–1048. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Bansal, A.; Hofman, F.; Chen, T.C.; Zada, G. A systematic review of inhaled intranasal therapy for central nervous system neoplasms: An emerging therapeutic option. J. Neurooncol. 2014, 116, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Ovadia, H.; Touitou, E. Nasal administration of drugs as a new non-invasive strategy for efficient treatment of multiple sclerosis. J. Neuroimmunol. 2013, 258, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Avachat, A.M. Pharmacodynamic and pharmacokinetic investigation of cyclodextrin-mediated asenapine maleate in situ nasal gel for improved bioavailability. Drug Dev. Ind. Pharm. 2017, 43, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.E.-E.; Waszczak, B.L. Intranasal gene delivery for treating Parkinsons disease: Overcoming the blood-brain barrier. Expert Opin. Drug Deliv. 2015, 12, 1923–1941. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jain, K.; Gowthamarajan, K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J. Drug Target. 2014, 22, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yi, X.; Zhao, Y.; Poon, C.-D.; Bullock, K.M.; Hansen, K.M.; Salameh, T.S.; Farr, S.A.; Banks, W.A.; Kabanov, A.V. Intranasal delivery of N-terminal modified leptin-pluronic conjugate for treatment of obesity. J. Control. Release 2017, 263, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Merkus, F.W.H.M.; van den Berg, M.P. Can Nasal Drug Delivery Bypass the Blood-Brain Barrier? Drugs R & D 2007, 8, 133–144. [Google Scholar]
- Illum, L. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2012, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, M.J.R.; de Lange, E.C.M. Emerging Insights for Translational Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Studies: Towards Prediction of Nose-to-Brain Transport in Humans. AAPS J. 2015, 17, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.R.; Breitner, J.C.S.; DeGroodt, W.; et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Westlye, L.T.; Hope, S.; Nærland, T.; Elvsåshagen, T.; Dørum, E.; Rustan, Ø.; Valstad, M.; Rezvaya, L.; Lishaugen, H.; et al. Dose-dependent social-cognitive effects of intranasal oxytocin delivered with novel Breath Powered device in adults with autism spectrum disorder: A randomized placebo-controlled double-blind crossover trial. Transl. Psychiatry 2017, 7, e1136. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, K.; Feifel, D. Helping oxytocin deliver: Considerations in the development of oxytocin-based therapeutics for brain disorders. Front. Neurosci. 2013, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Morimoto, B.H.; Tiong, J.; Fox, A.; Sutherland, K.; Dangoor, D.; Holser-Cochav, M.; Vered, K.; Newton, P.; Aisen, P.S.; et al. NAP: Research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP). CNS Drug Rev. 2005, 11, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, B.H.; Schmechel, D.; Hirman, J.; Blackwell, A.; Keith, J.; Gold, M. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2013, 35, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Lang, A.E.; Grossman, M.; Knopman, D.S.; Miller, B.L.; Schneider, L.S.; Doody, R.S.; Lees, A.; Golbe, L.I.; Williams, D.R.; et al. AL-108-231 Investigators Davunetide in patients with progressive supranuclear palsy: A randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014, 13, 676–685. [Google Scholar] [CrossRef]
- Landis, M.S.; Boyden, T.; Pegg, S. Nasal-to-CNS drug delivery: Where are we now and where are we heading? An industrial perspective. Ther. Deliv. 2012, 3, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Kürti, L.; Gáspár, R.; Márki, Á.; Kápolna, E.; Bocsik, A.; Veszelka, S.; Bartos, C.; Ambrus, R.; Vastag, M.; Deli, M.A.; et al. In vitro and in vivo characterization of meloxicam nanoparticles designed for nasal administration. Eur. J. Pharm. Sci. 2013, 50, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Sipos, P.; Budai-Szűcs, M.; Csányi, E.; Gáspár, R.; Márki, Á.; Seres, A.B.; Sztojkov-Ivanov, A.; Horváth, T.; et al. Study of sodium hyaluronate-based intranasal formulations containing micro- or nanosized meloxicam particles. Int. J. Pharm. 2015, 491, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.A.; Tadros, M.I. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: In vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int. J. Pharm. 2013, 452, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.A.; Abdelmalak, N.S.; Naguib, M.J.; Rashed, H.M.; Ibrahim, A.B. Intranasal brain-targeted clonazepam polymeric micelles for immediate control of status epilepticus: In vitro optimization, ex vivo determination of cytotoxicity, in vivo biodistribution and pharmacodynamics studies. Drug Deliv. 2016, 23, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability. Biomed. Res. Int. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: Formulation, optimization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2017, 11, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.; Pandit, J.; Vohora, D.; Aqil, M.; Ali, A.; Sultana, Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: In vitro characterization and in vivo efficacy in epilepsy. Expert Opin. Drug Deliv. 2014, 12, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Brain Targeting of Temozolomide via the Intranasal Route Using Lipid-Based Nanoparticles: Brain Pharmacokinetic and Scintigraphic Analyses. Mol. Pharm. 2016, 13, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Kubek, M.J.; Domb, A.J.; Veronesi, M.C. Attenuation of Kindled Seizures by Intranasal Delivery of Neuropeptide-Loaded Nanoparticles. Neurotherapeutics 2009, 6, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech 2015, 16, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, I.; Umar, S.; Iqbal, Z.; Samim, M.; Ahmad, F.J. PNIPAM nanoparticles for targeted and enhanced nose-to-brain delivery of curcuminoids: UPLC/ESI-Q-ToF-MS/MS-based pharmacokinetics and pharmacodynamic evaluation in cerebral ischemia model. Drug Deliv. 2016, 23, 2095–2114. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.R.; Ho, P.C. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: Implications for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2018, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.-T.; Sone, H.; Kurokawa, Y.; Zeng, Y.; Zeng, Q.; Nitta, H.; Hirano, S. Effects of PAMAM dendrimers in the mouse brain after a single intranasal instillation. Toxicol. Lett. 2014, 228, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Stepensky, D. Quantitative analysis of the brain-targeted delivery of drugs and model compounds using nano-delivery systems. J. Control. Release 2013, 171, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Mustafa, G.; Baboota, S.; Ali, J. Nanoneurotherapeutics approach intended for direct nose to brain delivery. Drug Dev. Ind. Pharm. 2015, 41, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery—A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: A mini review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Rinaldi, F.; Hanieh, P.N.; Paolino, D.; Marzio, L.D.; Carafa, M. Nose to Brain Delivery: New Trends in Amphiphile-Based “Soft” Nanocarriers. Curr. Pharm. Des. 2015, 21, 5225–5232. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T. Brain delivery of small interfering ribonucleic acid and drugs through intranasal administration with nano-sized polymer micelles. Med. Devices 2015, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Egusquiaguirre, S.P.; Bianco, J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M.; Préat, V.; Beloqui, A. Nanoparticle transport across in vitro olfactory cell monolayers. Int. J. Pharm. 2016, 499, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.-Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release 2014, 192, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Pellitteri, R.; Spatuzza, M.; Puglisi, G. Nose-to-brain delivery: Evaluation of polymeric nanoparticles on olfactory ensheathing cells uptake. J. Pharm. Sci. 2014, 103, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Murali, K.; Kenesei, K.; Li, Y.; Demeter, K.; Környei, Z.; Madarász, E. Uptake and bio-reactivity of polystyrene nanoparticles is affected by surface modifications, ageing and LPS adsorption: In vitro studies on neural tissue cells. Nanoscale 2015, 7, 4199–4210. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nose-to-Brain Delivery: Investigation of the Transport of Nanoparticles with Different Surface Characteristics and Sizes in Excised Porcine Olfactory Epithelium. Mol. Pharm. 2015, 12, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.-C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Kaneko, M.; Niide, T.; Akiyama, F.; Kakizaki, S.; Ibaraki, H.; Shiraishi, S.; Takashima, Y.; Suzuki, T.; Seta, Y. Enhancement of nose-to-brain delivery of hydrophilic macromolecules with stearate- or polyethylene glycol-modified arginine-rich peptide. Int. J. Pharm. 2017, 530, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Dorman, D.C.; Elder, A.; Veronesi, B. Neurological impacts from inhalation of pollutants and the nose-brain connection. NeuroToxicology 2012, 33, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Simkó, M.; Mattsson, M.O. Risks from accidental exposures to engineered nanoparticles and neurological health effects: A critical review. Part. Fibre Toxicol. 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Schipper, N.G.M.; Coos Verhoef, J.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Horvát, S.; Fehér, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Tóth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kürti, L.; et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Betbeder, D.; Spérandio, S.; Latapie, J.P.; de Nadai, J.; Etienne, A.; Zajac, J.M.; Francés, B. Biovector nanoparticles improve antinociceptive efficacy of nasal morphine. Pharm. Res. 2000, 17, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.N.; Betti, A.H.; Carvalho, F.C.; Gremião, M.P.D.; Dimer, F.A.; Guterres, S.S.; Tebaldi, M.L.; Rates, S.M.K.; Pohlmann, A.R. Mucoadhesive amphiphilic methacrylic copolymer-functionalized poly(ε-caprolactone) nanocapsules for nose-to-brain delivery of olanzapine. J. Biomed. Nanotechnol. 2014, 11, 1472–1481. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Devkar, T.B.; Tekade, A.R.; Khandelwal, K.R. Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer. Colloids Surf. B Biointerfaces 2014, 122, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Md, S.; Fazil, M.; Kumar, M.; Sahni, J.K.; Ali, J.; Baboota, S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012, 89, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, H.; Leng, W.; Tang, X. Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int. J. Pharm. 2006, 317, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Jafarieh, O.; Md, S.; Ali, M.; Baboota, S.; Sahni, J.K.; Kumari, B.; Bhatnagar, A.; Ali, J. Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2015, 41, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Md, S.; Hasan, Q.; Fazil, M.; Ali, A.; Baboota, S.; Ali, J. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. 2014, 23, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2017, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Javia, A.; Thakkar, H. Intranasal delivery of tapentadol hydrochloride–loaded chitosan nanoparticles: Formulation, characterisation and its in vivo evaluation. J. Microencapsul. 2017, 34, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pandey, R.S.; Patra, K.C.; Jain, S.K.; Soni, M.L.; Dangi, J.S.; Madan, J. Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int. J. Biol. Macromol. 2013, 61, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Shahnaz, G.; Vetter, A.; Barthelmes, J.; Rahmat, D.; Laffleur, F.; Iqbal, J.; Perera, G.; Schlocker, W.; Dünnhaput, S.; Augustijns, P.; et al. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: Bioavailability and pharmacokinetic characterization. Int. J. Pharm. 2012, 428, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Naik, S.; Misra, A. Improved transnasal transport and brain uptake of tizanidine HCl-loaded thiolated chitosan nanoparticles for alleviation of pain. J. Pharm. Sci. 2012, 101, 690–706. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Naik, S.; Chuttani, K.; Mathur, R.; Mishra, A.K.; Misra, A. Intranasal delivery of cyclobenzaprine hydrochloride-loaded thiolated chitosan nanoparticles for pain relief. J. Drug Target. 2013, 21, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2016, 44, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; De Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Chalikwar, S.S.; Mene, B.S.; Pardeshi, C.V.; Belgamwar, V.S.; Surana, S.J. Self-Assembled, Chitosan Grafted PLGA Nanoparticles for Intranasal Delivery: Design, Development and Ex Vivo Characterization. Polym. Plast. Technol. Eng. 2013, 52, 368–380. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef] [PubMed]
- Romana, B.; Batger, M.; Prestidge, C.; Colombo, G.; Sonvico, F. Expanding the Therapeutic Potential of Statins by Means of Nanotechnology Enabled Drug Delivery Systems. Curr. Top. Med. Chem. 2014, 14, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Zimetti, F.; Pohlmann, A.R.; Guterres, S.S. Drug delivery to the brain: How can nanoencapsulated statins be used in the clinic? Ther. Deliv. 2017, 8, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.L.; et al. The nasal delivery of nanoencapsulated statins—An approach for brain delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Samia, O.; Hanan, R.; Kamal, E.T. Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 2012, 19, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.P.; Mundiña-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Increased brain radioactivity by intranasal P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int. J. Nanomed. 2012, 7, 1373–1385. [Google Scholar]
- Jain, D.S.; Bajaj, A.N.; Athawale, R.B.; Shikhande, S.S.; Pandey, A.; Goel, P.N.; Gude, R.P.; Patil, S.; Raut, P. Thermosensitive PLA based nanodispersion for targeting brain tumor via intranasal route. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wavikar, P.R.; Vavia, P.R. Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery. J. Liposome Res. 2015, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhao, J.; Zhang, S.; Tong, T.; Zhuang, Q.; Jin, K.; Chen, W.; Tang, H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf. B Biointerfaces 2016, 147, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Vila, A.; Gill, H.; McCallion, O.; Alonso, M.J. Transport of PLA-PEG particles across the nasal mucosa: Effect of particle size and PEG coating density. J. Control. Release 2004, 98, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Suk, J.S.; Pace, A.; Wang, Y.-Y.; Yang, M.; Mert, O.; Chen, J.; Kim, J.; Hanes, J. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials 2011, 32, 6285–6290. [Google Scholar] [CrossRef] [PubMed]
- Mert, O.; Lai, S.K.; Ensign, L.; Yang, M.; Wang, Y.-Y.; Wood, J.; Hanes, J. NANOMEDICINE. J. Control. Release 2012, 157, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Boylan, N.J.; Cai, S.; Miao, B.; Patel, H.; Hanes, J. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J. Control. Release 2013, 170, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kirch, J.; Schneider, A.; Abou, B.; Hopf, A.; Schaefer, U.F.; Schneider, M.; Schall, C.; Wagner, C.; Lehr, C.-M. Optical tweezers reveal relationship between microstructure and nanoparticle penetration of pulmonary mucus. Proc. Natl. Acad. Sci. USA 2012, 109, 18355–18360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, R.K.; Bhatnagar, A.; Nishad, D.K.; Singh, T.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose to Brain Delivery of Midazolam Loaded PLGA Nanoparticles: In Vitro and In Vivo Investigations. Curr. Drug Deliv. 2016, 13, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Lüle, S.; Bozdağ Pehlivan, S.; Öztürk, N.; Kara, A.; Kaffashi, A.; Vural, I.; Işıkay, I.; Yavuz, B.; Oguz, K.K.; et al. A potential non-invasive glioblastoma treatment: Nose-to-brain delivery of farnesylthiosalicylic acid incorporated hybrid nanoparticles. J. Control. Release 2017, 261, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Zha, L.-S.; Zhang, Y.; Jiang, W.-M.; Lu, W.; Shi, Z.-Q.; Jiang, X.-G.; Fu, S.-K. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J. Drug Target. 2006, 14, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Pena, A.I.V.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Nabar, S.; Dandekar, P.; Patravale, V. Micellar nanocarriers: Potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm. Res. 2010, 27, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Nabar, S.; Dandekar, P.; Hassan, P.; Aswal, V.; Talmon, Y.; Shet, T.; Borde, L.; Ray, K.; Patravale, V. Formulation and evaluation of novel micellar nanocarrier for nasal delivery of sumatriptan. Nanomedicine 2010, 5, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A.S. Phospholipid based colloidal poloxamer-nanocubic vesicles for brain targeting via the nasal route. Colloids Surf. B Biointerfaces 2012, 100, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Elshafeey, A.H.; Mohamed, M.I. Response surface optimization, Ex vivo and In vivo investigation of nasal spanlastics for bioavailability enhancement and brain targeting of risperidone. Int. J. Pharm. 2017, 530, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Li, X.; Lu, C.-T.; Lin, M.; Chen, L.-J.; Xiang, Q.; Zhang, M.; Jin, R.R.; Jiang, X.; Shen, X.-T.; et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine 2014, 10, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery--possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.A.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In vitro preparation, optimization and in vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Saraf, S.K.; Saraf, S.A. SLN approach for nose-to-brain delivery of alprazolam. Drug Deliv. Transl. Res. 2012, 2, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Mishra, V.; Jain, N.K. Receptor-based targeting of therapeutics. Ther. Deliv. 2013, 4, 369–394. [Google Scholar] [CrossRef] [PubMed]
- Ruenraroengsak, P.; Cook, J.M.; Florence, A.T. Nanosystem drug targeting: Facing up to complex realities. J. Control. Release 2010, 141, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Bies, C.; Lehr, C.-M.; Woodley, J.F. Lectin-mediated drug targeting: History and applications. Adv. Drug Deliv. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Broadwell, R.D.; Balin, B.J. Endocytic and exocytic pathways of the neuronal secretory process and trans synaptic transfer of wheat germ agglutinin-horseradish peroxidasein vivo. J. Comp. Neurol. 1985, 242, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Emory, C.R.; Ala, T.A.; Frey, W.H. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995, 692, 278–282. [Google Scholar] [CrossRef]
- Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG-PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials 2006, 27, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, Y.; Chen, J.; Gao, X.; Feng, C.; Wang, L.; Zhang, Q.; Jiang, X. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm. Res. 2012, 29, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J.; Chen, J.; Wu, B.; Chen, H.; Jiang, X. Quantum Dots Bearing Lectin-Functionalized Nanoparticles as a Platform for In Vivo Brain Imaging. Bioconjug. Chem. 2008, 19, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosumlectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2011, 20, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J.; Tao, W.; Zhu, J.; Zhang, Q.; Chen, H.; Jiang, X. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int. J. Pharm. 2007, 340, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yan, Z.; He, R.; Pang, Z.; Guo, L.; Qian, Y.; Jiang, X.; Fang, L. Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv. 2011, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Piazza, J.; Hoare, T.; Molinaro, L.; Terpstra, K.; Bhandari, J.; Selvaganapathy, P.R.; Gupta, B.; Mishra, R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)–block-poly(d,l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014, 87, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shao, X.; Chen, J.; Shen, Y.; Feng, C.; Gao, X.; Zhao, Y.; Li, J.; Zhang, Q.; Jiang, X. In vivo toxicity and immunogenicity of wheat germ agglutinin conjugated poly(ethylene glycol)-poly(lactic acid) nanoparticles for intranasal delivery to the brain. Toxicol. Appl. Pharmacol. 2011, 251, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.B.; Pereira, M.P.; Kelley, S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, E.; He, H.; Shin, M.C.; Moon, C.; Yang, V.C.; Huang, Y. Nose-to-brain delivery of macromolecules mediated by cell-penetrating peptides. Acta Pharm. Sin. B 2016, 6, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Gao, X.; Gu, G.; Liu, Z.; Zeng, N.; Hu, Q.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Low molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials 2011, 32, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Taki, H.; Tanaka, K.; Takashima, Y.; Okada, H. Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration. Pharm. Res. 2011, 28, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Taki, H.; Kanazawa, T.; Akiyama, F.; Takashima, Y.; Okada, H. Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors. Pharmaceuticals 2012, 5, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Akiyama, F.; Kakizaki, S.; Takashima, Y.; Seta, Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials 2013, 34, 9220–9226. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Posadino, A.M.; Pintus, G.; Sarmento, B.; Giunchedi, P.; Gavini, E. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf. B Biointerfaces 2017, 152, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Solomon, B. Filamentous phage as vector-mediated antibody delivery to the brain. Proc. Natl. Acad. Sci. USA 2002, 99, 5675–5679. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.J. Delivery of Active Proteins to the Central Nervous System Using Phage Vectors. European Patent EP 1898701 A1, 19 March 2008. [Google Scholar]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Elfinger, M.; Maucksch, C.; Rudolph, C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials 2007, 28, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.C.; Wang, A.P.; Chu, Y.C.; Liu, S.; Mu, H.J.; Liu, W.H.; Wu, Z.M.; Sun, K.X.; Li, Y.X. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, J.; Ju, F.; Zhu, H. Intranasal delivery of α-asarone to the brain with lactoferrin-modified mPEG-PLA nanoparticles prepared by premix membrane emulsification. Drug Deliv. Transl. Res. 2018, 8, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Li, W.; Sun, H.; Lou, Y.; Zhang, X.; Li, F. Transferrin receptor antibody-modified α-cobrotoxin-loaded nanoparticles enable drug delivery across the blood–brain barrier by intranasal administration. J. Nanopart. Res. 2013, 15, 2059. [Google Scholar] [CrossRef]
- Hoekman, J.D.; Srivastava, P.; Ho, R.J.Y. Aerosol-stable peptide-coated liposome nanoparticles: A proof-of-concept study with opioid fentanyl in enhancing analgesic effects and reducing plasma drug exposure. J. Pharm. Sci. 2014, 103, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
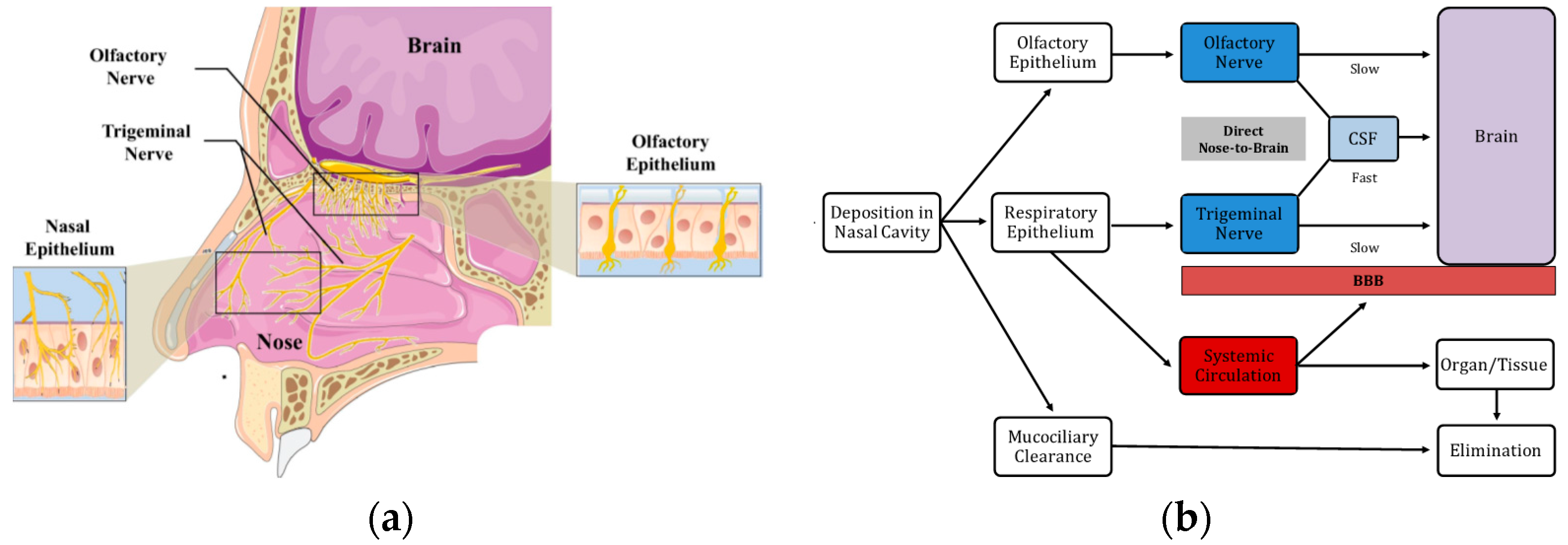

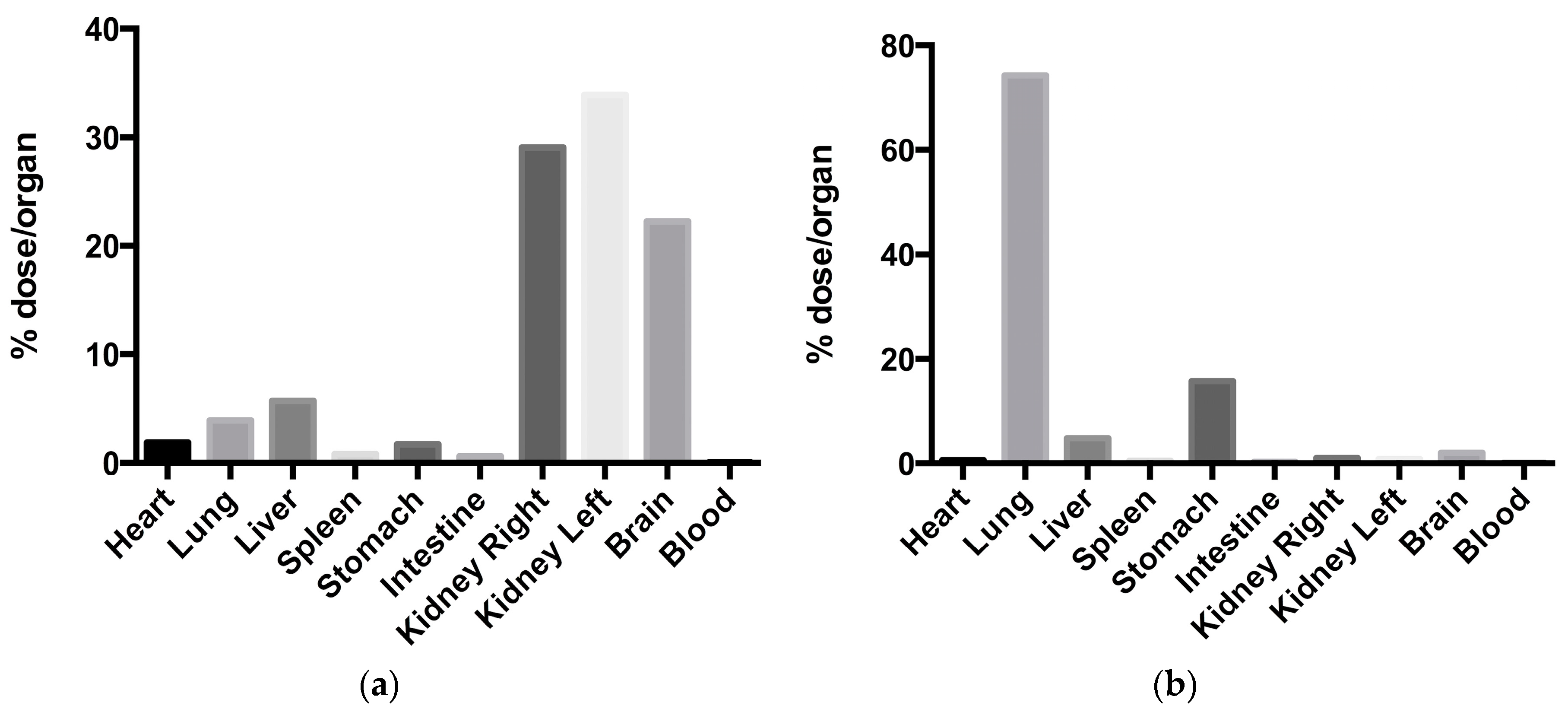
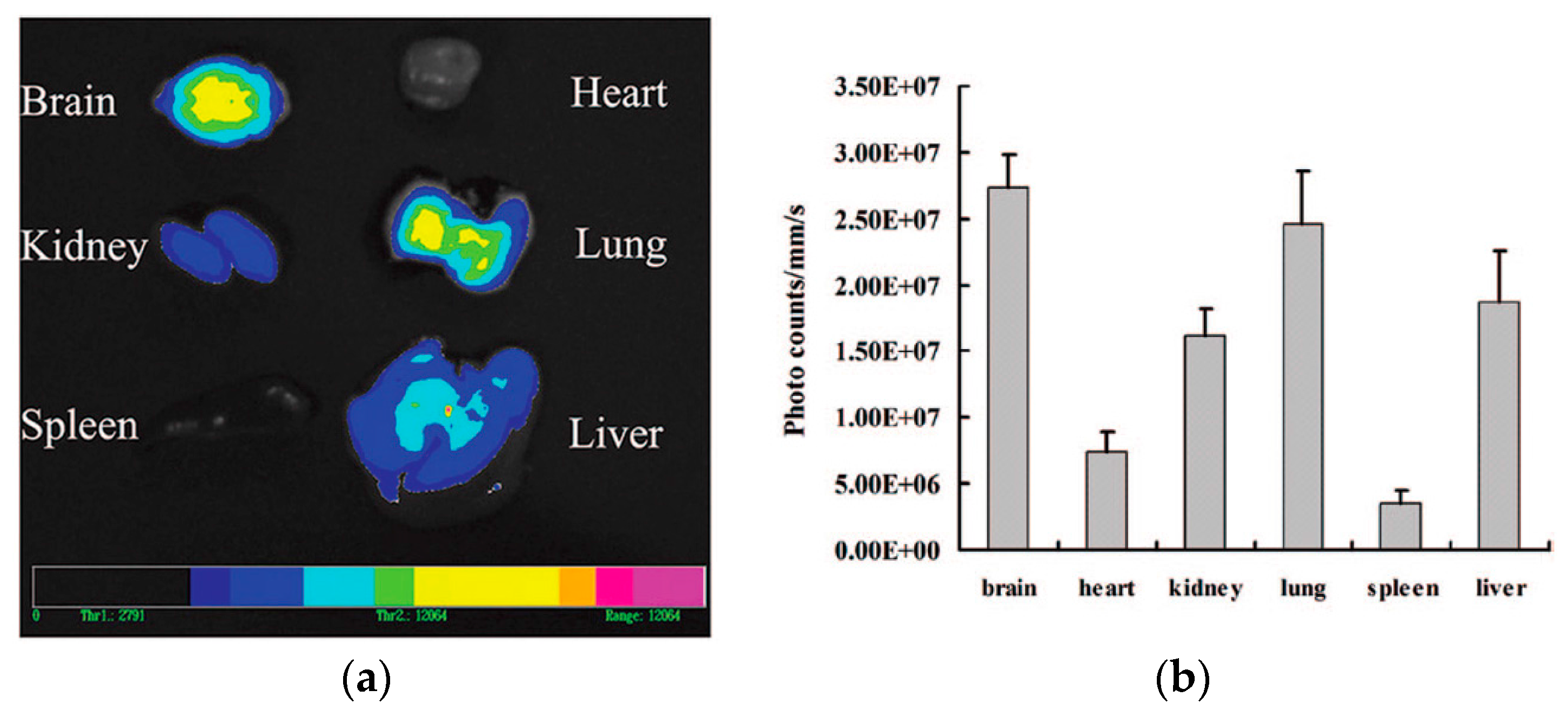
| Nanocarrier | Drug | Application | Size (nm) | PDI | ζ-potential (mV) | EE (%) | DL (%) | Biodistribution | DTE | DTP | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methacrylic-PCL Ncps | Olanzapine | Schizophrenia | 254.9 ± 12.1 | 0.030 | +22.0 ± 1.2 | 99.0 | - | HPLC-UV | - | - | [88] |
| β CD Albumin NPs | Tacrine | AD | 189.3 ± 10.0 | 0.228 | −10.2 ± 0.6 | - | 12.5 | - | - | - | [90] |
| Delonix regia gum NLC | Ondansetron | Nausea | 92.3 ± 13.0 | 0.360 | −11.5 ± 2.3 | 39.5 | 5.6 | HPLC-UV | 506.0 | 97.1 | [91] |
| Alginate NPs | Venlafaxine | Depression | 173.7 ± 2.5 | 0.391 | +37.4 ± 1.7 | 81.3 | 26.7 | CFM | 425.8 | 76.5 | [92] |
| Chitosan NPs | Estradiol | AD | 269.3 ± 31.6 | - | +25.4± 0.7 | 64.7 | 1.9 | HPLC-Fluo | 320.0 | 68.4 | [95] |
| Chitosan NPs | Rivastigmine | AD | 185.4 ± 8.4 | 0.391 | +38.4 ± 2.8 | 85.3 | 43.4 | CFM | 355.0 | 71.8 | [96] |
| Chitosan NPs | Thymoquinone | AD | 172.4 ± 7.4 | 0.130 | +30.3 ± 2.2 | 63.3 | 31.2 | γ scintigraphy | 3321.2 | 97.0 | [97] |
| Chitosan NPs | Bromocriptine | PD | 161.3 ± 4.7 | 0.440 | +40.3 ± 2.7 | 84.2 | 37.8 | γ scintigraphy | 633.0 | 84.2 | [98] |
| Chitosan NPs | Ropinirole | PD | 173.7 ± 2.3 | 0.390 | +32.7 ± 1.5 | 69.6 | 13.8 | γ scintigraphy | - | - | [99] |
| Chitosan NPs | Rasagiline | PD | 151.1 ± 10.3 | 0.380 | - | 96.4 | - | HPLC-UV | 325.0 | 69.3 | [100] |
| Chitosan NPs | Pramipexole | PD | 292.5 ± 8.8 | 0.292 | +14.0 ± 2.9 | 93.3 | - | - | - | - | [101] |
| Chitosan NPs | Tapentadol | Chronic pain | 201.2 ± 1.5 | 0.201 | +49.3 ± 1.2 | 63.5 | 17.2 | HPLC-UV | 321.0 | 68.8 | [102] |
| Trimethylchitosan NPs | Leu-Enk | Chronic pain | 443.0 ± 23.0 | 0.317 | +15.0 ± 2.0 | 78.3 | 14.0 | - | - | - | [103] |
| Thiolated Chitosan NPs | Tizanidine | Muscular pain | 276.2 ± 13.9 | - | +18.3 ± 1.4 | 75.6 | - | γ scintigraphy | 8523 | 98.8 | [105] |
| Thiolated Chitosan NPs | Cyclobenzaprine | Muscular pain | 272.1 ± 11.5 | - | +20.9 ± 1.7 | 70.4 | 5.4 | γ scintigraphy | 2471 | 96.0 | [106] |
| Thiolated Chitosan NPs | Selegiline | Depression | 215.0 ± 34.7 | 0.214 | +17.1 | 70.0 | - | - | - | - | [107] |
| GC SBE β CD NPs | Dopamine | PD | 372.0 ± 81.0 | 0.260 | +9.3 ± 1.3 | 54.5 | - | FM | - | - | [108] |
| Chitosan-PLGA NPs | Chlorpromazine | Schizophrenia | 463.9 ± 12.0 | 0.187 | +21 ± 2 | 36.7 | 4.6 | - | - | - | [109] |
| Chitosan-coated Liposomes | Ghrelin | Cachexia | 194.0 ± 6.1 | 0.198 | +6.0 ± 0.4 | 56.1 | - | - | - | - | [110] |
| Lecithin/Chitosan NPs | Simvastatin | AD | 204.5 ± 15.4 | 0.098 | +48.4 ± 4.1 | 98.5 | - | γ scintigraphy | - | - | [113] |
| Nanocarrier | Drug | Application | Size (nm) | PDI | ζ-potential (mV) | EE (%) | DL (%) | Biodistribution | DTE | DTP | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pluronic® F127 PLGA NPs | Diazepam | Epilepsy | 183.2 | <0.200 | <−15 | 87.8 | - | γ scintigraphy | 258.0 | 61.0 | [51] |
| Pluronic® F127 PLGA NPs | Midazolam | Epilepsy | 164.0 ± 4.5 | 0.099 | −16.6 ± 2.5 | 87.4 | 5.3 | γ scintigraphy | 234.7 | - | [129] |
| Lipid/PEG-PLGA NPs | FTA | Glioblastoma | 164.3 ± 10.3 | 0.192 | −12.0 ± 1.3 | 97.7 | 3.5 | HPLC-MS | - | - | [130] |
| TPGS Micelles | Zolmitriptan | Migraine | 24.2 ± 0.7 | 0.064 | - | - | - | γ scintigraphy | - | - | [136] |
| TPGS Micelles | Sumatriptan | Migraine | 23.1 ± 0.4 | 0.046 | - | - | - | γ scintigraphy | - | - | [137] |
| Poloxamer 188 Cubosomes | Olanzapine | Schizophrenia | 363.0 ± 31.8 | 0.088 | - | 67.3 | - | HPLC-MS/MS | 100 | - | [138] |
| Spanlastics | Risperidone | Schizophrenia | 103.4 | 0.341 | −45.92 | 63.9 | - | HPLC-MS/MS | 468.9 | 55.2 | [139] |
| Gelatin NLC | bFGF | PD | 172.0 ± 1.3 | 0.105 | −27.6 ± 1.1 | 86.7 | 4.6 | Western blot | - | - | [140] |
| Polysorbate 80 SLN | Rosmarinic acid | HD | 149.2 ± 18.2 | 0.290 | −38.27 | 61.9 | - | HPLC-UV | - | - | [141] |
| Novasomes | Zolmitriptan | Migraine | 149.9 ± 10.9 | 0.477 | −55.6 ± 1.0 | 92.9 | - | γ scintigraphy | - | 99.2 | [143] |
| Nanocarrier | Drug | Application | Size (nm) | PDI | ζ-potential (mV) | EE (%) | DL (%) | Biodistribution | DTE | DTP | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WGA PEG-PLA NPs | VIP | AD | 100–120 | - | - | 70.1 | 1.4 | Radiolabeling (125I) | - | - | [152] |
| WGA PEG-PLA NPs | Quantum Dots | Brain Imaging | 95.3 ± 41.0 | - | −22.7 ± 1.2 | - | - | Luminescence | - | - | [153] |
| STL PEG-PLGA NPs | Haloperidol | Schizophrenia | 132 ± 20 | 0.174 | −14.4 ± 0.1 | 73.2 | 0.85 | HPLC | - | - | [157] |
| OL PEG-PLGA NPs | Urocortin | PD | 114.8 ± 5.6 | 0.193 | - | 75.5 | 0.14 | Fluorescence imaging | - | - | [158] |
| Tat mPEG-PCL Micelles | Camptothecin | Glioma | 88.5 ± 20.2 | - | 10.4 ± 2.8 | 62.5 | - | - | - | - | [164] |
| Tat mPEG-PCL Micelles | siRNA | CNS Disorders | 51.0 | - | 11.3 | - | - | Fluorescence imaging | - | - | [165] |
| RVG SLN Chitosan | siRNA | AD | 358.4 ± 25.9 | 0.028 | +10.5 ± 0.8 | 75.5 | 0.14 | - | - | - | [166] |
| Lactoferrin PEG-PCL NPs | NAP | AD | 88.4 ± 7.8 | 0.220 | −23.6 ± 1.0 | 47.61 | 0.62 | Fluorescence imaging | - | - | [171] |
| Lactoferrin PEG-PCL NPs | Rotigotine | PD | 122.0 ± 19.3 | 0.194 | −21.3 ± 2.2 | 92.6 | ~7 | Fluorescence imaging | - | - | [173] |
| Lactoferrin PEG-PCL NPs | α-Asarone | Epilepsy | 360.1 ± 3.7 | 0.165 | −21.8 ± 1.0 | 86.3 | 7.3 | UPLC-MS | 261–734 | >80 | [173] |
| mAb OX26 PEG-PLA NPs | α-Cobrotoxin | Pain | 96.2 ± 6.3 | 0.112 | −33.4 ± 1.2 | 82.1 | - | Fluorescence analysis | - | - | [174] |
| RGD Liposomes | Fentanyl | Pain | 96.5 ± 6.1 | - | - | ~80 | 1.4 | HPLC-MS | - | - | [175] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. https://doi.org/10.3390/pharmaceutics10010034
Sonvico F, Clementino A, Buttini F, Colombo G, Pescina S, Stanisçuaski Guterres S, Raffin Pohlmann A, Nicoli S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics. 2018; 10(1):34. https://doi.org/10.3390/pharmaceutics10010034
Chicago/Turabian StyleSonvico, Fabio, Adryana Clementino, Francesca Buttini, Gaia Colombo, Silvia Pescina, Silvia Stanisçuaski Guterres, Adriana Raffin Pohlmann, and Sara Nicoli. 2018. "Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting" Pharmaceutics 10, no. 1: 34. https://doi.org/10.3390/pharmaceutics10010034